Quality of Life in Women Subjected to Surgical Treatment of Breast Cancer Depending on the Procedure Performed within the Breast and Axillary Fossa—A Single-Center, One Year Prospective Analysis
Abstract
1. Introduction
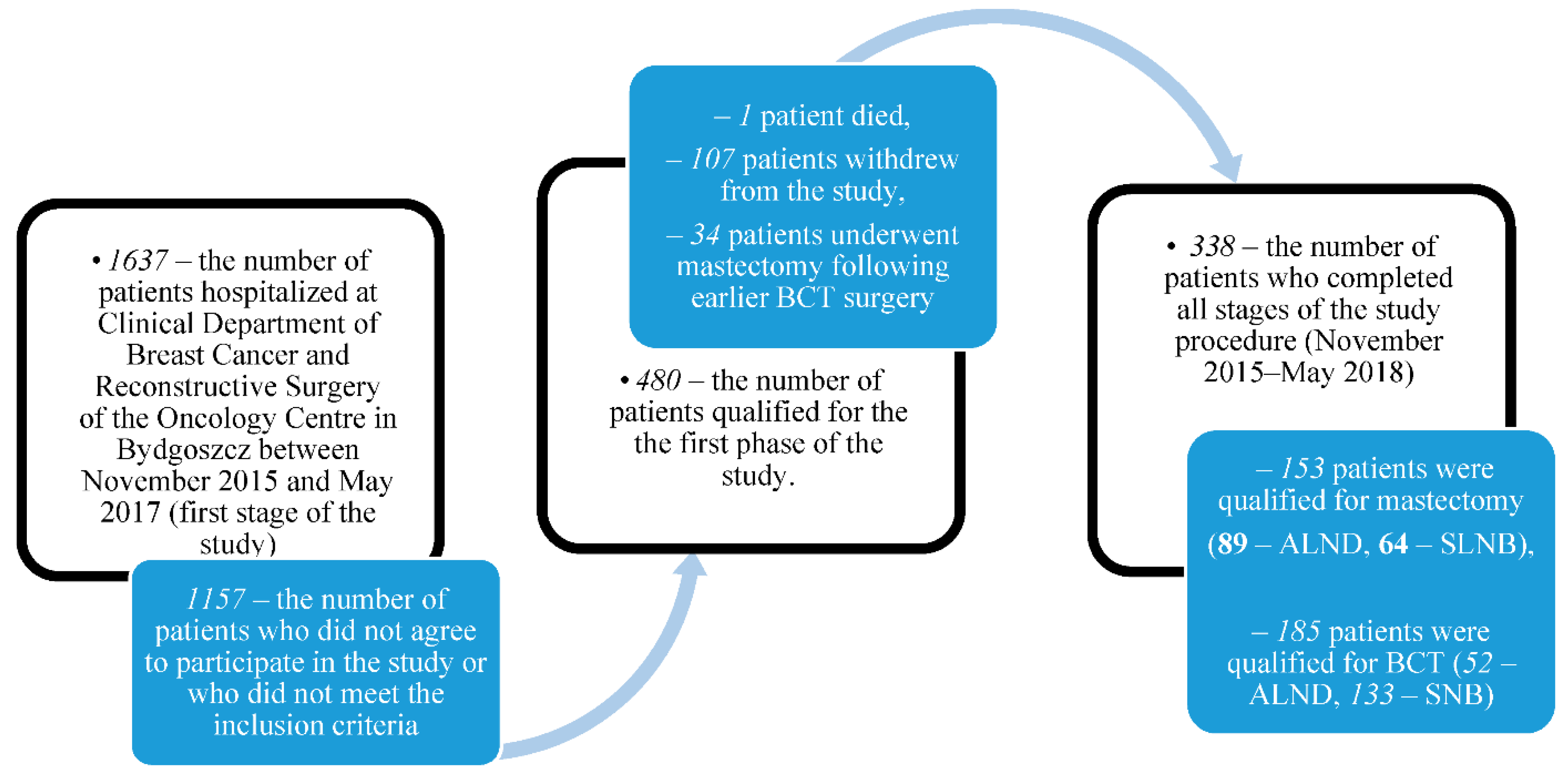
2. Material and Methods
- Written consent to participate in the study.
- Age of above 18.
- Hospitalization for surgical treatment of breast cancer at the Clinical Department of Breast Cancer and Reconstructive Surgery of the Oncology Centre in Bydgoszcz during the first stage of the study (November 2015–May 2017).
- Overall good performance status (ECOG 0-1).
- Exclusion criteria:
- Other severe comorbidities (above ASA II).
- Treatment modality being changed from BCT to mastectomy during the study.
- Distant metastases at the time of qualification for the study.
- Breast reconstruction surgery during the study.
- Lack of consent for breast-conserving treatment (according to patient’s preferences).
- Other malignancy diagnosed in the subject within the last 5 years.
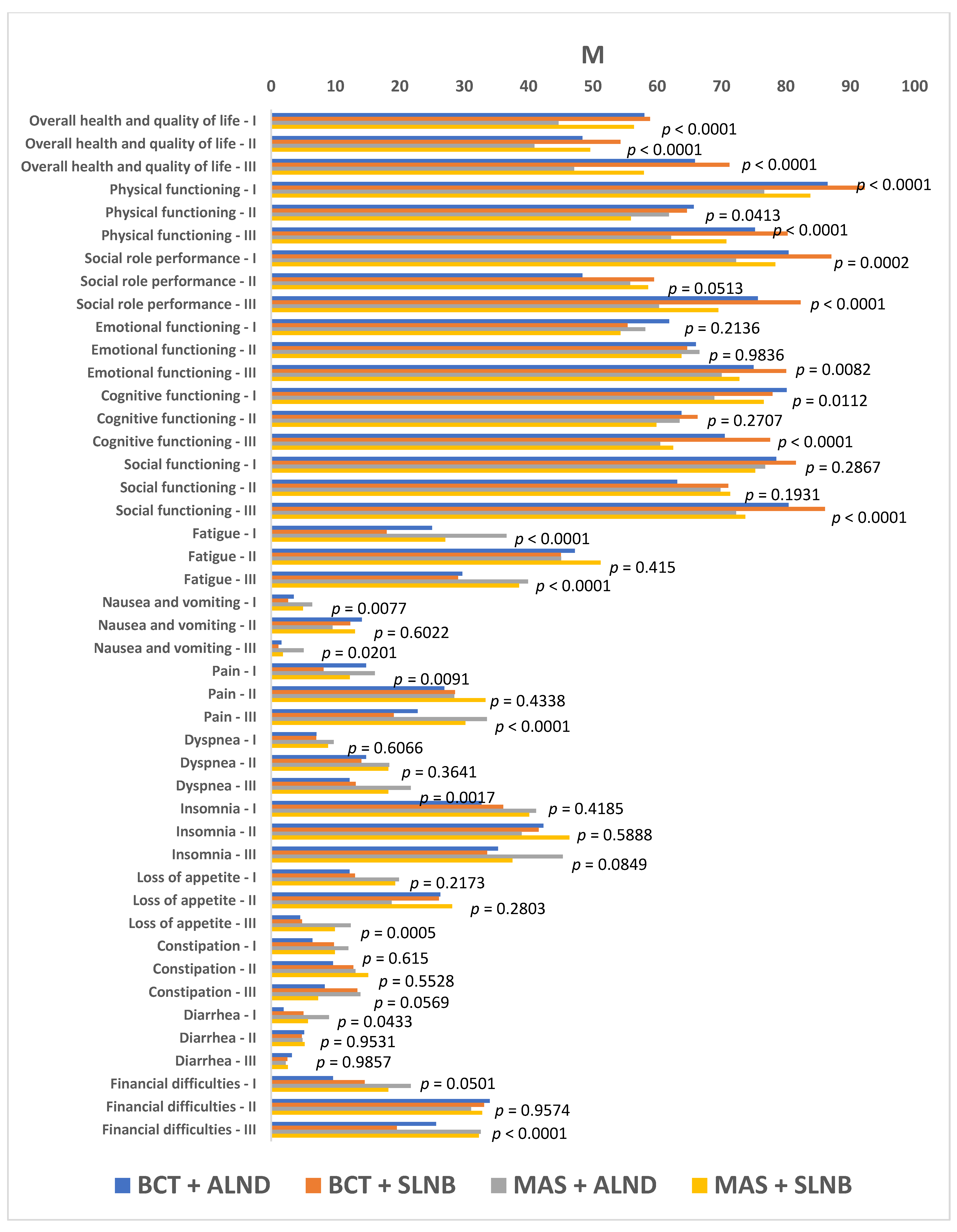
- The EORTC QLQ-C30 questionnaire, version 3.0: an international, standardized test tool developed by the European Organisation for Research and Treatment of Cancer (EORTC) to assess patients’ physical, social, and emotional functioning, role-based performance, memory and concentration, fatigue, pain, nausea and vomiting, loss of appetite, diarrhea, constipation, financial difficulties, dyspnea, insomnia, and overall quality of life. The QLQ-C30 a self-administered tool used in all patients diagnosed with cancer regardless of cancer stage, type, and location.
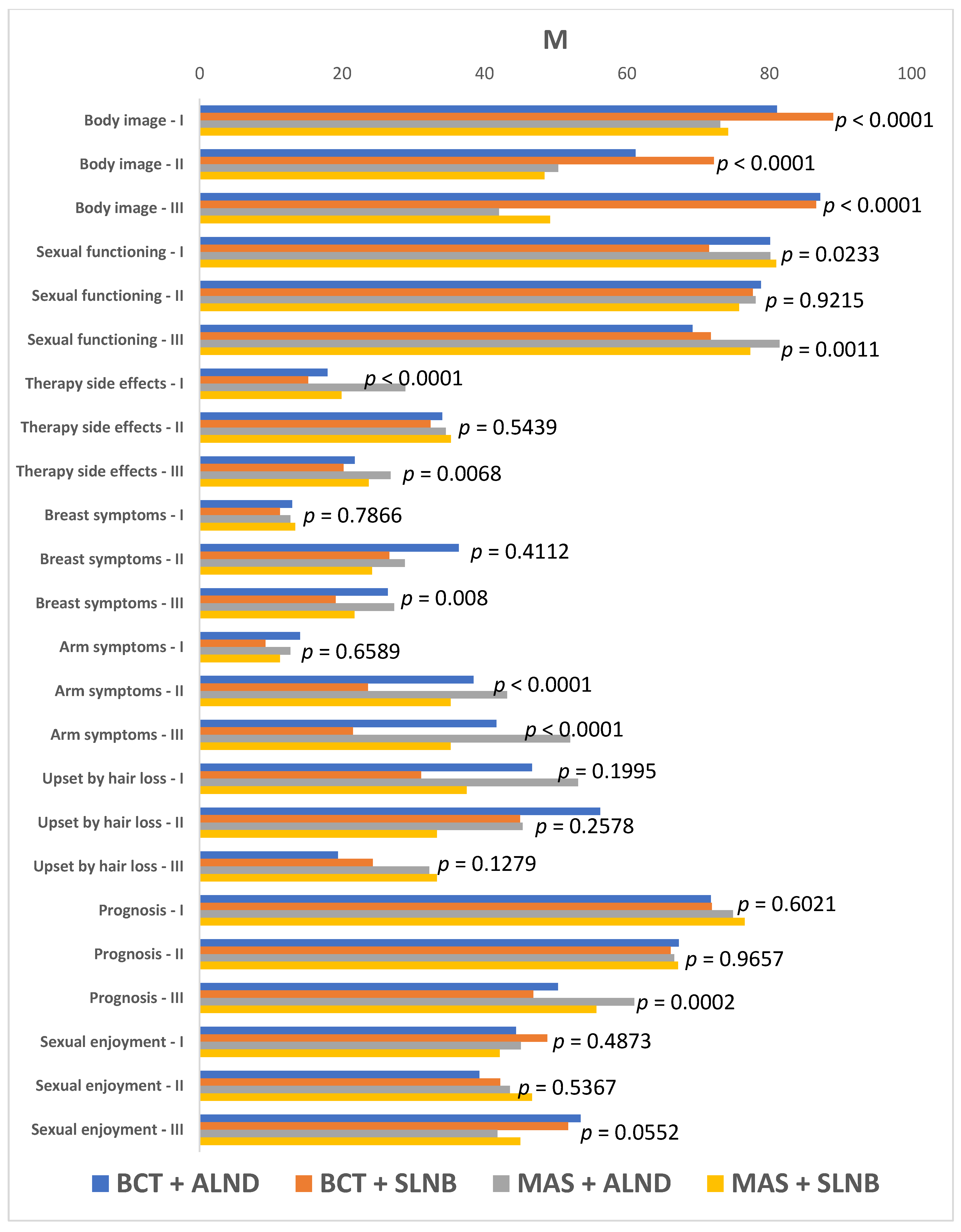
- The EORTC QLQ-BR23 questionnaire for more accurate assessment of the quality of life of women with breast cancer; the questionnaire consists of two functional status scales (body image and sexual functioning), three symptomatic scales (systemic therapy side effects, arm symptoms, and discomfort originating from the operated breast), and three questions regarding patient’s concerns about hair loss, future perspective, and sexual enjoyment. Results obtained for each subscale of QLQ-C30 and QLQ-BR23 questionnaires are in the range of 0–100. For functional scales, higher results mean better performance within the particular aspect whereas for symptomatic scales, the higher the result, the greater the severity of the condition and the poorer the quality of life.
- The Acceptance of Illness Scale (AIS) as developed at the Center for Community Research and Action, Department of Psychology, New York University. The scale consists of eight statements relating to the consequences of ill health. Individual claims pertain to illness-related restrictions, reduced self-esteem, and lack of self-sufficiency. A higher degree of acceptance contributes to faster adjustment to the new health condition and to a better quality of life. The scale is intended for adult patients regardless of their disease. The score range is 8–40 points. The higher the score, the better the acceptance of current illness and the less negative emotions associated with it.
- The Mini-Mental Adjustment to Cancer (Mini-MAC) scale consists of 29 statements that measure four methods of coping with cancer, including anxious preoccupation, fighting spirit, helplessness-hopelessness, and positive re-evaluation. The first two strategies account for a constructive coping style whereas the last two comprise a destructive style of coping with the disease. The scale can be used to assess patients’ response to the diagnosis of cancer as well as their adaptation to subsequent treatment and rehabilitation stages. The coping strategy may be a measure of the quality of life. Higher scores are indicative of a predominant contribution of a particular coping style.
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- Patients qualified for BCT presented with better self-perceived quality of life in many functional dimensions before surgery as well as threeand 12 months afterwards as compared to patients having undergone mastectomy.
- Axillary lymph node dissection contributed to reduced quality of life in numerous functional dimensions regardless of the type of breast surgery. With regard to most functional and symptomatic scales, the best quality of life was observed in women subjected to BCT sentinel lymph node biopsy while the worst quality of life was observed in patients subjected to mastectomy with axillary lymph node dissection.
- BCT with SLNB offered better acceptance of illness levels and contributed to patients choosing more constructive strategies for coping with breast cancer.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeSantis, C.; Siegel, R.; Bandi, P.; Jemal, A. Breast Cancer Statistics, 2011. CA Cancer J. Clin. 2011, 61, 409–418. [Google Scholar] [CrossRef]
- Głowacka-Mrotek, I.; Sowa, M.; Nowikiewicz, T.; Siedlecki, Z.; Hagner, W.; Zegarski, W. Foot Posture in Female patIents 5 Years After Breast-Conserving Surgery: A Case-Control Study. Breast Cancer 2018, 25, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, M.H.; Cruzado, J.A. A Longitudinal Study on Anxiety, Depressive and Adjustment Disorder, Suicide Ideation and Symptoms of Emotional Distress in Patients with Cancer Undergoing Radiotherapy. J. Psychosom. Res. 2016, 87, 14–21. [Google Scholar] [CrossRef]
- Czerw, A.; Religioni, U.; Deptała, A. Assessment of Pain, Acceptance of Illness, Adjustment to Life with Cancer and Coping Strategies in Breast Cancer Patients. Breast Cancer 2016, 23, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, K.; Mansour, E.; Vinluan, J. A Cross-Sectional Assessment of Quality of Life of Breast Cancer Patients in Saudi Arabia. Public Health 2016, 136, 117–125. [Google Scholar] [CrossRef] [PubMed]
- King, M.T.; Kenny, P.; Shiell, A.; Hall, J.; Boyages, J. Quality of Life Three Months and One Year after First Treatment for Early Stage Breast Cancer: Influence of Treatment and Patient Characteristics. Qual. Life Res. 2000, 9, 789–800. [Google Scholar] [CrossRef]
- Arndt, V.; Stegmaier, C.; Ziegler, H. Quality of Life over 5 Years in Women with Breast Cancer after Breast-Conserving Thera-py Versus Mastectomy: A Population-Based Study. J. Cancer Res. Clin. Oncol. 2008, 134, 1311–1318. [Google Scholar] [CrossRef]
- Aerts, L.; Christiaens, M.; Enzlin, P.; Neven, P.; Amant, F. Sexual Functioning in Women after Mastectomy Versus Breast Conserving Therapy for Early-Stage Breast Cancer: A Prospective Controlled Study. Breast 2014, 23, 629–636. [Google Scholar] [CrossRef]
- Rietman, J.S.; Dijkstra, P.U.; Hoekstra, H.J.; Eisma, W.H.; Szabo, B.G.; Groothoff, J.W.; Geertzen, J.H.B. Late Morbidity after Treatment of Breast Cancer in Relation to Daily Activities and Quality of Life: A Systematic Review. Eur. J. Surg. Oncol. 2003, 29, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Ueno, T.; Fujioka, T.; Fujitomi, Y.; Ueo, H. Psychosocial Factors Affecting the Therapeutic Decision-Making and Postoperative Mood States in Japanese Breast Cancer Patients who Underwent Various Types of Surgery: Body Image and Sexuality. J. Clin. Oncol. 2007, 37, 412–418. [Google Scholar] [CrossRef]
- Fobair, P.; Stewart, S.L.; Chang, S.; D’Onofrio, C.; Banks, P.J.; Bloom, J.R. Body Image and Sexual Problems in Young Women with Breast Cancer. Psycho Oncol. J. Psychol. Soc. Behav. Dimens. Cancer 2006, 15, 579–594. [Google Scholar] [CrossRef]
- Belmonte, R.; Garin, O.; Segura, M.; Pont, A.; Escalada, F.; Ferrer, M. Quality-of-Life Impact of Sentinel Lymph Node Biopsy Versus Axillary Lymph Node Dissection in Breast Cancer Patients. Value Health 2012, 15, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, P.; Li, R.; Wu, C.; Zhang, X.; Zhu, H. Axillary Lymph Node Dissection Versus Sentinel Lymph Node Biopsy Alone for Early Breast Cancer with Sentinel Node Metastasis: A Meta-Analysis. Eur. J. Surg. Oncol. 2015, 41, 958–966. [Google Scholar] [CrossRef]
- Peintinger, F.; Reitsamer, R.; Stranzl, H.; Ralph, G. Comparison of Quality of Life and Arm Complaints after Axillary Lymph Node Dissection vs. Sentinel Lymph Node Biopsy in Breast Cancer Patients. Br. J. Cancer 2003, 89, 648–652. [Google Scholar] [CrossRef]
- Kootstra, J.; Hoekstra-Weebers, J.E.H.M.; Rietman, H.; De Vries, J.; Baas, P.; Geertzen, J.H.B.; Hoekstra, H.J. Quality of Life After Sentinel Lymph Node Biopsy or Axillary Lymph Node Dissection in Stage I/II Breast Cancer Patients: A Prospective Longitudinal Study. Ann. Surg. Oncol. 2008, 15, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Chabowski, M.; Polański, J.; Jankowska-Polanska, B.; Lomper, K.; Janczak, D.; Rosinczuk, J. The Acceptance of Illness, the Intensity of Pain and the Quality of Life in Patients with Lung Cancer. J. Thorac. Dis. 2017, 9, 2952–2958. [Google Scholar] [CrossRef]
- Cieślak, K.; Golusiński, W. Coping with Loss of Ability vs. Acceptance of Disease in Women after Breast Cancer Treatment. Rep. Pract. Oncol. Radiother. 2017, 22, 231–236. [Google Scholar] [CrossRef][Green Version]
- Religioni, U.; Czerw, A.; Deptała, A. Acceptance of Cancer in Patients Diagnosed with Lung, Breast, Colorectal and Prostate Carcinoma. Iran. J. Public Health 2015, 44, 1135–1142. [Google Scholar] [PubMed]
- Geyer, S.; Koch-Giesselmann, H.; Noeres, D. Coping with Breast Cancer and Relapse: Stability of Coping and Long-Term Outcomes in an Observational Study over 10 Years. Soc. Sci. Med. 2015, 135, 92–98. [Google Scholar] [CrossRef]
- Johansson, M.; Rydén, A.; Finizia, C. Mental Adjustment to Cancer and its Relation Toanxiety, Depression, HRQL and Survival in Patients with Laryngeal Cancer—A Longitudinal Study. BMC Cancer 2011, 11, 283. [Google Scholar] [CrossRef]
- Deepa, K.V.; Gadgil, A.; Löfgren, J.; Mehare, S.; Bhandarkar, P.; Roy, N. Is Quality of Life after Mastectomy Comparable to that after Breast Conservation Surgery? A 5-Year Follow Up Study from MUMBAI, India. Qual. Life Res. 2020, 29, 683–692. [Google Scholar] [CrossRef]
- Okoli, C.; Anyanwu, S.N.C.; Ochomma, A.O.; Emegoakor, C.D.; Chianakwana, G.U.; Nzeako, H.; Ihekwoaba, E. Assessing the Quality of Life of Patients with Breast Cancer Treated in a Tertiary Hospital in a Resource-Poor Country. World J. Surg. 2018, 43, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Słowik, A.J.; Jabłoński, M.J.; Michałowska-Kaczmarczyk, A.M.; Jach, R. Evaluation of Quality of Life in Women with Breast Cancer, with Particular Emphasis on Sexual Satisfaction, Future Perspectives and Body Image, Depending on the Method of Surgery. Psychiatr. Pol. 2017, 51, 871–888. [Google Scholar] [CrossRef]
- Marinkovic, M.; Djordjevic, N.; Djordjevic, L.; Ignjatovic, N.; Djordjevic, M.; Karanikolic, V. Assessment of the Quality of Life in Breast Cancer Depending on the Surgical Treatment. Support. Care Cancer 2020, 1–10. [Google Scholar] [CrossRef]
| BCT (n = 185) | MAS (n = 153) | |||||
|---|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |||
| Operated side | left | 89 | 48.11% | 81 | 52.94% | χ2 = 0.78, p = 0.3764 |
| right | 96 | 51.89% | 72 | 47.06% | ||
| Neoadjuvant treatment | no | 171 | 92.43% | 86 | 56.21% | χ2 = 60.42, p < 0.0001 |
| yes | 14 | 7.57% | 67 | 43.79% | ||
| Staging | IA | 105 | 56.76% | 32 | 20.91% | χ2 = 80.57, p < 0.0001 |
| IIA | 58 | 31.35% | 45 | 29.41% | ||
| IIB | 20 | 10.81% | 30 | 19.61% | ||
| IIIA | 1 | 0.541% | 21 | 13.72% | ||
| IIIB | 1 | 0.541% | 25 | 16.34% | ||
| Adjuvant treatment | CHTH | 75 | 40.54% | 57 | 37.25% | χ2 = 0.38, p = 0.5377 |
| RTH | 185 | 100.0% | 92 | 60.13% | χ2 = 90.00, p < 0.0001 | |
| HTH | 146 | 78.92% | 104 | 67.97% | χ2 = 5.21, p = 0.0225 | |
| herceptin | 20 | 10.81% | 32 | 20.91% | χ2 = 6.57, p = 0.0104 | |
| none | 0 | 0.00% | 4 | 2.61% | χ2 = 4.89, p = 0.0269 | |
| ER | positive | 146 | 78.92% | 104 | 67.97% | χ2 = 5.21, p = 0.0225 |
| negative | 39 | 21.081 | 49 | 32.03% | ||
| PR | positive | 139 | 75.13% | 90 | 58.83% | χ2 = 10.20, p = 0.0014 |
| negative | 46 | 24.84% | 63 | 41.17% | ||
| HER2 | positive | 20 | 10.81% | 32 | 20.91% | χ2 = 6.57, p = 0.0104 |
| negative | 165 | 89.19% | 121 | 79.09% | ||
| Menopause | no | 40 | 21.62% | 51 | 33.33% | χ2 = 5.84, p = 0.0157 |
| yes | 145 | 78.38% | 102 | 66.67% | ||
| Family history | negative | 144 | 77.84% | 117 | 76.47% | χ2 = 0.09, p = 0.7655 |
| positive | 41 | 22.16% | 36 | 23.53% | ||
| Age | >35 | 4 | 2.16% | 4 | 2.61% | χ2 = 9.48, p = 0.0501 |
| 35 to 44 | 12 | 6.49% | 23 | 15.03% | ||
| 45 to 54 | 48 | 25.95% | 37 | 24.18% | ||
| 55 to 64 | 75 | 40.54% | 45 | 29.41% | ||
| 65 and over | 46 | 24.87% | 44 | 28.76% | ||
| Educational background | elementary | 23 | 12.43% | 12 | 7.84% | χ2 = 6.33, p = 0.0965 |
| vocational | 34 | 18.38% | 43 | 28.10% | ||
| secondary | 76 | 41.08% | 64 | 41.83% | ||
| higher | 52 | 28.11% | 34 | 22.22% | ||
| Area of residence | rural | 48 | 25.95% | 50 | 32.68% | χ2 = 4.33, p = 0.1151 |
| urban (population up to 50,000 residents) | 63 | 34.05% | 58 | 37.91% | ||
| urban (population above 50,000 residents) | 74 | 40.00% | 45 | 29.41% | ||
| Employment status | employed | 65 | 35.13% | 41 | 26.80% | χ2 = 10.56, p = 0.0609 |
| self-employed | 11 | 5.95% | 5 | 3.27% | ||
| housewife | 11 | 5.95% | 9 | 5.88% | ||
| retired | 75 | 40.54% | 61 | 39.87% | ||
| disability allowance or family pension | 12 | 6.49% | 24 | 15.69% | ||
| unemployed | 11 | 5.95% | 13 | 8.50% | ||
| Marital status | unmarried | 11 | 5.95% | 11 | 7.19% | χ2 = 2.34, p = 0.5046 |
| married | 131 | 70.81% | 109 | 71.24% | ||
| divorced | 20 | 10.81% | 10 | 6.54% | ||
| widowed | 23 | 12.43% | 23 | 15.03% | ||
| Socioeconomic status | very high | 9 | 4.86% | 5 | 3.27% | χ2 = 1.12, p = 0.7714 |
| high | 75 | 40.54% | 65 | 42.48% | ||
| medium | 90 | 48.65% | 71 | 46.40% | ||
| low | 11 | 5.95% | 12 | 7.84% | ||
| BCT | MAS | ||||
|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | ||
| ALND | 52 | 28.11% | 89 | 58.17% | χ2 = 31.13, p < 0.0001 |
| SLNB | 133 | 71.89% | 64 | 41.83% | |
| Time Point | Group | M | Me | SD | Kruskall-Wallis Test | POST-HOC (Dunn Bonferroni) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| BCT + ALND | BCT + SLNB | MAS + ALND | MAS + SLNB | |||||||
| General acceptance of illness | I | BCT + ALND | 28.62 | 29.00 | 7.47 | H = 37.41, p < 0.0001 | 0.5133 | 0.0089 | 1 | |
| BCT + SLNB | 30.62 | 32.00 | 7.42 | 0.5133 | <0.0001 | 0.3021 | ||||
| MAS + ALND | 24.34 | 22.00 | 7.54 | 0.0089 | <0.0001 | 0.0062 | ||||
| MAS + SLNB | 28.48 | 29.00 | 7.19 | 1 | 0.3021 | 0.0062 | ||||
| II | BCT + ALND | 23.90 | 23.00 | 7.25 | H = 86.3292, p < 0.0001 | <0.0001 | 1 | 0.0725 | ||
| BCT + SLNB | 30.38 | 31.00 | 5.77 | <0.0001 | <0.0001 | 0.0056 | ||||
| MAS + ALND | 22.98 | 22.00 | 4.86 | 1 | <0.0001 | 0.0002 | ||||
| MAS + SLNB | 27.03 | 27.00 | 5.13 | 0.0725 | 0.0056 | 0.0002 | ||||
| III | BCT + ALND | 27.77 | 28.00 | 6.85 | H = 123.4095, p < 0.0001 | <0.0001 | 0.0001 | 1 | ||
| BCT + SLNB | 32.80 | 33.00 | 5.43 | <0.0001 | <0.0001 | <0.0001 | ||||
| MAS + ALND | 22.73 | 23.00 | 4.43 | 0.0001 | <0.0001 | 0.0001 | ||||
| MAS + SLNB | 27.44 | 26.50 | 5.54 | 1 | <0.0001 | 0.0001 | ||||
| Time Point | Group | M | Me | SD | Kruskall-Wallis Test | POST-HOC (Dunn Bonferroni) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| BCT + ALND | BCT + SLNB | MAS + ALND | MAS + SLNB | |||||||
| CON | I | BCT + ALND | 44.10 | 44.00 | 4.24 | H = 11.6415, p = 0.0087 | 0.2222 | 1 | 0.4262 | |
| BCT + SLNB | 45.53 | 46.00 | 4.79 | 0.2222 | 0.0227 | 1 | ||||
| MAS + ALND | 43.83 | 44.00 | 4.42 | 1 | 0.0227 | 0.0998 | ||||
| MAS + SLNB | 45.64 | 45.00 | 4.73 | 0.4262 | 1 | 0.0998 | ||||
| II | BCT + ALND | 42.75 | 43.00 | 4.14 | H = 10.932, p = 0.0121 | 0.064 | 0.0288 | 0.014 | ||
| BCT + SLNB | 44.26 | 44.00 | 3.88 | 0.064 | 1 | 1 | ||||
| MAS + ALND | 44.56 | 45.00 | 4.08 | 0.0288 | 1 | 1 | ||||
| MAS + SLNB | 44.66 | 46.00 | 4.77 | 0.014 | 1 | 1 | ||||
| III | BCT + ALND | 44.40 | 45.00 | 5.54 | H = 1.816, p = 0.6115 | 1 | 1 | 1 | ||
| BCT + SLNB | 44.58 | 44.00 | 4.78 | 1 | 1 | 1 | ||||
| MAS + ALND | 43.65 | 44.00 | 5.09 | 1 | 1 | 1 | ||||
| MAS + SLNB | 44.00 | 43.00 | 4.82 | 1 | 1 | 1 | ||||
| DES | I | BCT + ALND | 30.71 | 32.00 | 5.90 | H = 0.7573, p = 0.8597 | 1 | 1 | 1 | |
| BCT + SLNB | 30.45 | 30.00 | 6.30 | 1 | 1 | 1 | ||||
| MAS + ALND | 30.74 | 31.00 | 6.56 | 1 | 1 | 1 | ||||
| MAS + SLNB | 30.16 | 30.00 | 5.65 | 1 | 1 | 1 | ||||
| II | BCT + ALND | 32.17 | 33.50 | 5.94 | H = 4.4939, p = 0.2128 | 0.2123 | 0.6629 | 1 | ||
| BCT + SLNB | 30.17 | 30.00 | 6.17 | 0.2123 | 1 | 1 | ||||
| MAS + ALND | 30.67 | 30.00 | 6.39 | 0.6629 | 1 | 1 | ||||
| MAS + SLNB | 30.75 | 30.00 | 5.91 | 1 | 1 | 1 | ||||
| III | BCT + ALND | 27.19 | 27.00 | 6.38 | H = 25.1802, p < 0.0001 | 1 | 0.0052 | 1 | ||
| BCT + SLNB | 26.38 | 26.00 | 6.43 | 1 | <0.0001 | 0.2348 | ||||
| MAS + ALND | 31.46 | 31.00 | 7.26 | 0.0052 | <0.0001 | 0.2007 | ||||
| MAS + SLNB | 28.89 | 29.00 | 8.08 | 1 | 0.2348 | 0.2007 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarkowska, M.; Głowacka-Mrotek, I.; Nowikiewicz, T.; Goch, A.; Zegarski, W. Quality of Life in Women Subjected to Surgical Treatment of Breast Cancer Depending on the Procedure Performed within the Breast and Axillary Fossa—A Single-Center, One Year Prospective Analysis. J. Clin. Med. 2021, 10, 1339. https://doi.org/10.3390/jcm10071339
Tarkowska M, Głowacka-Mrotek I, Nowikiewicz T, Goch A, Zegarski W. Quality of Life in Women Subjected to Surgical Treatment of Breast Cancer Depending on the Procedure Performed within the Breast and Axillary Fossa—A Single-Center, One Year Prospective Analysis. Journal of Clinical Medicine. 2021; 10(7):1339. https://doi.org/10.3390/jcm10071339
Chicago/Turabian StyleTarkowska, Magdalena, Iwona Głowacka-Mrotek, Tomasz Nowikiewicz, Aleksander Goch, and Wojciech Zegarski. 2021. "Quality of Life in Women Subjected to Surgical Treatment of Breast Cancer Depending on the Procedure Performed within the Breast and Axillary Fossa—A Single-Center, One Year Prospective Analysis" Journal of Clinical Medicine 10, no. 7: 1339. https://doi.org/10.3390/jcm10071339
APA StyleTarkowska, M., Głowacka-Mrotek, I., Nowikiewicz, T., Goch, A., & Zegarski, W. (2021). Quality of Life in Women Subjected to Surgical Treatment of Breast Cancer Depending on the Procedure Performed within the Breast and Axillary Fossa—A Single-Center, One Year Prospective Analysis. Journal of Clinical Medicine, 10(7), 1339. https://doi.org/10.3390/jcm10071339






