Ectopic Fat Accumulation in Pancreas and Heart
Abstract
1. Introduction
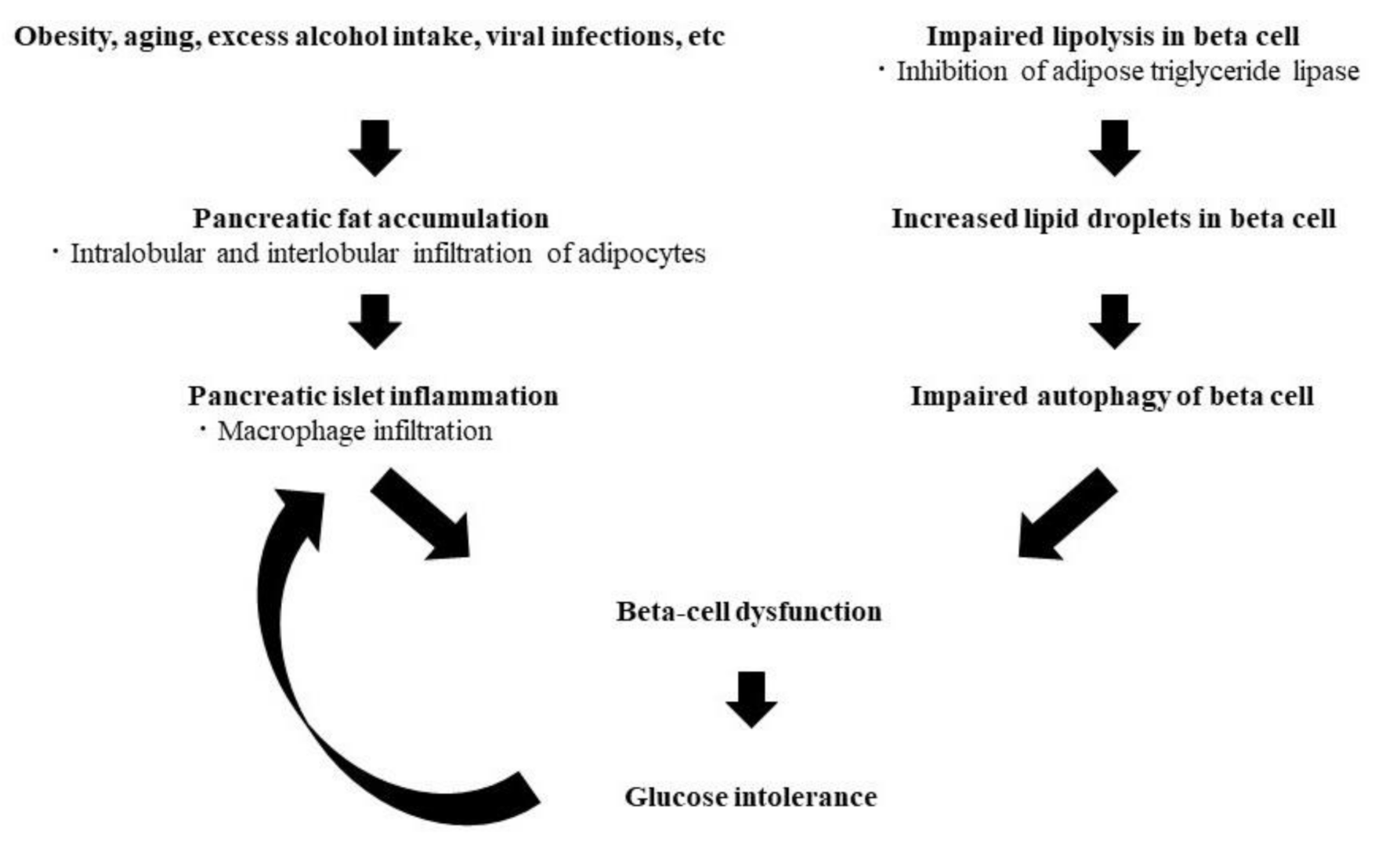
2. Ectopic Fat in Pancreas
3. Ectopic Fat in Heart
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sattar, N.; Gill, J.M. Type 2 diabetes as a disease of ectopic fat? BMC Med. 2014, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Dewidar, B.; Kahl, S.; Pafili, K.; Roden, M. Metabolic liver disease in diabetes—From mechanisms to clinical trials. Metabolism 2020, 111, 154299. [Google Scholar] [CrossRef]
- Yu, T.Y.; Wang, C.Y. Impact of non-alcoholic fatty pancreas disease on glucose metabolism. J. Diabetes Investig. 2017, 8, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Meigs, J.B. Links between ectopic fat and vascular disease in humans. Arter. Thromb. Vasc. Biol. 2014, 34, 1820–1826. [Google Scholar] [CrossRef]
- Baron, A.D.; Brechtel, G.; Wallace, P.; Edelman, S.V. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am. J. Physiol. Endocrinol. Metab. 1988, 255, E769–E774. [Google Scholar] [CrossRef]
- Jacob, S.; Machann, J.; Rett, K.; Brechtel, K.; Volk, A.; Renn, W.; Maerker, E.; Matthaei, S.; Schick, F.; Claussen, C.D.; et al. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes 1999, 48, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; He, J.; Watkins, S.; Kelley, D.E. Skeletal muscle lipid content and insulin resistance: Evidence for a paradox in endur-ance-trained athletes. J. Clin. Endocrinol. Metab. 2001, 86, 5755–5761. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.C.; Hwang, S.J.; Porter, S.A.; Massaro, J.M.; Hoffmann, U. Fox CS. Fatty kidney, hypertension, and chronic kidney disease: The Framingham Heart Study. Hypertension 2011, 58, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, H.L.; Morgan, T.M.; Rocco, M.; Stacey, B.; Brinkley, T.E.; Ding, J.; Nicklas, B.; Hamilton, C.; Hundley, W.G. Renal sinus fat and poor blood pressure control in middle-aged and elderly individuals at risk for cardiovascular events. Hypertension 2010, 56, 901–906. [Google Scholar] [CrossRef]
- Irazabal, M.V.; Eirin, A. Role of Renal Sinus Adipose Tissue in Obesity-induced Renal Injury. EBioMedicine 2016, 13, 21–22. [Google Scholar] [CrossRef]
- Shen, F.C.; Cheng, B.C.; Chen, J.F. Peri-renal fat thickness is positively associated with the urine albumin excretion rate in patients with type 2 diabetes. Obes. Res. Clin. Pract. 2020, 14, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Schwenzer, N.F.; Machann, J.; Martirosian, P.; Stefan, N.; Schraml, C.; Fritsche, A.; Claussen, C.D.; Schick, F. Quantification of pan-creatic lipomatosis and liver steatosis by MRI: Comparison of in/opposed-phase and spectral-spatial excitation techniques. Invest. Radiol. 2008, 43, 330–337. [Google Scholar] [CrossRef]
- Ji, J.; Petropavlovskaia, M.; Khatchadourian, A.; Patapas, J.; Makhlin, J.; Rosenberg, L.; Maysinger, D. Type 2 diabetes is associated with suppression of autophagy and lipid accumulation in β-cells. J. Cell. Mol. Med. 2019, 23, 2890–2900. [Google Scholar] [CrossRef]
- Tong, X.; Dai, C.; Walker, J.T.; Nair, G.G.; Kennedy, A.; Carr, R.M.; Hebrok, M.; Powers, A.C.; Stein, R. Lipid Droplet Accumulation in Human Pancreatic Islets Is Dependent On Both Donor Age and Health. Diabetes 2020, 69, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Promes, J.A.; Harata, M.; Mishra, A.; Stephens, S.B.; Taylor, E.B.; Burand, A.J., Jr.; Sivitz, W.I.; Fink, B.D.; Ankrum, J.A.; et al. Adipose Triglyceride Lipase Is a Key Lipase for the Mobilization of Lipid Droplets in Human beta-Cells and Critical for the Maintenance of Syntaxin 1a Levels in beta-Cells. Diabetes 2020, 69, 1178–1192. [Google Scholar] [CrossRef] [PubMed]
- Saisho, Y.; Butler, A.E.; Meier, J.J.; Monchamp, T.; Allen-Auerbach, M.; Rizza, R.A.; Butler, P.C. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin. Anat. 2007, 20, 933–942. [Google Scholar] [CrossRef]
- Tariq, H.; Nayudu, S.; Akella, S.; Glandt, M.; Chilimuri, S. Non-Alcoholic Fatty Pancreatic Disease: A Review of Literature. Gastro-Enterol. Res. 2016, 9, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Dite, P.; Blaho, M.; Bojkova, M.; Jabandziev, P.; Kunovsky, L. Nonalcoholic Fatty Pancreas Disease: Clinical Consequences. Dig. Dis. 2020, 38, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, C.; Kozawa, J.; Fujita, Y.; Yoneda, S.; Uno, S.; Kimura, T.; Fukui, K.; Nojima, S.; Morii, E.; Eguchi, H.; et al. Glucose Intolerance After Pancreatectomy Was Associated With Preoperative Hemoglobin A1c, Insulin Resistance, and Histological Pancreatic Fatty Infiltration. Pancreas 2018, 47, e48–e50. [Google Scholar] [CrossRef]
- Horii, T.; Fujita, Y.; Ishibashi, C.; Fukui, K.; Eguchi, H.; Kozawa, J.; Shimomura, I. Islet inflammation is associated with pancreatic fatty infiltration and hyperglycemia in type 2 diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001508. [Google Scholar] [CrossRef]
- Ishibashi, C.; Kozawa, J.; Hosakawa, Y.; Yoneda, S.; Kimura, T.; Fujita, Y.; Fukui, K.; Iwahashi, H.; Shimomura, I. Pancreatic fat is related to the longitudinal decrease in the increment of C-peptide in glucagon stimulation test in type 2 diabetes patients. J. Diabetes Investig. 2020, 11, 80–87. [Google Scholar] [CrossRef]
- Petit, J.M.; Cercueil, J.P.; Loffroy, R.; Denimal, D.; Bouillet, B.; Fourmont, C.; Chevallier, O.; Duvillard, L.; Vergès, B. Effect of Li-raglutide Therapy on Liver Fat Content in Patients With Inadequately Controlled Type 2 Diabetes: The Lira-NAFLD Study. J. Clin. Endocrinol. Metab. 2017, 102, 407–415. [Google Scholar]
- Eguchi, Y.; Kitajima, Y.; Hyogo, H.; Takahashi, H.; Kojima, M.; Ono, M.; Araki, N.; Tanaka, K.; Yamaguchi, M.; Matsuda, Y.; et al. Japan Study Group for NAFLD (JSG-NAFLD). Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J). Hepatol. Res. 2015, 45, 269–278. [Google Scholar] [CrossRef]
- Shibuya, T.; Fushimi, N.; Kawai, M.; Yoshida, Y.; Hachiya, H.; Ito, S.; Kawai, H.; Ohashi, N.; Mori, A. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective randomized con-trolled pilot study. Diabetes Obes. Metab. 2018, 20, 438–442. [Google Scholar] [CrossRef]
- Fujimori, N.; Tanaka, N.; Kimura, T.; Sano, K.; Horiuchi, A.; Kato, N.; Takahashi, Y.; Kuribayashi, N.; Sugiura, A.; Yamazaki, T.; et al. Long-term luseogliflozin therapy improves histological activity of non-alcoholic steato-hepatitis accompanied by type 2 diabetes mellitus. Clin. J. Gastroenterol. 2020, 13, 83–89. [Google Scholar] [CrossRef]
- Honda, Y.; Kessoku, T.; Ogawa, Y.; Tomeno, W.; Imajo, K.; Fujita, K.; Yoneda, M.; Takizawa, T.; Saito, S.; Nagashima, Y.; et al. Pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator, improves the pathogenesis in a rodent model of nonalcoholic steatohepatitis. Sci. Rep. 2017, 7, 42477. [Google Scholar] [CrossRef] [PubMed]
- Shiba, K.; Tsuchiya, K.; Komiya, C.; Miyachi, Y.; Mori, K.; Shimazu, N.; Yamaguchi, S.; Ogasawara, N.; Katoh, M.; Itoh, M.; et al. Canagliflozin, an SGLT2 inhibitor, attenuates the development of hepatocellular carcinoma in a mouse model of human NASH. Sci. Rep. 2018, 8, 2362. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Hori, M.; Ishigamori, R.; Mutoh, M.; Imai, T.; Nakagama, H. Fatty pancreas: A possible risk factor for pancreatic cancer in animals and humans. Cancer Sci. 2018, 109, 3013–3023. [Google Scholar] [CrossRef]
- Hori, M.; Takahashi, M.; Hiraoka, N.; Yamaji, T.; Mutoh, M.; Ishigamori, R.; Furuta, K.; Okusaka, T.; Shimada, K.; Kosuge, T.; et al. Association of pancreatic Fatty infiltration with pancreatic ductal adenocarcinoma. Clin. Transl. Gastroenterol. 2014, 5, e53. [Google Scholar] [CrossRef]
- Horii, T.; Kozawa, J.; Fujita, S.; Hosokawa, Y.; Kimura, T.; Fujita, Y.; Tokunaga, A.; Fukui, K.; Shimomura, I. Amelioration of pancreatic fat accumulation in Japanese type 2 diabetes patients treated with sodium-glucose cotransporter 2 inhibitors: A retrospective study. Obes. Sci. Pract. 2021. [Google Scholar] [CrossRef]
- Iacobellis, G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat. Rev. Endocrinol. 2015, 11, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Milanese, G.; Silva, M.; Ledda, R.E.; Goldoni, M.; Nayak, S.; Bruno, L.; Rossi, E.; Maffei, E.; Cademartiri, F.; Sverzellati, N. Validity of epicardial fat volume as biomarker of coronary artery disease in symptomatic individuals: Results from the ALTER-BIO registry. Int. J. Cardiol. 2020, 314, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, Y.; Nakamura, N.; Itatani, R.; Oda, S.; Kusunoki, S.; Takahashi, H.; Nakaura, T.; Utsunomiya, D.; Yamashita, Y. Epicardial fat volume measured on nongated chest CT is a predictor of coronary artery disease. Eur. Radiol. 2019, 29, 3638–3646. [Google Scholar] [CrossRef]
- Hirano, K.; Ikeda, Y.; Zaima, N.; Sakata, Y.; Matsumiya, G. Triglyceride deposit cardiomyovasculopathy. N. Engl. J. Med. 2008, 359, 2396–2398. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, R.W.; Doornbos, J.; Kozerke, S.; Schär, M.; Bax, J.J.; Hammer, S.; Smit, J.W.; Romijn, J.A.; Diamant, M.; Rijzewijk, L.J.; et al. Metabolic imaging of myocardial triglyceride content: Reproducibility of 1H MR spectroscopy with respiratory navigator gating in volunteers. Radiology 2007, 245, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Rijzewijk, L.J.; van der Meer, R.W.; Smit, J.W.; Diamant, M.; Bax, J.J.; Hammer, S.; Romijn, J.A.; de Roos, A.; Lamb, H.J. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J. Am. Coll. Cardiol. 2008, 52, 1793–1799. [Google Scholar] [CrossRef]
- Astorri, E.; Fiorina, P.; Gavaruzzi, G.; Astorri, A.; Magnati, G. Left ventricular function in insulin-dependent and in non-insulin-dependent diabetic patients: Radionuclide assessment. Cardiology 1997, 88, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Perseghin, G.; Fiorina, P.; De Cobelli, F.; Scifo, P.; Esposito, A.; Canu, T.; Danna, M.; Gremizzi, C.; Secchi, A.; Luzi, L.; et al. Cross-sectional assessment of the effect of kidney and kidney-pancreas transplantation on resting left ventricular energy metabolism in type 1 diabetic-uremic patients: A phosphorous-31 magnetic resonance spectroscopy study. J. Am. Coll. Cardiol. 2005, 46, 1085–1092. [Google Scholar] [CrossRef][Green Version]
- Scheuermann-Freestone, M.; Madsen, P.L.; Manners, D.; Blamire, A.M.; Buckingham, R.E.; Styles, P.; Radda, G.K.; Neubauer, S.; Clarke, K. Ab-normal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation 2003, 107, 3040–3046. [Google Scholar] [CrossRef]
- Levelt, E.; Pavlides, M.; Banerjee, R.; Mahmod, M.; Kelly, C.; Sellwood, J.; Ariga, R.; Thomas, S.; Francis, J.; Rodgers, C.; et al. Ectopic and Visceral Fat Deposition in Lean and Obese Patients With Type 2 Diabetes. J. Am. Coll. Cardiol. 2016, 68, 53–63. [Google Scholar] [CrossRef]
- Natali, A.; Vichi, S.; Landi, P.; Severi, S.; L’Abbate, A.; Ferrannini, E. Coronary atherosclerosis in Type II diabetes: Angiographic findings and clinical outcome. Diabetologia 2000, 43, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Yasuda, S.; Morii, I.; Otsuka, Y.; Kawamura, A.; Miyazaki, S. Quantitative coronary angiographic studies of patients with angina pectoris and impaired glucose tolerance. Diabetes Care 2005, 28, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.J.; Waltenberger, J.; Rogers, J.H. Percutaneous coronary intervention in patients with diabetes: Current concepts and future directions. J. Diabetes Sci. Technol. 2014, 8, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Kozawa, J.; Higashi, M.; Shimomura, I.; Hirano, K.I. Intractable Coronary Artery Disease in a Patient With Type 2 Diabetes Presenting With Triglyceride Deposit Cardiomyovasculopathy. Diabetes Care 2019, 42, 983–986. [Google Scholar] [CrossRef]
- Higashi, M.; Ikeda, Y.; Miyauchi, H.; Zaima, N.; Suzuki, A.; Li, M.; Kobayashi, K.; Naito, H.; Hirano, K. Imaging modalities for triglyceride deposit Cardiomyovasculopathy. Ann. Nucl. Cardiol. 2017, 3, 94–102. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Thanassoulis, G.; Glavinovic, T.; Navar, A.M.; Pencina, M.; Catapano, A.; Ference, B.A. Apolipoprotein B Particles and Cardiovascular Disease: A Narrative Review. JAMA Cardiol. 2019, 4, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Dubland, J.A.; Francis, G.A. So Much Cholesterol: The unrecognized importance of smooth muscle cells in atherosclerotic foam cell formation. Curr. Opin. Lipidol. 2016, 27, 155–161. [Google Scholar] [CrossRef]
- Watanabe, S.; Kumazaki, S.; Yamamoto, S.; Sato, I.; Kitamori, K.; Mori, M.; Yamori, Y.; Hirohata, S. Non-alcoholic steatohepatitis aggravates nitric oxide synthase inhibition-induced arteriosclerosis in SHRSP5/Dmcr rat model. Int. J. Exp. Pathol. 2018, 99, 282–294. [Google Scholar] [CrossRef]
- Paroni, R.; Fermo, I.; Fiorina, P.; Cighetti, G. Determination of asymmetric and symmetric dimethylarginines in plasma of hyperhomocysteinemic subjects. Amino Acids 2005, 28, 389–394. [Google Scholar] [CrossRef]
- Yagi, S.; Hirata, Y.; Ise, T.; Kusunose, K.; Yamada, H.; Fukuda, D.; Salim, H.M.; Maimaituxun, G.; Nishio, S.; Takagawa, Y.; et al. Canagliflozin reduces epicardial fat in patients with type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2017, 9, 78. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozawa, J.; Shimomura, I. Ectopic Fat Accumulation in Pancreas and Heart. J. Clin. Med. 2021, 10, 1326. https://doi.org/10.3390/jcm10061326
Kozawa J, Shimomura I. Ectopic Fat Accumulation in Pancreas and Heart. Journal of Clinical Medicine. 2021; 10(6):1326. https://doi.org/10.3390/jcm10061326
Chicago/Turabian StyleKozawa, Junji, and Iichiro Shimomura. 2021. "Ectopic Fat Accumulation in Pancreas and Heart" Journal of Clinical Medicine 10, no. 6: 1326. https://doi.org/10.3390/jcm10061326
APA StyleKozawa, J., & Shimomura, I. (2021). Ectopic Fat Accumulation in Pancreas and Heart. Journal of Clinical Medicine, 10(6), 1326. https://doi.org/10.3390/jcm10061326




