Hypoperfusion Index Ratio as a Surrogate of Collateral Scoring on CT Angiogram in Large Vessel Stroke
Abstract
1. Introduction
2. Materials and Methods
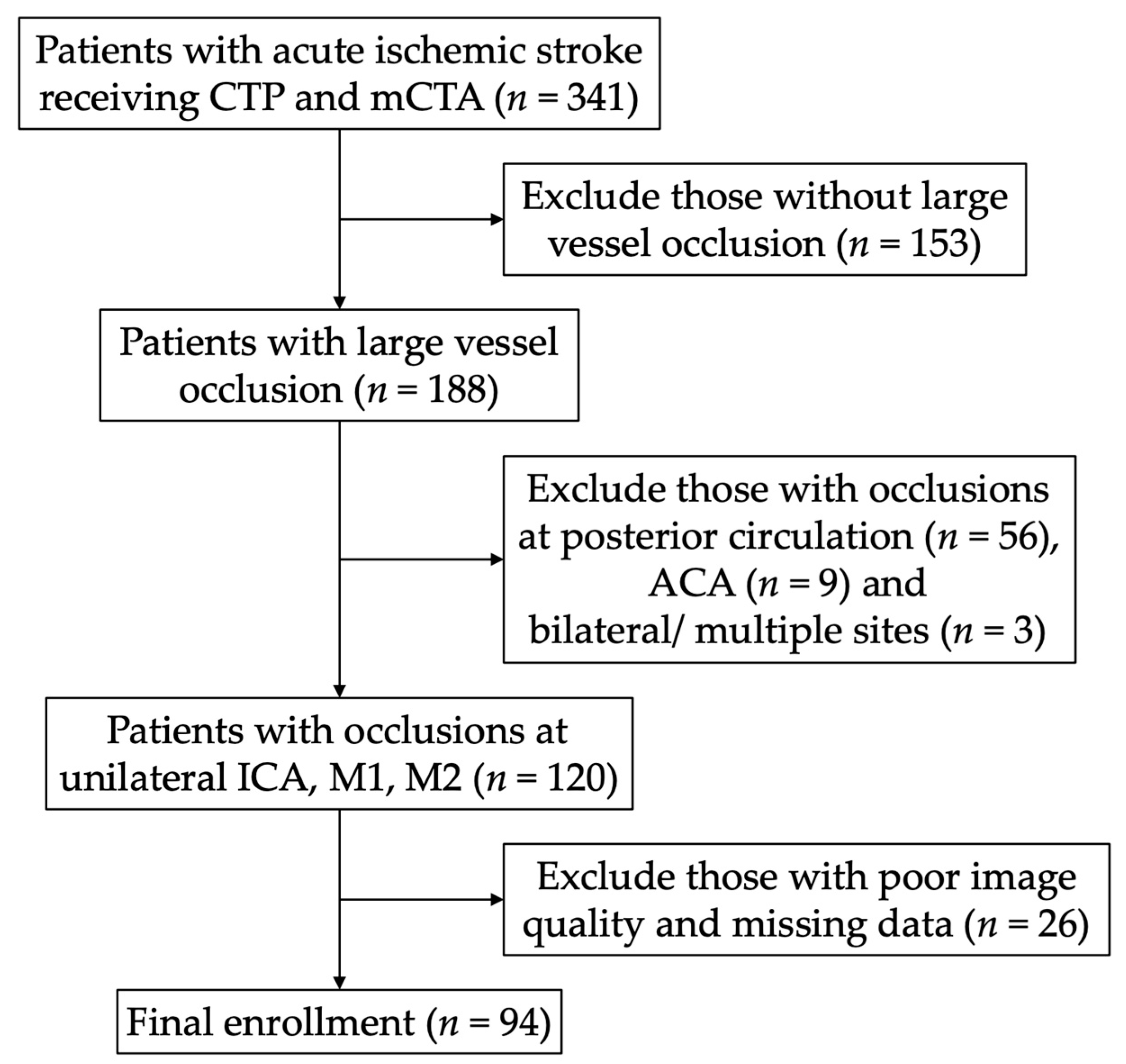
2.1. Patient Inclusion, Population, and Clinical Data
2.2. Multiphase Computed Tomography Angiography Collateral Score, Hypoperfusion Intensity Ratio, and the Eligibility of EVT
2.3. Outcome and Statistical Analysis
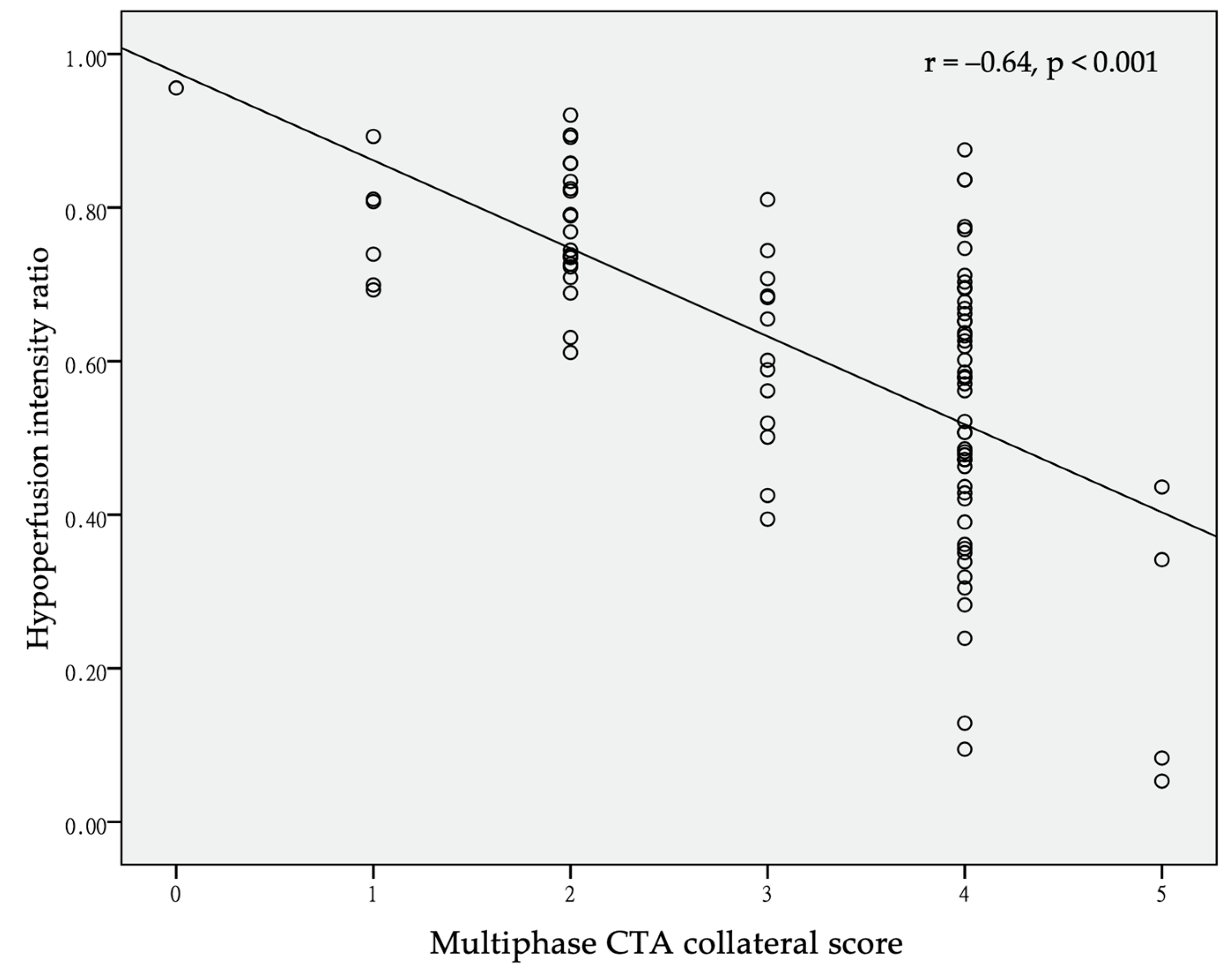
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christoforidis, G.A.; Mohammad, Y.; Kehagias, D.; Avutu, B.; Slivka, A.P. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. Am. J. Neuroradiol. 2005, 26, 1789–1797. [Google Scholar] [PubMed]
- Gerber, J.C.; Petrova, M.; Krukowski, P.; Kuhn, M.; Abramyuk, A.; Bodechtel, U.; Dzialowski, I.; Engellandt, K.; Kitzler, H.; Pallesen, L.P. Collateral state and the effect of endovascular reperfusion therapy on clinical outcome in ischemic stroke patients. Brain Behav. 2016, 6, e00513. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.G.; Jung, C.; Sunwoo, L.; Bae, Y.J.; Choi, B.S.; Kim, J.H.; Kim, B.J.; Han, M.-K.; Bae, H.-J.; Jung, S. Dichotomizing level of pial collaterals on multiphase CT angiography for endovascular treatment in acute ischemic stroke: Should it be refined for 6-hour time window? Neurointervention 2019, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- McVerry, F.; Liebeskind, D.; Muir, K. Systematic review of methods for assessing leptomeningeal collateral flow. Am. J. Neuroradiol. 2012, 33, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Piedade, G.S.; Schirmer, C.M.; Goren, O.; Zhang, H.; Aghajanian, A.; Faber, J.E.; Griessenauer, C.J. Cerebral collateral circulation: A review in the context of ischemic stroke and mechanical thrombectomy. World Neurosurg. 2019, 122, 33–42. [Google Scholar] [CrossRef]
- Menon, B.K.; d’Esterre, C.D.; Qazi, E.M.; Almekhlafi, M.; Hahn, L.; Demchuk, A.M.; Goyal, M. Multiphase CT angiography: A new tool for the imaging triage of patients with acute ischemic stroke. Radiology 2015, 275, 510–520. [Google Scholar] [CrossRef]
- Demeestere, J.; Wouters, A.; Christensen, S.; Lemmens, R.; Lansberg, M.G. Review of perfusion imaging in acute ischemic stroke: From time to tissue. Stroke 2020, 51, 1017–1024. [Google Scholar] [CrossRef]
- Olivot, J.M.; Mlynash, M.; Inoue, M.; Marks, M.P.; Wheeler, H.M.; Kemp, S.; Straka, M.; Zaharchuk, G.; Bammer, R.; Lansberg, M.G. Hypoperfusion intensity ratio predicts infarct progression and functional outcome in the DEFUSE 2 Cohort. Stroke 2014, 45, 1018–1023. [Google Scholar] [CrossRef]
- Guenego, A.; Marcellus, D.G.; Martin, B.W.; Christensen, S.; Albers, G.W.; Lansberg, M.G.; Marks, M.P.; Wintermark, M.; Heit, J.J. Hypoperfusion intensity ratio is correlated with patient eligibility for thrombectomy. Stroke 2019, 50, 917–922. [Google Scholar] [CrossRef]
- Guenego, A.; Fahed, R.; Albers, G.W.; Kuraitis, G.; Sussman, E.S.; Martin, B.W.; Marcellus, D.G.; Olivot, J.M.; Marks, M.P.; Lansberg, M.G. Hypoperfusion intensity ratio correlates with angiographic collaterals in acute ischaemic stroke with M1 occlusion. Eur. J. Neurol. 2020, 27, 864–870. [Google Scholar] [CrossRef]
- Austein, F.; Riedel, C.; Kerby, T.; Meyne, J.; Binder, A.; Lindner, T.; Huhndorf, M.; Wodarg, F.; Jansen, O. Comparison of perfusion CT software to predict the final infarct volume after thrombectomy. Stroke 2016, 47, 2311–2317. [Google Scholar] [CrossRef]
- Koopman, M.S.; Berkhemer, O.A.; Geuskens, R.R.; Emmer, B.J.; van Walderveen, M.A.; Jenniskens, S.F.; van Zwam, W.H.; van Oostenbrugge, R.J.; van der Lugt, A.; Dippel, D.W. Comparison of three commonly used CT perfusion software packages in patients with acute ischemic stroke. J. Neurointerventional Surg. 2019, 11, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-Y.; Wu, D.P.; Sung, S.-F. Registry-based stroke research in Taiwan: Past and future. Epidemiol. Health 2018, 40, e2018004. [Google Scholar] [CrossRef]
- García-Tornel, A.; Carvalho, V.; Boned, S.; Flores, A.; Rodríguez-Luna, D.; Pagola, J.; Muchada, M.; Sanjuan, E.; Coscojuela, P.; Juega, J. Improving the evaluation of collateral circulation by multiphase computed tomography angiography in acute stroke patients treated with endovascular reperfusion therapies. Intervig. Neurol. 2016, 5, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Calamante, F.; Christensen, S.; Desmond, P.M.; Østergaard, L.; Davis, S.M.; Connelly, A. The physiological significance of the time-to-maximum (Tmax) parameter in perfusion MRI. Stroke 2010, 41, 1169–1174. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [PubMed]
- Tang, S.; Tsai, L.; Chen, C.; Lee, C.; Wang, K.; Lai, Y. Taiwan Stroke Society guideline for endovascular thrombectomy in acute ischemic stroke patients. J. Stroke 2019, 1, 77–89. [Google Scholar]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N. Engl. J. Med. 2018, 378, 708–718. [Google Scholar] [CrossRef]
- Arenillas, J.F.; Cortijo, E.; García-Bermejo, P.; Levy, E.I.; Jahan, R.; Liebeskind, D.; Goyal, M.; Saver, J.L.; Albers, G.W. Relative cerebral blood volume is associated with collateral status and infarct growth in stroke patients in SWIFT PRIME. J. Cereb. Blood Flow Metab. 2018, 38, 1839–1847. [Google Scholar] [CrossRef]
- Wufuer, A.; Wubuli, A.; Mijiti, P.; Zhou, J.; Tuerxun, S.; Cai, J.; Ma, J.; Zhang, X. Impact of collateral circulation status on favorable outcomes in thrombolysis treatment: A systematic review and meta-analysis. Exp. Ther. Med. 2018, 15, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Olivot, J.-M.; Mlynash, M.; Thijs, V.N.; Kemp, S.; Lansberg, M.G.; Wechsler, L.; Bammer, R.; Marks, M.P.; Albers, G.W. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke 2009, 40, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Mlynash, M.; Lansberg, M.G.; De Silva, D.A.; Lee, J.; Christensen, S.; Straka, M.; Campbell, B.C.; Bammer, R.; Olivot, J.-M.; Desmond, P. Refining the definition of the malignant profile: Insights from the DEFUSE-EPITHET pooled data set. Stroke 2011, 42, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Al-Dasuqi, K.; Payabvash, S.; Torres-Flores, G.A.; Strander, S.M.; Nguyen, C.K.; Peshwe, K.U.; Kodali, S.; Silverman, A.; Malhotra, A.; Johnson, M.H. Effects of collateral status on infarct distribution following endovascular therapy in large vessel occlusion stroke. Stroke 2020, 51, e193–e202. [Google Scholar] [CrossRef]
- Bathla, G.; Limaye, K.; Policeni, B.; Klotz, E.; Juergens, M.; Derdeyn, C. Achieving comparable perfusion results across vendors. The next step in standardizing stroke care: A technical report. J. Neurointerventional Surg. 2019, 11, 1257–1260. [Google Scholar] [CrossRef]
- Bathla, G.; Ortega-Gutierrez, S.; Klotz, E.; Juergens, M.; Zevallos, C.B.; Ansari, S.; Ward, C.E.; Policeni, B.; Samaniego, E.; Derdeyn, C. Comparing the outcomes of two independent computed tomography perfusion softwares and their impact on therapeutic decisions in acute ischemic stroke. J. Neurointerventional Surg. 2020, 12, 1028–1032. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, X.; Zhang, Z.; Fang, Q.; Hu, C. Relationship between CT angiography-derived collateral status and CT perfusion-derived tissue viability. Clin. Radiol. 2019, 74, 956–961. [Google Scholar] [CrossRef]
- de Havenon, A.; Mlynash, M.; Kim-Tenser, M.A.; Lansberg, M.G.; Leslie-Mazwi, T.; Christensen, S.; McTaggart, R.A.; Alexander, M.; Albers, G.; Broderick, J. Results from DEFUSE 3: Good collaterals are associated with reduced ischemic core growth but not neurologic outcome. Stroke 2019, 50, 632–638. [Google Scholar] [CrossRef]
- Seker, F.; Potreck, A.; Möhlenbruch, M.; Bendszus, M.; Pham, M. Comparison of four different collateral scores in acute ischemic stroke by CT angiography. J. Neurointerventional Surg. 2016, 8, 1116–1118. [Google Scholar] [CrossRef]

| Characteristics | All (n = 94) | Poor Collaterals (n = 42) | Good Collaterals (n = 52) | p Value |
|---|---|---|---|---|
| Age (years) (mean (SD)) | 71.9 (12.9) | 73.0 (11.3) | 71.0 (14.1) | 0.439 |
| Male | 55 (58.5%) | 23 (54.8%) | 32 (61.5%) | 0.507 |
| Medical history | ||||
| Hypertension | 71 (75.5%) | 32 (76.2%) | 39 (75%) | 0.894 |
| Diabetes Mellitus | 35 (37.2%) | 15 (35.7%) | 20 (38.5%) | 0.784 |
| Hyperlipidemia | 64 (68.1%) | 25 (59.5%) | 39 (75%) | 0.110 |
| Atrial fibrillation | 42 (44.7%) | 24 (57.1%) | 18 (34.6%) | 0.029 |
| Coronary artery disease | 19 (20.2%) | 12 (28.6%) | 7 (13.5%) | 0.070 |
| Congestive heart failure | 10 (10.6%) | 7 (16.7%) | 3 (5.8%) | 0.088 |
| Prior AP | 25 (26.9%) | 11 (26.2%) | 14 (26.9%) | 0.936 |
| Prior AC | 13 (13.8%) | 7 (16.7%) | 6 (11.5%) | 0.474 |
| Smoker | 24 (25.8%) | 7 (16.7%) | 17 (33.3%) | 0.095 |
| Initial NIHSS (median (IQR)) | 20.5 (14–27) | 25 (21–30) | 14 (10–21) | <0.001 |
| Lesion site | 0.021 | |||
| ICA | 23 (24.5%) | 11 (26.2%) | 12 (23.1%) | |
| M1 | 42 (44.7%) | 24 (57.1%) | 18 (34.6%) | |
| M2 | 29 (30.9%) | 7 (16.7%) | 22 (42.3%) | |
| Initial SBP (mmHg) (median (IQR)) | 152.5 (137.75–171.25) | 154 (137–180) | 151.5(137.5–167.75) | 0.451 |
| IV-tPA | 30 (31.9%) | 7 (16.7%) | 23 (44.2%) | 0.004 |
| EVT | 32 (34.0%) | 12 (28.6%) | 20 (38.5%) | 0.314 |
| Onset to ER (median (IQR)) | 154 (61–311.75) | 172.5 (123–277.75) | 111.50 (37.5–364.25) | 0.050 |
| Onset to CTP (median (IQR)) | 40.50 (23.75–75.25) | 30.50 (20–49.5) | 52 (31.50–82.75) | 0.005 |
| Characteristics | All (n = 94) | Poor Collaterals (n = 42) | Good Collaterals (n = 52) | p Value |
|---|---|---|---|---|
| Core volume (mL) (SD) | 72.7 (63.7) | 116.5 (70.0) | 37.3 (24.7) | <0.001 |
| Tmax > 6 volume (mL) (SD) | 157.3 (86.4) | 203.5 (88.0) | 120.0 (64.9) | <0.001 |
| Tmax > 10 volume (mL) (SD) | 100.7 (75.2) | 152.0 (82.6) | 59.2 (31.1) | <0.001 |
| HIR (SD) | 0.61 (0.20) | 0.73 (0.13) | 0.51 (0.20) | <0.001 |
| Discharge mRS (IQR) | 4 (1.25–5) | 5 (2–6) | 2 (1–3.75) | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-M.; Chang, Y.-M.; Sung, P.-S.; Chen, C.-H. Hypoperfusion Index Ratio as a Surrogate of Collateral Scoring on CT Angiogram in Large Vessel Stroke. J. Clin. Med. 2021, 10, 1296. https://doi.org/10.3390/jcm10061296
Wang C-M, Chang Y-M, Sung P-S, Chen C-H. Hypoperfusion Index Ratio as a Surrogate of Collateral Scoring on CT Angiogram in Large Vessel Stroke. Journal of Clinical Medicine. 2021; 10(6):1296. https://doi.org/10.3390/jcm10061296
Chicago/Turabian StyleWang, Chun-Min, Yu-Ming Chang, Pi-Shan Sung, and Chih-Hung Chen. 2021. "Hypoperfusion Index Ratio as a Surrogate of Collateral Scoring on CT Angiogram in Large Vessel Stroke" Journal of Clinical Medicine 10, no. 6: 1296. https://doi.org/10.3390/jcm10061296
APA StyleWang, C.-M., Chang, Y.-M., Sung, P.-S., & Chen, C.-H. (2021). Hypoperfusion Index Ratio as a Surrogate of Collateral Scoring on CT Angiogram in Large Vessel Stroke. Journal of Clinical Medicine, 10(6), 1296. https://doi.org/10.3390/jcm10061296






