Exacerbations and Changes in Physical Activity and Sedentary Behaviour in Patients with Bronchiectasis after 1 Year
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Measurements
2.3. Statistical Analysis
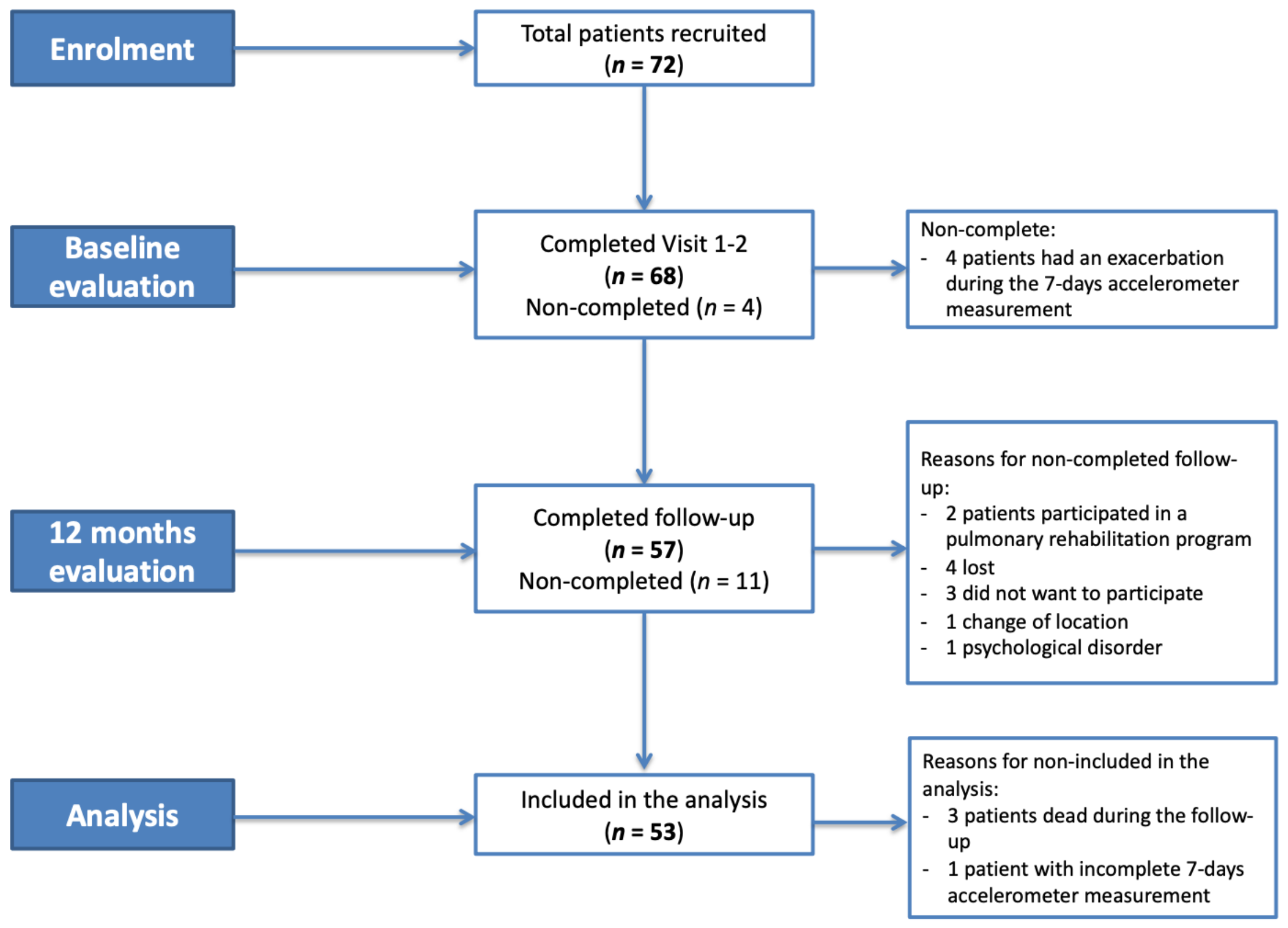
3. Results
3.1. Baseline Data
3.2. Follow-Up Assessment at 1 Year
3.3. Factors Associated with the Shift to Reduced Activity Levels
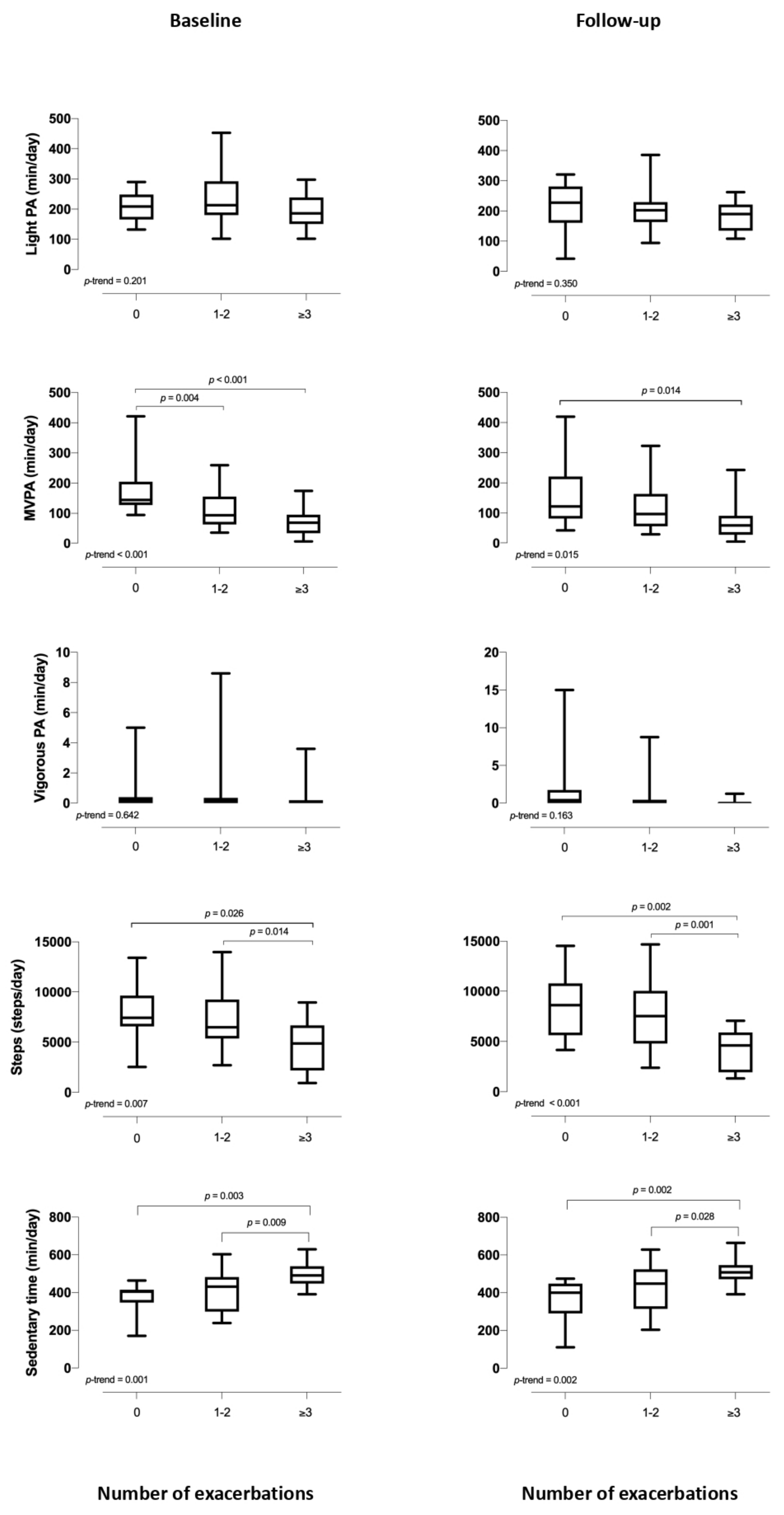
3.4. Relationship between Baseline and Follow-Up Activity Levels by Number of Exacerbations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- King, P.T.; Holdsworth, S.R.; Freezer, N.J.; Villanueva, E.; Holmes, P.W. Characterisation of the onset and presenting clinical features of adult bronchiectasis. Respir. Med. 2006, 100, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.T.; Haworth, C.S.; Aliberti, S.; Barker, A.; Blasi, F.; Boersma, W.; Chalmers, J.D.; de Soyza, A.; Dimakou, K.; Elborn, J.S.; et al. Pulmonary exacerbation in adults with bronchiectasis: A consensus definition for clinical research. Eur. Respir. J. 2017, 49, 1700051. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.C.; Elborn, J.S.; Byrnes, C.A. Bronchiectasis: Treatment decisions for pulmonary exacerbations and their prevention. Respirology 2018, 11, 1006–1022. [Google Scholar] [CrossRef]
- Cantón, R.; Máiz, L.; Escribano, A.; Olveira, C.; Oliver, A.; Asensio, O.; Gartner, S.; Roma, E.; Quintana-Gallego, E.; Salcedo, A.; et al. Consenso español para la prevención y el tratamiento de la infección bronquial por Pseudomonas aeruginosa en el paciente con fibrosis quística. Arch. Bronconeumol. 2015, 51, 140–150. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.Á.; Athanazio, R.; Gramblicka, G.; Corso, M.; Cavalcanti Lundgren, F.; Fernandes de Figueiredo, M.; Arancibia, F.; Rached, S.; Girón, R.; Máiz Carro, L.; et al. Prognostic Value of Frequent Exacerbations in Bronchiectasis: The Relationship with Disease Severity. Arch. Bronconeumol. 2019, 55, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Santos, E.; Frei, A.; Steurer-Stey, C.; de Batlle, J.; Rabinovich, R.A.; Raste, Y.; Hopkinson, N.S.; Polkey, M.I.; van Remoortel, H.; Troosters, T.; et al. Determinants and outcomes of physical activity in patients with COPD: A systematic review. Thorax 2014, 69, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.; Lamotte, M.; Gerlier, L.; Svangren, P.; Miquel-Cases, A.; Haughney, J. Cost-effectiveness of physical activity in the management of COPD patients in the UK. Int. J. COPD 2019, 14, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz-Serrano, V.; Gimeno-Santos, E.; Scioscia, G.; Gabarrús, A.; Navarro, A.; Herrero-Cortina, B.; Amaro, R.; Fernández-Barat, L.; Torres, A. Association between physical activity and risk of hospitalisation in bronchiectasis. Eur. Respir. J. 2020, 55, 1–9. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines on physical activity and sedentary behaviour. Available online: https://www.who.int/publications/i/item/9789240015128 (accessed on 24 December 2020).
- Schneider, L.P.; Furlanetto, K.C.; Rodrigues, A.; Lopes, J.R.; Hernandes, N.A.; Pitta, F. Sedentary Behaviour and Physical Inactivity in Patients with Chronic Obstructive Pulmonary Disease: Two Sides of the Same Coin? COPD 2018, 15, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Hester, K.L.M.; Macfarlane, J.G.; Tedd, H.; Jary, H.; McAlinden, P.; Rostron, L.; Small, T.; Newton, J.L.; De Soyza, A. Fatigue in bronchiectasis. QJM 2012, 105, 235–240. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Olveira, C.; Olveira, G.; Espildora, F.; Giron, R.M.; Muñoz, G.; Quittner, A.L.; Martinez-Garcia, M.-A. Validation of a Quality of Life Questionnaire for Bronchiectasis: Psychometric analyses of the Spanish QOL-B-V3.0. Qual. Life Res. 2014, 23, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, G.; Buxó, M.; De Gracia, J.; Olveira, C.; Martinez-Garcia, M.A.; Giron, R.; Polverino, E.; Alvarez, A.; Birring, S.S.; Vendrell, M. Validation of a Spanish version of the Leicester Cough Questionnaire in non-cystic fibrosis bronchiectasis. Chron. Respir. Dis. 2016, 13, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Goeminne, P.; Aliberti, S.; McDonnel, M.J.; Lonni, S.; Davidson, J.; Poppelwell, L.; Salih, W.; Pesci, A.; Dupont, L.J.; et al. The bronchiectasis severity index. An international derivation and validation study. Am. J. Respir. Crit. Care Med. 2014, 189, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Aliberti, S.; Filonenko, A.; Shteinberg, M.; Goeminne, P.C.; Hill, A.T.; Fardon, T.C.; Obradovic, D.; Gerlinger, C.; Sotgiu, G.; et al. Characterization of the “frequent exacerbator phenotype” in bronchiectasis. Am. J. Respir. Crit. Care Med. 2018, 197, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Demeyer, H.; Burtin, C.; Van Remoortel, H.; Hornikx, M.; Langer, D.; Decramer, M.; Gosselink, R.; Janssens, W.; Troosters, T. Standardizing the Analysis of Physical Activity in Patients with COPD Following a Pulmonary Rehabilitation Program. Chest 2014, 146, 318–327. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S504. [Google Scholar] [CrossRef]
- Hosmer, D.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Collett, D. Modelling Binary Data; Chapman and Hall: London, UK, 1991. [Google Scholar]
- Bradley, J.M.; Wilson, J.J.; Hayes, K.; Kent, L.; McDonough, S.; Tully, M.A.; Bradbury, I.; Kirk, A.; Cosgrove, D.; Convery, R.; et al. Sedentary behaviour and physical activity in bronchiectasis: A cross-sectional study. BMC Pulm. Med. 2015, 15, 61. [Google Scholar] [CrossRef]
- Moy, M.L.; Teylan, M.; Weston, N.A.; Gagnon, D.R.; Garshick, E. Daily Step Count Predicts Acute Exacerbations in a US Cohort with COPD. PLoS ONE 2013, 8, e60400. [Google Scholar] [CrossRef]
- Donaire-Gonzalez, D.; Gimeno-Santos, E.; Balcells, E.; De Batlle, J.; Ramon, M.A.; Rodriguez, E.; Farrero, E.; Benet, M.; Guerra, S.; Sauleda, J.; et al. Benefits of physical activity on COPD hospitalisation depend on intensity. Eur. Respir. J. 2015, 46, 1281–1289. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Chu, L.; Liu, I.L.A.; Lee, J.S.; Suh, D.; Korotzer, B.; Yuen, G.; Desai, S.; Coleman, K.J.; Xiang, A.H.; et al. Associations between physical activity and 30-day readmission risk in chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2014, 11, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.E.; Boezen, H.M.; de Greef, M.H.; Ten Hacken, N.H. Physical and psychosocial factors associated with physical activity in patients with chronic obstructive pulmonary disease. Arch. Phys. Med. Rehabil. 2013, 94, 2396–2402. [Google Scholar] [CrossRef]
- Furlanetto, K.C.; Donária, L.; Schneider, L.P.; Lopes, J.R.; Ribeiro, M.; Fernandes, K.B.; Hernandes, N.A.; Pitta, F. Sedentary Behavior Is an Independent Predictor of Mortality in Subjects With COPD. Respir. Care 2017, 62, 579–587. [Google Scholar] [CrossRef]
- Garcia-Aymerich, J.; Lange, P.; Benet, M.; Schnohr, P.; Antó, J.M. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: A population based cohort study. Thorax 2006, 61, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rio, F.; Rojo, B.; Casitas, R.; Lores, V.; Madero, R.; Romero, D.; Galera, R.; Villasante, C. Prognostic value of the objective measurement of daily physical activity in patients with COPD. Chest 2012, 142, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Vaes, A.W.; Garcia-Aymerich, J.; Marott, J.L.; Benet, M.; Groenen, M.T.J.; Schnohr, P.; Franssen, F.M.E.; Vestbo, J.; Wouters, E.F.M.; Lange, P.; et al. Changes in physical activity and all-cause mortality in COPD. Eur. Respir. J. 2014, 44, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Esteban, C.; Garcia-Gutierrez, S.; Legarreta, M.J.; Anton-Ladislao, A.; Gonzalez, N.; Lafuente, I.; Fernandez de Larrea, N.; Vidal, S.; Bare, M.; Quintana, J.M. One-year Mortality in COPD After an Exacerbation: The Effect of Physical Activity Changes during the Event. COPD 2016, 13, 718–725. [Google Scholar] [CrossRef]
- Waschki, B.; Kirsten, A.; Holz, O.; Müller, K.C.; Meyer, T.; Watz, H.; Magnussen, H. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: A prospective cohort study. Chest 2011, 140, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.L.; Hill, C.J.; McDonald, C.F.; Holland, A.E. Pulmonary rehabilitation in individuals with non-cystic fibrosis bronchiectasis: A systematic review. Arch. Phys. Med. Rehabil. 2017, 98, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Quittner, A.L.; O’Donnell, A.E.; Salathe, M.A.; Lewis, S.A.; Li, X.; Montgomery, A.B.; O’Riordan, T.G.; Barker, A.F. Quality of life questionnaire-bronchiectasis: Final psychometric analyses and determination of minimal important difference scores. Thorax 2015, 70, 12–20. [Google Scholar] [CrossRef]
- Pitta, F.; Troosters, T.; Probst, V.S.; Spruit, M.A.; Decramer, M.; Gosselink, R. Physical activity and hospitalization for exacerbation of COPD. Chest 2006, 129, 536–544. [Google Scholar] [CrossRef]
- Alahmari, A.D.; Patel, A.R.C.; Kowlessar, B.S.; Mackay, A.J.; Singh, R.; Wedzicha, J.A.; Donaldson, G.C. Daily activity during stability and exacerbation of chronic obstructive pulmonary disease. BMC Pulm. Med. 2014, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.C.; Wilkinson, T.M.A.; Hurst, J.R.; Perera, W.R.; Wedzicha, J.A. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005, 171, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.C.; Carvalho, C.R.F. Physical activity in daily life in Brazilian COPD patients during and after exacerbation. COPD 2012, 9, 596–602. [Google Scholar] [CrossRef]
- Demeyer, H.; Costilla-Frias, M.; Louvaris, Z.; Gimeno-Santos, E.; Tabberer, M.; Rabinovich, R.A.; de Jong, C.; Polkey, M.I.; Hopkinson, N.S.; Karlsson, N.; et al. Both moderate and severe exacerbations accelerate physical activity decline in COPD patients. Eur. Respir. J. 2018, 51, 1702110. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, M.; Khan, R.; Wakefield, D.; Qureshi, A.; Murray, L.; ZuWallack, R.; Leidy, N.K. A longitudinal study evaluating the effect of exacerbations on physical activity in patients with chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2013, 10, 559–564. [Google Scholar] [CrossRef]
- Sievi, N.A.; Brack, T.; Brutsche, M.H.; Frey, M.; Irani, S.; Leuppi, J.D.; Thurnheer, R.; Kohler, M.; Clarenbach, C.F. “Can do, don’t do” are not the lazy ones: A longitudinal study on physical functioning in patients with COPD. Respir. Res. 2020, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Koreny, M.; Demeyer, H.; Benet, M.; Arbillaga-Etxarri, A.; Balcells, E.; Barberan-Garcia, A.; Gimeno-Santos, E.; Hopkinson, N.S.; De Jong, C.; Karlsson, N.; et al. Patterns of Physical Activity Progression in Patients With COPD. Arch. Bronconeumol. 2020, S0300-2896, 30280–30285. [Google Scholar] [CrossRef] [PubMed]
- Clarenbach, C.F.; Sievi, N.A.; Haile, S.R.; Brack, T.; Brutsche, M.H.; Frey, M.; Irani, S.; Leuppi, J.D.; Thurnheer, R.; Kohler, M. Determinants of annual change in physical activity in COPD. Respirology 2017, 22, 1133–1139. [Google Scholar] [CrossRef]
- Sievi, N.A.; Brack, T.; Brutsche, M.H.; Frey, M.; Irani, S.; Leuppi, J.D.; Thurnheer, R.; Kohler, M.; Clarenbach, C.F. Physical activity declines in COPD while exercise capacity remains stable: A longitudinal study over 5 years. Respir. Med. 2018, 141, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sievi, N.A.; Kohler, M.; Thurnheer, R.; Leuppi, J.D.; Irani, S.; Frey, M.; Brutsche, M.; Brack, T.; Clarenbach, C.F. No impact of exacerbation frequency and severity on the physical activity decline in COPD: A long-term observation. Int. J. COPD 2019, 14, 431–437. [Google Scholar] [CrossRef]
- Arbillaga-Etxarri, A.; Gimeno-Santos, E.; Barberan-Garcia, A.; Benet, M.; Borrell, E.; Dadvand, P.; Foraster, M.; Marín, A.; Monteagudo, M.; Rodríguez-Roisin, R.; et al. Socio-environmental correlates of physical activity in patients with chronic obstructive pulmonary disease (COPD). Thorax 2017, 72, 796–802. [Google Scholar] [CrossRef] [PubMed]
| All Patients | ‘Active + Not Sedentary’ | ‘Active + Sedentary’ | ‘Inactive + Not Sedentary’ | ‘Inactive + Sedentary’ | p Value | |
|---|---|---|---|---|---|---|
| N = 53 | 24 (45) | 3 (6) | 11 (21) | 15 (28) | ||
| Demographics | ||||||
| Female | 37 (70) | 19 (79) | 1 (33.3) | 7 (63.6) | 10 (66.6) | 0.368 |
| Age, years | 62.3 (15.9) | 58.7 (14.9) | 62.7 (6.4) | 64.6 (21.9) | 66.1 (13.9) | 0.523 |
| BMI, Kg/m2 | 24.3 (4.1) | 23.8 (3.9) | 29.4 (5.1) | 23.9 (2.9) | 24.1 (4.6) | 0.174 |
| Work activity | 0.263 | |||||
| Active | 19 (36) | 12 (50) | 1 (33.3) | 2 (18.2) | 4 (26.7) | |
| Retired | 34 (64) | 12 (50) | 2 (66.6) | 9 (81.8) | 11 (73.3) | |
| Smoking habit | 0.538 | |||||
| Active smoker | 1 (2) | 1 (4.2) | 0 (0) | 0 (0) | 0 (0) | |
| Former smokers | 14 (26) | 4 (16.6) | 2 (66.6) | 3 (27.2) | 5 (33.3) | |
| Non-smoker | 38 (71.7) | 19 (79.2) | 1 (33.3) | 8 (72.7) | 10 (66.6) | |
| Chronic colonisation | 20 (37) | 5 (20.8) | 1 (33.3) | 6 (51.5) | 8 (53.3) | 0.121 |
| Pseudomonas aeruginosa | 16 (30) | 3 (60) | 1 (100) | 5 (83.3) | 7 (87.5) | 0.081 |
| Dyspnoea (mMRC Scale, 0–4) | 1 [1,1] | 1 [0,1] | 1 [1,1] | 1 [1,2] | 1 [1,2] | 0.263 |
| No. exacerbations previous year | 1 [0,1,2] | 1 [0,1,2] | 1 [0,1] | 2 [0,1,2] | 1 [0,1,2,3,4] | 0.283 |
| No. hospitalizations previous year | 0 [0,0] | 0 [0,0] | 0 [0,0] | 0 [0,0] | 0 [0,1] | 0.653 |
| Number of lobes affected in CT scan | 3.62 (1.6) | 3.50 (1.7) | 2.33 (0.6) | 3.91 (1.3) | 3.86 (1.7) | 0.350 |
| Aetiology | ||||||
| Post-infectious | 24 (45) | 15 (62.5) | 0 (0) | 6 (54.5) | 3 (20) | 0.022 |
| Idiopathic | 13 (24) | 3 (12.5) | 2 (66.6) | 2 (18) | 6 (40) | 0.074 |
| Others | 16 (30.2) | 6 (25) | 1 (33.3) | 3 (27.3) | 6 (40) | 0.789 |
| Severity | ||||||
| BSI stages | 2 [1,2,3] | 2 [1,2] | 2 [1,2] | 2 [2,3] | 3 [1,2,3] | 0.086 |
| Pulmonary function | ||||||
| FEV1, % predicted | 73.2 (20) | 80.9 (18.2) | 67 (5.56) | 68.9 (23.1) | 65.4 (19.9) | 0.086 |
| FVC, % predicted | 81 (18.3) | 87.2 (16.4) | 90.3 (38.6) | 72.36 (14.3) | 75.6 (16.5) | 0.059 |
| FEV1/FVC, % | 85.9 (18) | 89.1 (17.2) | 73 (17.7) | 84.9 (19.4) | 84.2 (18.6) | 0.490 |
| 6MWT, metres | 516.8 (97.8) | 534.3 (80.03) | 564.8 (40.5) | 512.8 (103.4) | 482.3 (122.01) | 0.345 |
| Physical activity | ||||||
| Light (min per day) | 216.7 (77) | 237.2 (71.6) | 138.8 (64.4) | 256.6 (91.9) | 170.2 (37.6) | 0.002 a,b |
| Moderate (min per day) | 112 (76) | 143.3 (91.1) | 97.2 (24.5) | 117.5 (57.4) | 60.8 (30.1) | 0.008 a |
| Vigorous (min per day) | 0.66 (1.7) | 1.1 (2.3) | 0 (0) | 0.26 (0.31) | 0.44 (1.0) | 0.445 |
| MVPA (min per day) | 112.5 (77) | 144 (92.1) | 97.2 (24.5) | 117.7 (57.5) | 61.2 (30.5) | 0.009 a |
| Steps per day | 6759 (3530) | 9441 (3014) | 8912 (549) | 4840 (1120) | 3444 (1563) | <0.001 |
| Sedentary time (min) | 430 (99.8) | 374.4 (75.7) | 487.5 (18.4) | 384.9 (79.8) | 541 (46.6) | <0.001 a,b |
| Quality of Life Bronchiectasis Questionnaire | ||||||
| Physical Function | 60.4 (32.7) | 62.3 (33.3) | 55.5 (19.2) | 45.5 (34.2) | 64.4 (32) | 0.382 |
| Role Function | 76.7 (26.6) | 83.3 (26) | 66.6 (0) | 75.7 (26.2) | 68.9 (29.5) | 0.367 |
| Vitality | 59.4 (26.8) | 62.5 (24.7) | 55.6 (19.2) | 65.2 (24) | 51.1 (33) | 0.521 |
| Emotional Function | 74.2 (28.9) | 75.7 (27.4) | 61.1 (25.4) | 69.7 (34) | 77.8 (30) | 0.771 |
| Social Function | 69.8 (30.8) | 76.4 (24.5) | 55.6 (38.5) | 65.2 (36.9) | 65.6 (34.7) | 0.530 |
| Treatment Burden | 72.9 (39.8) | 72.2 (42.5) | 66.7 (57.7) | 69.7 (37.8) | 77.7 (37) | 0.948 |
| Health Perceptions | 53.1 (26.7) | 61.8 (28.4) | 50 (16.7) | 51.5 (24.1) | 41.4 (24.3) | 0.129 |
| Respiratory Symptoms | 76.7 (23.2) | 77.7 (23.4) | 66.7 (33.3) | 78.8 (22.5) | 75.6 (23.5) | 0.981 |
| Leicester Cough Questionnaire | ||||||
| Total | 15.3 (4.64) | 16.04 (4.2) | 12.9 (7.2) | 15.1 (5.5) | 14.8 (4.5) | 0.678 |
| Physical | 4.9 (1.34) | 5.2 (1.2) | 4.37 (2.3) | 5.03 (1.3) | 4.74 (1.5) | 0.616 |
| Psychological | 5.01 (1.74) | 5.2 (1.6) | 4.19 (2.4) | 4.78 (2.2) | 5.04 (1.6) | 0.773 |
| Social | 5.44 (1.8) | 5.67 (1.6) | 4.41 (2.7) | 5.3 (2.2) | 5.4 (1.6) | 0.710 |
| All Patients | ‘Active + Not Sedentary’ | ‘Active + Sedentary’ | ‘Inactive + Not Sedentary’ | ‘Inactive + Sedentary’ | p Value | |
|---|---|---|---|---|---|---|
| N = 53 | 18 (34) | 7 (13) | 8 (15) | 20 (38) | ||
| Demographics | ||||||
| BMI, Kg/m2 | 24.2 (4.3) | 25.1 (4.2) | 24.2 (4.6) | 21.6 (4.1) | 24.7 (4.4) | 0.287 |
| Dyspnoea (mMRC Scale, 0–4) | 1 [0,1] | 1 [0,1,2] | 1 [0.75–2.25] | 2 [0,1,2,3] | 3 [2,3,4] | 0.075 |
| No. exacerbations during FUP | 2 [1,2,3] | 1 [0,1,2] | 1 [0.75–2.25] | 2 [0,1,2,3] | 3 [2,3,4] | <0.001 |
| No. hospitalisations during FUP | 0 [0–0.5] | 0 [0,0] | 0 [0,0] | 0 [0–0.75] | 0 [0,1] | 0.125 |
| Pulmonary function | ||||||
| FEV1, % predicted | 69.5 (20.4) | 75.5 (19.4) | 73.8 (22.4) | 64.1 (23.2) | 66.7 (19.1) | 0.363 |
| FVC, % predicted | 77.7 (17.8) | 82.4 (16.6) | 77.4 (13.5) | 72.7 (23.1) | 76.5 (18.2) | 0.256 |
| FEV1/FVC, % | 83.7 (18.3) | 83.9 (14.8) | 93 (20.5) | 87.4 (30.7) | 81.9 (13.3) | 0.532 |
| 6MWT, metres | 520.4 (98.5) | 540.3 (77.2) | 582.9 (81.2) | 505.4 (73.9) | 470.8 (111.8) | 0.068 |
| Physical activity | ||||||
| Light (min per day) | 205.4 (75.5) | 248.2 (86.1) | 164.8 (37.1) | 206.1 (86.4) | 179.2 (53.7) | 0.016 a |
| Moderate (min per day) | 107.6 (84.2) | 160.2 (95.7) | 86.2 (36.3) | 155.7 (88.8) | 49.8 (24.6) | <0.001 a,c |
| Vigorous (min per day) | 0.89 (2.51) | 1.9 (3.9) | 1.14 (2.3) | 0.5 (0.67) | 0.04 (0.14) | 0.123 |
| MVPA (min per day) | 108.5 (85.1) | 162.2 (96.6) | 87.3 (37.4) | 156.2 (89.4) | 49.9 (24.6) | <0.001 |
| Steps per day | 6781 (5799) | 10443 (2681) | 8073 (1624) | 4383 (1764) | 3983 (1484) | <0.001 a,c |
| Sedentary time (min) | 441.2 (115.6) | 337.9 (72.1) | 501.3 (30.3) | 372.6 (117.1) | 540.2 (53.3) | <0.001 |
| Quality of Life Bronchiectasis Questionnaire | ||||||
| Physical Function | 53.14 (31.8) | 61.1 (23.6) | 72.2 (25.1) | 50 (34.7) | 35.1 (32.3) | 0.026 |
| Role Function | 72.01 (29.56) | 81.5 (23.5) | 94.4 (13.6) | 61.9 (12.6) | 57 (35.7) | 0.012 a,b |
| Vitality | 59.4 (22.98) | 61.1 (21.4) | 63.8 (16.4) | 66.7 (21.5) | 50.8 (25.7) | 0.215 |
| Emotional Function | 74.5 (27.5) | 73.1 (24.3) | 88.9 (17.2) | 80.9 (26.2) | 66.7 (33.3) | 0.295 |
| Social Function | 64.5 (31.4) | 78.7 (23.4) | 75 (20.4) | 54.7 (20.9) | 48.2 (37.6) | 0.030 a |
| Treatment Burden | 69.9 (37.8) | 75.9 (35.8) | 72.2 (44.3) | 52.4 (42.4) | 68.4 (37.6) | 0.590 |
| Health Perceptions | 55.3 (28.8) | 62.9 (27.1) | 63.9 (6.8) | 57.1 (26.9) | 42.1 (32.6) | 0.101 |
| Respiratory Symptoms | 74.8 (23.5) | 77.8 (19.8) | 72.2 (13.6) | 85.7 (17.8) | 66.7 (29.4) | 0.164 |
| Leicester Cough Questionnaire | ||||||
| Total | 15.5 (4.4) | 15.9 (4.4) | 17.3 (2.2) | 16.1 (3.8) | 14.2 (5.1) | 0.348 |
| Physical | 4.98 (1.4) | 5.24 (1.2) | 5.6 (0.8) | 5.1 (1.3) | 4.5 (1.6) | 0.238 |
| Psychological | 5.23 (1.7) | 5.4 (1.7) | 5.8 (0.8) | 5.4 (1.5) | 4.7 (1.9) | 0.426 |
| Social | 5.36 (1.6) | 5.5 (1.5) | 5.9 (0.9) | 5.6 (1.3) | 4.9 (1.8) | 0.348 |
| Variable | Univariate a | Multivariable b | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age (+1 year) | 1.003 | 0.96 to 1.05 | 0.906 | - | - | - |
| 6-min walking distance (+1 m) | 0.998 | 0.99 to 1.00 | 0.518 | - | - | - |
| FEV1 (+1% predicted) | 1.005 | 0.97 to 1.04 | 0.796 | - | - | - |
| Number of hospitalisations during FUP (+1 unit) | 1.200 | 0.80 to 1.793 | 0.089 | - | - | - |
| Number of exacerbations during FUP (+1 unit) | 1.540 | 1.23 to 2.65 | 0.045 | 2.192 | 1.12 to 4.28 | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcaraz-Serrano, V.; Arbillaga-Etxarri, A.; Oscanoa, P.; Fernández-Barat, L.; Bueno, L.; Amaro, R.; Gimeno-Santos, E.; Torres, A. Exacerbations and Changes in Physical Activity and Sedentary Behaviour in Patients with Bronchiectasis after 1 Year. J. Clin. Med. 2021, 10, 1190. https://doi.org/10.3390/jcm10061190
Alcaraz-Serrano V, Arbillaga-Etxarri A, Oscanoa P, Fernández-Barat L, Bueno L, Amaro R, Gimeno-Santos E, Torres A. Exacerbations and Changes in Physical Activity and Sedentary Behaviour in Patients with Bronchiectasis after 1 Year. Journal of Clinical Medicine. 2021; 10(6):1190. https://doi.org/10.3390/jcm10061190
Chicago/Turabian StyleAlcaraz-Serrano, Victoria, Ane Arbillaga-Etxarri, Patricia Oscanoa, Laia Fernández-Barat, Leticia Bueno, Rosanel Amaro, Elena Gimeno-Santos, and Antoni Torres. 2021. "Exacerbations and Changes in Physical Activity and Sedentary Behaviour in Patients with Bronchiectasis after 1 Year" Journal of Clinical Medicine 10, no. 6: 1190. https://doi.org/10.3390/jcm10061190
APA StyleAlcaraz-Serrano, V., Arbillaga-Etxarri, A., Oscanoa, P., Fernández-Barat, L., Bueno, L., Amaro, R., Gimeno-Santos, E., & Torres, A. (2021). Exacerbations and Changes in Physical Activity and Sedentary Behaviour in Patients with Bronchiectasis after 1 Year. Journal of Clinical Medicine, 10(6), 1190. https://doi.org/10.3390/jcm10061190








