The Pathogenic Role of Interferons in the Hyperinflammatory Response on Adult-Onset Still’s Disease and Macrophage Activation Syndrome: Paving the Way towards New Therapeutic Targets
Abstract
1. Introduction
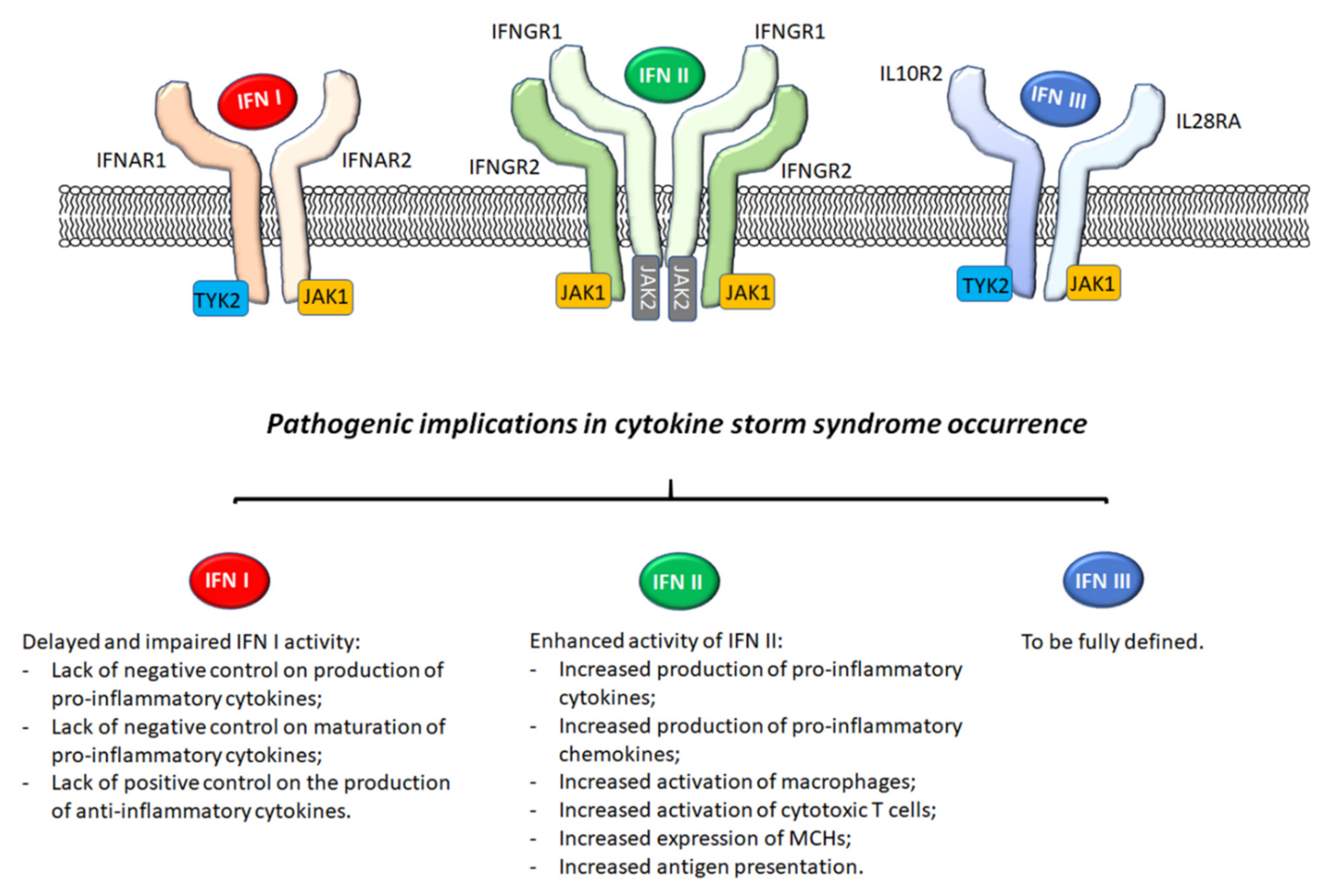
2. Interferons
3. IFN I
3.1. Generalities
3.2. Pathogenic Implications in AOSD and MAS
4. IFN II
4.1. Generalities
4.2. Pathogenic Implications, Ex Vivo Observations
4.3. Pathogenic Implications and In Vivo Observations
4.4. Therapeutic Strategies
5. IFN III
6. Discussion and Appraisal of Literature
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feist, E.; Mitrovic, S.; Fautrel, B. Mechanisms, biomarkers and targets for adult-onset Still’s disease. Nat. Rev. Rheumatol. 2018, 14, 603–618. [Google Scholar] [CrossRef]
- Giacomelli, R.; Ruscitti, P.; Shoenfeld, Y. A comprehensive review on adult onset Still’s disease. J. Autoimmun. 2018, 93, 24–36. [Google Scholar] [CrossRef]
- Ruscitti, P.; Iacono, D.; Ciccia, F.; Emmi, G.; Cipriani, P.; Grembiale, R.D.; Perosa, F.; Emmi, L.; Triolo, G.; Giacomelli, R.; et al. Macrophage Activation Syndrome in Patients Affected by Adult-onset Still Disease: Analysis of Survival Rates and Predictive Factors in the Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale Cohort. J. Rheumatol. 2018, 45, 864–872. [Google Scholar] [CrossRef]
- La Rosée, P.; Horne, A.; Hines, M.; Greenwood, T.V.B.; Machowicz, R.; Berliner, N.; Birndt, S.; Gil-Herrera, J.; Girschikofsky, M.; Jordan, M.B.; et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood 2019, 133, 2465–2477. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zerón, P.; López-Guillermo, A.; Khamashta, M.A.; Bosch, X. Adult haemophagocytic syndrome. Lancet 2014, 383, 1503–1516. [Google Scholar] [CrossRef]
- Grom, A.A.; Horne, A.; De Benedetti, F. Macrophage activation syndrome in the era of biologic therapy. Nat. Rev. Rheumatol. 2016, 12, 259–268. [Google Scholar] [CrossRef]
- Efthimiou, P.; Kadavath, S.; Mehta, B. Life-threatening complications of adult-onset Still’s disease. Clin. Rheumatol. 2014, 33, 305–314. [Google Scholar] [CrossRef]
- Rosário, C.; Zandman-Goddard, G.; Meyron-Holtz, E.G.; D’Cruz, D.P.; Shoenfeld, Y. The Hyperferritinemic Syndrome: Macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Gerfaud-Valentin, M.; Jamilloux, Y.; Iwaz, J.; Sève, P. Adult-onset Still’s disease. Autoimmun. Rev. 2014, 13, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Ruscitti, P.; Ursini, F.; Cipriani, P.; De Sarro, G.; Giacomelli, R. Biologic drugs in adult onset Still’s disease: A systematic review and meta-analysis of observational studies. Expert Rev. Clin. Immunol. 2017, 13, 1089–1097. [Google Scholar] [CrossRef]
- Ruscitti, P.; Cipriani, P.; Di Benedetto, P.; Liakouli, V.; Carubbi, F.; Berardicurti, O.; Ciccia, F.; Guggino, G.; Triolo, G.; Giacomelli, R. Advances in immunopathogenesis of macrophage activation syndrome during rheumatic inflammatory diseases: Toward new therapeutic targets? Expert Rev. Clin. Immunol. 2017, 13, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, A.; Lindenmann, J. Pillars Article: Virus Interference. I. The Interferon. Proc. R. Soc. Lond. B Biol. Sci. 1957, 147, 258–267. [Google Scholar]
- Pestka, S.; Krause, C.D.; Walter, M.R. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 2004, 202, 8–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.X.; Fish, E.N. Global virus outbreaks: Interferons as 1st responders. Semin. Immunol. 2019, 43, 101300. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, S.; Lee-Kirsch, M.A. Type I interferon-mediated autoinflammation and autoimmunity. Curr. Opin. Immunol. 2017, 49, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Ludigs, K.; Parfenov, V.; Du Pasquier, R.A.; Guarda, G. Type I IFN-mediated regulation of IL-1 production in inflammatory disorders. Cell. Mol. Life Sci. 2012, 69, 3395–3418. [Google Scholar] [CrossRef] [PubMed]
- Goodbourn, S.; Didcock, L.; Randall, R.E. Interferons: Cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 2000, 81, 2341–2364. [Google Scholar] [CrossRef]
- Barrat, F.J.; Crow, M.K.; Ivashkiv, L.B. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 2019, 20, 1574–1583. [Google Scholar] [CrossRef]
- Randall, R.E.; Goodbourn, S. Interferons and viruses: An interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 2008, 89, 1–47. [Google Scholar] [CrossRef]
- Sadler, A.J.; Williams, B.R.G. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 2008, 8, 559–568. [Google Scholar] [CrossRef]
- Billiau, A. Anti-inflammatory properties of Type I interferons. Antivir. Res. 2006, 71, 108–116. [Google Scholar] [CrossRef]
- Theofilopoulos, A.N.; Baccala, R.; Beutler, B.; Kono, D.H. Type I Interferons (α/β) in Immunity and Autoimmunity. Annu. Rev. Immunol. 2005, 23, 307–335. [Google Scholar] [CrossRef]
- Guarda, G.; Braun, M.; Staehli, F.; Tardivel, A.; Mattmann, C.; Förster, I.; Farlik, M.; Decker, T.; Du Pasquier, R.A.; Romero, P.; et al. Type I Interferon Inhibits Interleukin-1 Production and Inflammasome Activation. Immunity 2011, 34, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Schindler, R.; Ghezzi, P.; Dinarello, C.A. IL-1 induces IL-IV. IFN-gamma suppresses IL-1 but not lipopolysaccha-ride-induced transcription of IL-1. J. Immunol. 1990, 144, 2216–2222. [Google Scholar]
- Ruscitti, P.; Berardicurti, O.; Di Benedetto, P.; Cipriani, P.; Iagnocco, A.; Shoenfeld, Y.; Giacomelli, R. Severe COVID-19, Another Piece in the Puzzle of the Hyperferritinemic Syndrome. An Immunomodulatory Perspective to Alleviate the Storm. Front. Immunol. 2020, 11, 1130. [Google Scholar] [CrossRef] [PubMed]
- Ruscitti, P.; Bruno, F.; Berardicurti, O.; Acanfora, C.; Pavlych, V.; Palumbo, P.; Conforti, A.; Carubbi, F.; Di Cola, I.; Di Benedetto, P.; et al. Lung involvement in macrophage activation syndrome and severe COVID-19: Results from a cross-sectional study to assess clinical, laboratory and artificial intelligence–radiological differences. Ann. Rheum. Dis. 2020, 79, 1152–1155. [Google Scholar] [CrossRef]
- Ruscitti, P.; Berardicurti, O.; Barile, A.; Cipriani, P.; Shoenfeld, Y.; Iagnocco, A.; Giacomelli, R. Severe COVID-19 and related hyperferritinaemia: More than an innocent bystander? Ann. Rheum. Dis. 2020, 79, 1515–1516. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Suh, C.-H.; Jung, J.-Y.; Ahn, M.-H.; Han, M.H.; Kwon, J.E.; Yim, H.; Kim, H.-A. Elevated circulating levels of the interferon-γ–induced chemokines are associated with disease activity and cutaneous manifestations in adult-onset Still’s disease. Sci. Rep. 2017, 7, srep46652. [Google Scholar] [CrossRef] [PubMed]
- Canna, S.W.; Wrobel, J.; Chu, N.; Kreiger, P.A.; Paessler, M.; Behrens, E.M. Interferon-γ Mediates Anemia but Is Dispensable for Fulminant Toll-like Receptor 9-Induced Macrophage Activation Syndrome and Hemophagocytosis in Mice. Arthritis Rheum. 2013, 65, 1764–1775. [Google Scholar] [CrossRef]
- Young, H.A.; Bream, J.H. IFN-γ: Recent Advances in Understanding Regulation of Expression, Biological Functions, and Clinical Applications. Curr. Top. Microbiol. Immunol. 2007, 316, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Platanias, L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005, 5, 375–386. [Google Scholar] [CrossRef]
- Behrens, E.M.; Canna, S.W.; Slade, K.; Rao, S.; Kreiger, P.A.; Paessler, M.; Kambayashi, T.; Koretzky, G.A. Repeated TLR9 stimulation results in macrophage activation syndrome–like disease in mice. J. Clin. Investig. 2011, 121, 2264–2277. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.K.; Chu, N.; Behrens, E.M. Interferon-γ–Mediated Immunopathology Potentiated by Toll-Like Receptor 9 Activation in a Murine Model of Macrophage Activation Syndrome. Arthritis Rheumatol. 2019, 71, 161–168. [Google Scholar] [CrossRef]
- Prencipe, G.; Caiello, I.; Pascarella, A.; Grom, A.A.; Bracaglia, C.; Chatel, L.; Ferlin, W.G.; Marasco, E.; Strippoli, R.; de Min, C.; et al. Neutralization of IFN-γ reverts clinical and laboratory features in a mouse model of macrophage activation syndrome. J. Allergy Clin. Immunol. 2018, 141, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, M.F.; Klein-Gitelman, M.; Morgan, E.; Proytcheva, M.; Sullivan, C.; Morgan, G.; Pachman, L.M.; O’Gorman, M.R.G. Serum Neopterin Levels as a Diagnostic Marker of Hemophagocytic Lymphohistiocytosis Syndrome. Clin. Vaccine Immunol. 2011, 18, 609–614. [Google Scholar] [CrossRef]
- Buatois, V.; Chatel, L.; Cons, L.; Lory, S.; Richard, F.; Guilhot, F.; Johnson, Z.; Bracaglia, C.; De Benedetti, F.; De Min, C.; et al. Use of a mouse model to identify a blood biomarker for IFNγ activity in pediatric secondary hemophagocytic lymphohistiocytosis. Transl. Res. 2017, 180, 37–52. [Google Scholar] [CrossRef]
- Jordan, M.B.; Hildeman, D.; Kappler, J.; Marrack, P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood 2004, 104, 735–743. [Google Scholar] [CrossRef]
- Schmid, J.P.; Ho, C.-H.; Chrétien, F.; Lefebvre, J.M.; Pivert, G.; Kosco-Vilbois, M.; Ferlin, W.; Geissmann, F.; Fischer, A.; Basile, G.D.S. Neutralization of IFNγ defeats haemophagocytosis in LCMV-infected perforin- and Rab27a-deficient mice. EMBO Mol. Med. 2009, 1, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Zoller, E.E.; Lykens, J.E.; Terrell, C.E.; Aliberti, J.; Filipovich, A.H.; Henson, P.M.; Jordan, M.B. Hemophagocytosis causes a consumptive anemia of inflammation. J. Exp. Med. 2011, 208, 1203–1214. [Google Scholar] [CrossRef]
- Hatterer, E.; Richard, F.; Malinge, P.; Serge, A.; Startchick, S.; Kosco-Vilbois, M.; Deehan, M.; Ferlin, W.; Guilhot, F. P156 Investigating the novel mechanism of action for NI-0501, a human interferon gamma monoclonal antibody. Cytokine 2012, 59, 570. [Google Scholar] [CrossRef]
- Henter, J.-I.; von Bahr Greenwood, T.; Bergsten, E. Emapalumab in Primary Hemophagocytic Lymphohistiocytosis. N. Engl. J. Med. 2020, 383, 596–599. [Google Scholar] [CrossRef]
- Lounder, D.T.; Bin, Q.; De Min, C.; Jordan, M.B. Treatment of refractory hemophagocytic lymphohistiocytosis with emapalumab despite severe concurrent infections. Blood Adv. 2019, 3, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Merli, P.; Algeri, M.; Gaspari, S.; Locatelli, F. Novel Therapeutic Approaches to Familial HLH (Emapalumab in FHL). Front. Immunol. 2020, 11, 608492. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Lucarelli, B.; Merli, P. Current and future approaches to treat graft failure after allogeneic hematopoietic stem cell transplantation. Expert Opin. Pharmacother. 2013, 15, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Coccia, E.M.; Severa, M.; Giacomini, E.; Monneron, D.; Remoli, M.E.; Julkunen, I.; Cella, M.; Lande, R.; Uzé, G. Viral infection and Toll-like receptor agonists induce a differential expression of type I and λ interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur. J. Immunol. 2004, 34, 796–805. [Google Scholar] [CrossRef]
- Kotenko, S.V.; Gallagher, G.; Baurin, V.V.; Lewis-Antes, A.; Shen, M.; Shah, N.K.; Langer, J.A.; Sheikh, F.; Dickensheets, H.; Donnelly, R.P. IFN-λs mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 2002, 4, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Bracaglia, C.; Prencipe, G.; De Benedetti, F. Macrophage Activation Syndrome: Different mechanisms leading to a one clinical syndrome. Pediatr. Rheumatol. 2017, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, R.; Sota, J.; Ruscitti, P.; Campochiaro, C.; Colafrancesco, S.; Dagna, L.; Iacono, D.; Iannone, F.; Lopalco, G.; Sfriso, P.; et al. The treatment of adult-onset Still’s disease with anakinra, a recombinant human IL-1 receptor antagonist: A systematic review of literature. Clin. Exp. Rheumatol. 2020, 39, 187–195. [Google Scholar]
- Castañeda, S.; Martínez-Quintanilla, D.; Martín-Varillas, J.L.; García-Castañeda, N.; Atienza-Mateo, B.; González-Gay, M.A. Tocilizumab for the treatment of adult-onset Still’s disease. Expert Opin. Biol. Ther. 2019, 19, 273–286. [Google Scholar] [CrossRef]
- Grom, A.A.; Ilowite, N.T.; Pascual, V.; Brunner, H.I.; Martini, A.; Lovell, D.J.; Ruperto, N.; Leon, K.; Lheritier, K.; Abrams, K.; et al. Rate and Clinical Presentation of Macrophage Activation Syndrome in Patients With Systemic Juvenile Idiopathic Arthritis Treated With Canakinumab. Arthritis Rheumatol. 2016, 68, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Itoh, Y.; Morio, T.; Sumitomo, N.; Daimaru, K.; Minota, S. Macrophage Activation Syndrome in Patients with Systemic Juvenile Idiopathic Arthritis under Treatment with Tocilizumab. J. Rheumatol. 2015, 42, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Behrens, E.M.; Koretzky, G.A. Review: Cytokine Storm Syndrome: Looking Toward the Precision Medicine Era. Arthritis Rheumatol. 2017, 69, 1135–1143. [Google Scholar] [CrossRef]
- Ruscitti, P.; Cipriani, P.; Ciccia, F.; Masedu, F.; Liakouli, V.; Carubbi, F.; Berardicurti, O.; Guggino, G.; Di Benedetto, P.; Di Bartolomeo, S.; et al. Prognostic factors of macrophage activation syndrome, at the time of diagnosis, in adult patients affected by autoimmune disease: Analysis of 41 cases collected in 2 rheumatologic centers. Autoimmun. Rev. 2017, 16, 16–21. [Google Scholar] [CrossRef]
- Ruscitti, P.; Cipriani, P.; Masedu, F.; Iacono, D.; Ciccia, F.; Liakouli, V.; Guggino, G.; Carubbi, F.; Berardicurti, O.; Di Benedetto, P.; et al. Adult-onset Still’s disease: Evaluation of prognostic tools and validation of the systemic score by analysis of 100 cases from three centers. BMC Med. 2016, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Berardicurti, O.; Ruscitti, P.; Ursini, F.; D’Andrea, S.; Ciaffi, J.; Meliconi, R.; Iagnocco, A.; Cipriani, P.; Giacomelli, R. Mortal-ity in tocilizumab-treated patients with COVID-19: A systematic review and meta-analysis. Clin. Exp. Rheumatol. 2020, 38, 1247–1254. [Google Scholar]
- Klopfenstein, T.; Zayet, S.; Lohse, A.; Selles, P.; Zahra, H.; Kadiane-Oussou, N.J.; Toko, L.; Royer, P.-Y.; Balblanc, J.-C.; Gendrin, V.; et al. Impact of tocilizumab on mortality and/or invasive mechanical ventilation requirement in a cohort of 206 COVID-19 patients. Int. J. Infect. Dis. 2020, 99, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Maschalidi, S.; Sepulveda, F.E.; Garrigue, A.; Fischer, A.; Basile, G.D.S. Therapeutic effect of JAK1/2 blockade on the manifestations of hemophagocytic lymphohistiocytosis in mice. Blood 2016, 128, 60–71. [Google Scholar] [CrossRef]
- Das, R.; Guan, P.; Sprague, L.; Verbist, K.; Tedrick, P.; An, Q.A.; Cheng, C.; Kurachi, M.; Levine, R.; Wherry, E.J.; et al. Janus kinase inhibition lessens inflammation and ameliorates disease in murine models of hemophagocytic lymphohistiocytosis. Blood 2016, 127, 1666–1675. [Google Scholar] [CrossRef]
- Favoino, E.; Prete, M.; Catacchio, G.; Ruscitti, P.; Navarini, L.; Giacomelli, R.; Perosa, F. Working and safety profiles of JAK/STAT signaling inhibitors. Are these small molecules also smart? Autoimmun. Rev. 2021, 20, 102750. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, R.; Wu, X.; Li, F.; Yang, H.; Liu, L.; Guo, H.; Zhang, X.; Mai, H.; Li, H.; et al. Ruxolitinib-combined doxorubicin-etoposide-methylprednisolone regimen as a salvage therapy for refractory/relapsed haemophagocytic lymphohistiocytosis: A single-arm, multicentre, phase 2 trial. Br. J. Haematol. 2021. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, A.; Ma, H.H.; Zhang, L.; Lian, H.Y.; Wang, D.; Zhao, Y.Z.; Cui, L.; Li, W.J.; Yang, Y.; et al. A pilot study of ruxolitinib as a front-line therapy for 12 children with secondary hemophagocytic lymphohistiocytosis. Haematologica 2020. [Google Scholar] [CrossRef]
- Hansen, S.; Alduaij, W.; Biggs, C.M.; Belga, S.; Luecke, K.; Merkeley, H.; Chen, L.Y.C. Ruxolitinib as adjunctive therapy for secondary hemophagocytic lymphohistiocytosis: A case series. Eur. J. Haematol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bode, S.F.; Ammann, S.; Al-Herz, W.; Bataneant, M.; Dvorak, C.C.; Gehring, S.; Gennery, A.; Gilmour, K.C.; Gonzalez-Granado, L.I.; Gross-Wieltsch, U.; et al. The syndrome of hemophagocytic lymphohistiocytosis in primary immunodeficiencies: Implications for differential diagnosis and pathogenesis. Haematologica 2015, 100, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Dorman, S.E.; Picard, C.; Lammas, D.; Heyne, K.; Van Dissel, J.T.; Baretto, R.; Rosenzweig, S.D.; Newport, M.; Levin, M.; Roesler, J.; et al. Clinical features of dominant and recessive interferon γ receptor 1 deficiencies. Lancet 2004, 364, 2113–2121. [Google Scholar] [CrossRef]
- Olbrich, P.; Martínez-Saavedra, M.T.; Pérez-Hurtado, J.M.; Sanchez, C.; Sánchez, B.; Deswarte, C.; Obando, I.; Casanova, J.-L.; Speckmann, C.; Bustamante, J.; et al. Diagnostic and therapeutic challenges in a child with complete Interferon-γ Receptor 1 deficiency. Pediatr. Blood Cancer 2015, 62, 2036–2039. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Cola, I.; Ruscitti, P.; Giacomelli, R.; Cipriani, P. The Pathogenic Role of Interferons in the Hyperinflammatory Response on Adult-Onset Still’s Disease and Macrophage Activation Syndrome: Paving the Way towards New Therapeutic Targets. J. Clin. Med. 2021, 10, 1164. https://doi.org/10.3390/jcm10061164
Di Cola I, Ruscitti P, Giacomelli R, Cipriani P. The Pathogenic Role of Interferons in the Hyperinflammatory Response on Adult-Onset Still’s Disease and Macrophage Activation Syndrome: Paving the Way towards New Therapeutic Targets. Journal of Clinical Medicine. 2021; 10(6):1164. https://doi.org/10.3390/jcm10061164
Chicago/Turabian StyleDi Cola, Ilenia, Piero Ruscitti, Roberto Giacomelli, and Paola Cipriani. 2021. "The Pathogenic Role of Interferons in the Hyperinflammatory Response on Adult-Onset Still’s Disease and Macrophage Activation Syndrome: Paving the Way towards New Therapeutic Targets" Journal of Clinical Medicine 10, no. 6: 1164. https://doi.org/10.3390/jcm10061164
APA StyleDi Cola, I., Ruscitti, P., Giacomelli, R., & Cipriani, P. (2021). The Pathogenic Role of Interferons in the Hyperinflammatory Response on Adult-Onset Still’s Disease and Macrophage Activation Syndrome: Paving the Way towards New Therapeutic Targets. Journal of Clinical Medicine, 10(6), 1164. https://doi.org/10.3390/jcm10061164




