Comparing the Clinical and Laboratory Features of Remitting Seronegative Symmetrical Synovitis with Pitting Edema and Seronegative Rheumatoid Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Compliance with Ethical Standards
2.2. Study Design
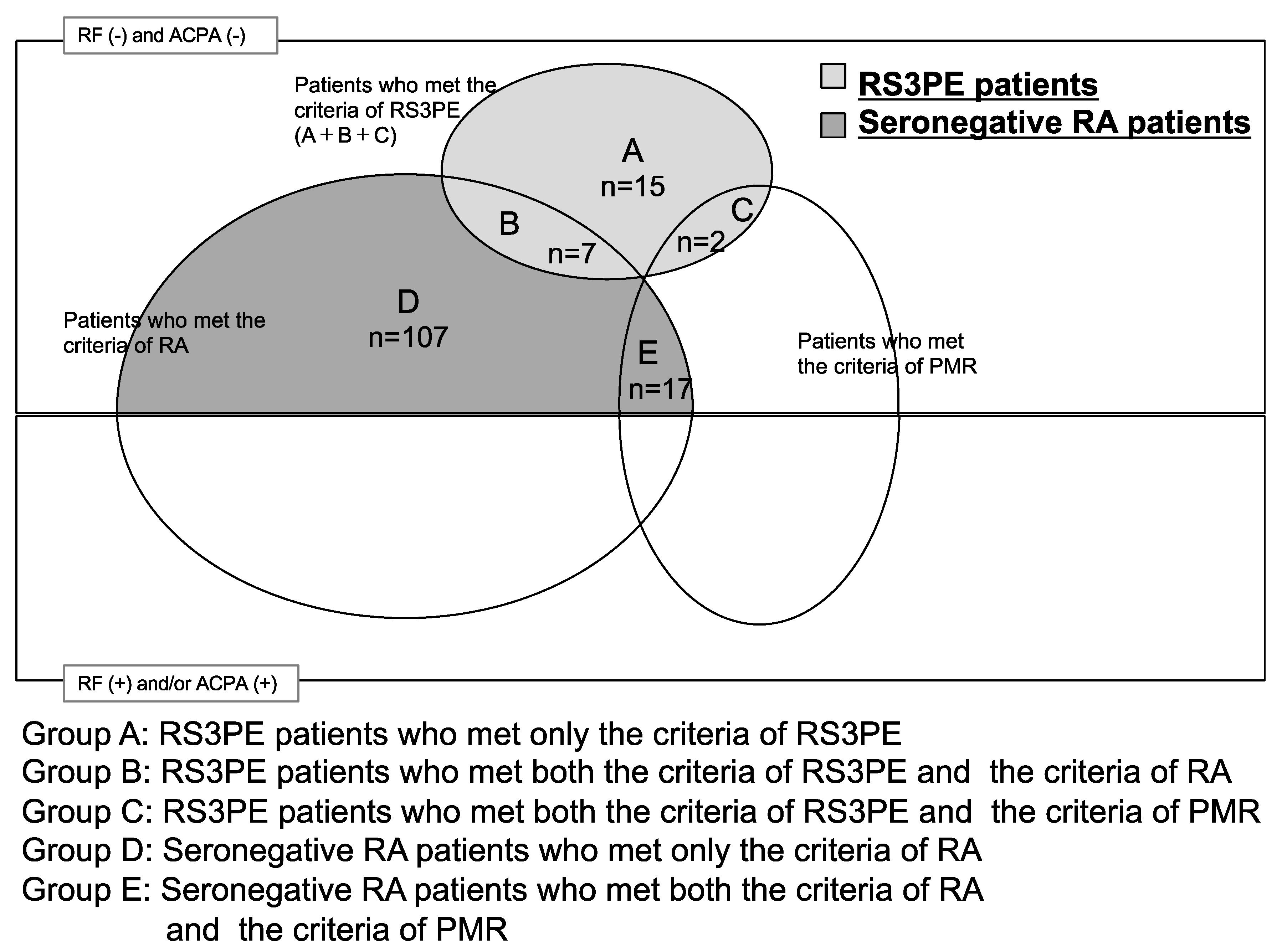
2.3. Patients
2.4. RS3PE Diagnosis
2.5. Seronegative RA Diagnosis
2.6. Clinical and Laboratory Features
2.7. Statistical Analysis
3. Results
3.1. Comparison of Clinical and Laboratory Features of RS3PE and Seronegative RA
3.2. Comparison of Clinical and Laboratory Features of RS3PE and Seronegative RA with a 1:2 Matching for Age and Sex
3.3. Comparison of Clinical Features of Patients with and without Malignancies among the RS3PE and Seronegative RA Patients
3.4. Comparison of Baseline Characteristics between RS3PE Patients with and without Malignancies
3.5. Comparison of Baseline Characteristics between Seronegative RA Patients with and without Malignancies
4. Discussion
4.1. Comparison of Clinical and Laboratory Features of RS3PE and Seronegative RA
4.2. Comparison between RS3PE/Seronegative RA with and without Malignancies
4.3. Comparison between RS3PE Patients with and without Malignancies
4.4. Comparison between Seronegative RA Patients with and without Malignancies
4.5. Comparison between Seronegative RA and RS3PE Patients with and without Malignancies
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCarty, D.J.; O’Duffy, J.D.; Pearson, L.; Hunter, J.B. Remitting seronegative symmetrical synovitis with pitting edema. RS3PE syndrome. JAMA 1985, 254, 2763–2767. [Google Scholar] [CrossRef]
- Olivé, A.; Del Blanco, J.; Pons, M.; Vaquero, M.; Tena, X. The clinical spectrum of remitting seronegative symmetrical synovitis with pitting edema. The Catalán Group for the Study of RS3PE. J. Rheumatol. 1997, 24, 333–336. [Google Scholar]
- Arima, K.; Origuchi, T.; Tamai, M.; Iwanaga, N.; Izumi, Y.; Huang, M.; Tanaka, F.; Kamachi, M.; Aratake, K.; Nakamura, H.; et al. RS3PE syndrome presenting as vascular endothelial growth factor associated disorder. Ann. Rheum. Dis. 2005, 64, 1653–1655. [Google Scholar] [CrossRef]
- Mavragani, C.P.; Moutsopoulos, H.M. Rheumatoid arthritis in the elderly. Exp. Gerontol. 1999, 34, 463–471. [Google Scholar] [CrossRef]
- Healy, L.A. RS3PE syndrome. J. Rheumatol. 1990, 17, 414. [Google Scholar]
- Kimura, M.; Tokuda, Y.; Oshiawa, H.; Yoshida, K.; Utsunomiya, M.; Kobayashi, T.; Deshpande, G.A.; Matsui, K.; Kishimoto, M. Clinical characteristics of patients with remitting seronegative symmetrical synovitis with pitting edema compared to patients with pure polymyalgia rheumatica. J. Rheumatol. 2012, 39, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Kawashiri, S.; Suzuki, T.; Okada, A.; Tsuji, A.; Takatani, A.; Shimizu, T.; Koga, T.; Iwamoto, N.; Ichinose, K.; Nakamura, H.; et al. Differences in musculoskeletal ultrasound findings between RS3PE syndrome and elderly-onset rheumatoid arthritis. Clin. Rheumatol. 2020, 39, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Sibilia, J.; Friess, S.; Schaeverbeke, T.; Maloisel, F.; Bertin, P.; Goichot, B.; Kuntz, J.L. Remitting seronegative symmetrical synovitis with pitting edema (RS3PE): A form of paraneoplastic polyarthritis? J. Rheumatol. 1991, 26, 115–120. [Google Scholar]
- Elizabeth, B.R. Remitting seronegative symmetrical synovitis with pitting edema syndrome: Follow up for neoplasia. J. Rheumatol. 2005, 32, 1760–1761. [Google Scholar]
- Morel, J.; Deschamps, V.; Toussirot, E.; Pertuiset, E.; Sordet, C.; Kieffer, P.; Berthelot, J.M.; Champagne, H.; Mariette, X.; Combe, B. Characteristics and survival of 26 patients with paraneoplastic arthritis. Ann. Rheum. Dis. 2008, 67, 244–247. [Google Scholar] [CrossRef]
- Dasgupta, B.; Cimmino, M.A.; Maradit-Kremers, H.; Schmidt, W.A.; Schirmer, M.; Salvarani, C.; Bachta, A.; Dejaco, C.; Duftner, C.; Jensen, H.S.; et al. Provisional classification criteria for polymyalgia rheumatica: A European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann. Rheum. Dis. 2012, 71, 484–492. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Yao, Q.; Su, X.; Altman, R.D. Is remitting seronegative symmetrical synovitis with pitting edema (RS3PE) a subset of rheumatoid arthritis? Semin. Arthritis Rheum. 2010, 40, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, P.K.; Silver, J.; Winchester, R.J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987, 30, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Fan, K.; Gong, Y.; Huang, Q.; Yang, C.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; Luo, G.; et al. The CRP/Albumin Ratio Predicts Survival and Monitors Chemotherapeutic Effectiveness in Patients With Advanced Pancreatic Cancer. Cancer Manag. Res. 2019, 11, 8781–8788. [Google Scholar] [CrossRef]
- Saito, K.; Kihara, K. Role of C-reactive protein as a biomarker for renal cell carcinoma. Expert Rev. Anticancer Ther. 2010, 10, 1979–1989. [Google Scholar] [CrossRef]
- Salvarani, C.; Gabriel, S.; Hunder, G. Distal extremity swelling with pitting edema in polymyalgia rheumatica. Report of nineteen cases. Arthritis Rheum. 1996, 39, 73–80. [Google Scholar] [CrossRef]
- Salvarani, C.; Cantini, F.; Macchioni, P.; Olivieri, I.; Niccoli, L.; Padula, A.; Boiardi, L. Distal musculoskeletal manifestations in polymyalgia rheumatica: A prospective followup study. Arthritis Rheum. 1998, 41, 1221–1226. [Google Scholar] [CrossRef]
- Bucaloiu, I.D.; Olenginski, T.P.; Harrington, T.M. Remitting seronegative symmetrical synovitis with pitting edema syndrome in a rural tertiary care practice: A retrospective analysis. Mayo Clin. Proc. 2007, 82, 1510–1515. [Google Scholar] [CrossRef]
- Manger, B.; Schett, G. Paraneoplastic syndromes in rheumatology. Nat. Rev. Rheumatol. 2014, 10, 662–670. [Google Scholar] [CrossRef]
- Muller, S.; Hider, S.; Helliwell, T.; Partington, R.; Mallen, C. The real evidence for polymyalgia rheumatica as a paraneoplastic syndrome. Reumatismo 2018, 70, 23–34. [Google Scholar] [CrossRef]
- Cancer Statistics. Available online: https://ganjoho.jp/reg_stat/statistics/stat/index.html (accessed on 9 September 2020). (In Japanese).
- Emamifar, A.; Hess, S.; Gildberg-Mortensen, R.; Hansen, I.M.J. Association of remitting seronegative symmetrical synovitis with pitting edema, polymyalgia rheumatica, and adenocarcinoma of the prostate. Am. J. Case Rep. 2016, 17, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Origuchi, T.; Arima, K.; Kawashiri, S.Y.; Tamai, M.; Yamasaki, S.; Nakamura, H.; Tsukada, T.; Aramaki, T.; Furuyama, M.; Miyashita, T.; et al. High serum matrix metalloproteinase 3 is characteristic of patients with paraneoplastic remitting seronegative symmetrical synovitis with pitting edema syndrome. Mod. Rheumatol. 2012, 22, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.; Salisbury, C.; Taylor, D.J.; Kirwan, J.R. Changes in biochemical markers of joint tissue metabolism in a randomized controlled trial of glucocorticoid in early rheumatoid arthritis. Arthritis Rheum. 1998, 41, 1203–1209. [Google Scholar] [CrossRef]
- Ohuchi, E.; Iwata, K.; Yamanaka, H. Serum MMP-3 in rheumatoid arthritis. Inflamm. Regenerat. 2004, 24, 154–160. (In Japanese) [Google Scholar] [CrossRef]
- Nagasawa, H.; Kameda, H.; Amano, K.; Takeuchi, T. Clinical significance of serum matrix metalloproteinase-3 level in patients with rheumatoid arthritis. Inflamm. Regenerat. 2004, 25, 60–64. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Karmacharya, P.; Donato, A.A.; Aryal, M.R.; Ghimire, S.; Pathak, R.; Shah, K.; Shrestha, P.; Poudel, D.; Wasser, T.; Subedi, A.; et al. RS3PE revisited: A systematic review and meta-analysis of 331 cases. Clin. Exp. Rheumatol. 2016, 34, 404–415. [Google Scholar]
| Characteristics | RS3PE Patients (n = 24) | Seronegative RA Patients (n = 124) | p Value | ||
|---|---|---|---|---|---|
| Age, median (IQR), years | 79.5 (73.8–86.5) | 68.5 (58.5–78.0) | <0.001 | ||
| Length of follow-up, median (IQR), months | 31.5 (12.0–109.0) | 62.9 (30.7–98.4) | 0.09 | ||
| Male sex, n (%) | 13 (54.2) | 46 (37.1) | 0.17 | ||
| Smoking, n (%) | 5 (20.8) | 23 (18.6) | 0.78 | ||
| Diabetes, n (%) | 6 (25.0) | 14 (11.3) | 0.10 | ||
| Hypertension, n (%) | 12 (50.0) | 41 (33.1) | 0.16 | ||
| Hyperlipidemia, n (%) | 5 (20.8) | 33 (26.1) | 0.62 | ||
| Swollen or/and tender joints, n (%) | |||||
| Shoulders | 8 (33.3) | 67 (54.3) | 0.08 | ||
| Elbows | 2 (8.3) | 53 (42.7) | 0.001 | ||
| Wrists | 17 (70.8) | 100 (80.7) | 0.28 | ||
| Fingers | 19 (79.2) | 120 (96.8) | 0.022 | ||
| Hips | 4 (16.7) | 13 (10.5) | 0.48 | ||
| Knees | 9 (37.5) | 59 (47.6) | 0.38 | ||
| Ankles | 18 (75.0) | 65 (52.4) | 0.046 | ||
| Toes | 8 (33.3) | 35 (28.2) | 0.63 | ||
| Patients with swollen large joints, n (%) | 17 (70.8) | 64 (51.6) | 0.12 | ||
| Patients with swollen small joints, n (%) | 21 (87.5) | 124 (100.0) | 0.024 | ||
| Number of swollen large joints, median (IQR), n | 2.0 (0.0–2.8) | 1.0 (0.0–2.0) | 0.17 | ||
| Number of swollen small joints, median (IQR), n | 3.0 (1.3–13.3) | 9.0 (5.0–15.0) | 0.33 | ||
| 28 swollen joints, median (IQR), n | 4.0 (1.3–10.8) | 8.0 (5.0–14.0) | 0.29 | ||
| 28 tender joints, median (IQR), n | 6.5 (4.3–12.0) | 11.0 (7.3–15.0) | 0.15 | ||
| Patients with erosion, n (%) | 0 (0.0) | 39 (31.5) | <0.001 | ||
| Systemic signs and symptoms, n (%) | |||||
| Temperature ≥38 °C | 2 (8.3) | 7 (5.7) | 0.64 | ||
| Malaise or fatigue | 3 (12.5) | 8 (6.5) | 0.39 | ||
| Weight loss | 5 (20.8) | 12 (9.7) | 0.16 | ||
| Morning stiffness (lasting at least 1 h) | 2 (8.3) | 31 (25.0) | 0.11 | ||
| Edema (both hands and feet) | 24 (100.0) | 0(0) | <0.001 | ||
| Edema (only hands) | 0 (0.0) | 1 (0.8) | 1.0 | ||
| Edema (only feet) | 0 (0.0) | 19 (15.3) | <0.001 | ||
| CRP, median (IQR), mg/dL | 8.2 (4.0–14) | 2.8 (0.7–6.6) | 0.004 | ||
| ESR, median (IQR), mm/h | |||||
| Men+Women | 91.0 (59–112.5) | 55.0 (32.0–90.0) | 0.07 | ||
| Men | 85.0 (28.5–114.5) | 57.0 (31.0–90.0) | 0.36 | ||
| Women | 91.0 (82–113) | 54.0 (32.0–88.0) | 0.010 | ||
| Alb, median (IQR), g/dL | 3.5 (3.0–3.7) | (n = 23) * | 3.9 (3.4–4.1) | (n = 100) * | 0.012 |
| LDH, median (IQR), U/L | 197.0 (161–234) | 176.0 (155.5–195) | 0.07 | ||
| MMP-3, median (IQR), ng/mL | |||||
| Men+Women | 378.5(243.3–662.2) | (n = 16) * | 162.0 (82.2–401.1) | (n = 115) * | 0.022 |
| Men | 359.4(269.1–435.4) | (n = 7) * | 211.0 (115.3–420.9) | (n = 45) * | 0.08 |
| Women | 414.1(92.8–997.2) | (n = 9) * | 151.0 (47.2–348.5) | (n = 70) * | 0.07 |
| Hb, mean ± SD, g/dL | |||||
| Men + Women | 10.7 ± 1.8 | 11.9 ± 1.8 | 0.024 | ||
| Men | 10.8 ± 2.0 | 12.2 ± 1.7 | 0.10 | ||
| Women | 10.6 ± 1.5 | 11.7 ± 1.8 | 0.12 | ||
| Malignancy (within 2 years before and after the diagnosis of RS3PE or seronegative RA), n (%) | 6 (25.0) | 8 (6.5) | 0.034 | ||
| Patients fulfilling the classification criteria for RA [11,12], n (%) | 7 (29.2) | 124 (100.0) | <0.001 | ||
| Patients fulfilling the classification criteria for PMR [10], n (%) | 2 (8.3) | 17 (13.7) | 0.74 | ||
| Patients fulfilling the classification criteria for RA [11,12] + PMR [10], n (%) | 0 (0.0) | 17 (13.7) | 0.08 | ||
| Sex, Age (years) | Interval between Diagnosis of RS3PE/Seronegative RA and Malignancies (Months) | Malignancy Type |
|---|---|---|
| RS3PE | ||
| M, 81 | –24 | Prostate cancer |
| M, 78 | –24 | Prostate cancer |
| M, 78 | –11 | Rectal cancer |
| F, 87 | 0 (+5 days) | Pancreatic cancer |
| M, 79 | 0 (+6 days) | Stomach cancer |
| M, 80 | 3 | Rectal cancer |
| Seronegative RA | ||
| M, 84 | –20 | Rectal cancer |
| F, 64 | –17 | Uterine cancer |
| M, 82 | –6 | Ascending colon cancer |
| M, 69 | –5 | Small cell lung cancer |
| F, 58 | –4 | Breast cancer |
| F, 80 | 1 | Breast cancer |
| M, 67 | 9 | Diffuse large B cell lymphoma |
| M, 83 | 18 | Pancreatic cancer |
| Characteristic | RS3PE Patients (n = 24) | Seronegative RA Patients (n = 48) | p Value |
|---|---|---|---|
| Age, median (IQR), years | 79.5 (73.8–86.5) | 79.5 (73.3–85.3) | 0.58 |
| Male sex, n (%) | 13 (54.2) | 23 (47.9) | 0.80 |
| Swollen or/and tender joint, n (%) | |||
| Shoulders | 8 (33.3) | 33 (68.8) | 0.006 |
| Elbows | 2 (8.3) | 19 (39.6) | 0.006 |
| Wrists | 17 (70.8) | 42 (87.5) | 0.11 |
| Fingers | 19 (79.2) | 46 (95.8) | 0.037 |
| Hips | 4 (16.7) | 6 (12.5) | 0.72 |
| Knees | 9 (37.5) | 19 (39.6) | 1.00 |
| Ankles | 18 (75.0) | 25 (52.1) | 0.08 |
| Toes | 8 (33.3) | 11 (22.9) | 0.40 |
| Patients with swollen large joints, n (%) | 17 (70.8) | 26 (54.2) | 0.21 |
| Patients with swollen small joints, n (%) | 21 (87.5) | 48 (100.0) | 0.034 |
| Number of swollen small joints, median (IQR), n | 3.0 (1.3–13.3) | 9.0 (6.0–15.0) | 0.021 |
| 28 swollen joints, median (IQR), n | 4.0 (1.3–10.8) | 9.5 (6.0–15.0) | 0.008 |
| 28 tender joints, median (IQR), n | 6.5 (4.3–12.0) | 11.0 (8.3–15.0) | 0.019 |
| Patients with erosion, n (%) | 0 (0.0) | 15 (31.3) | 0.001 |
| CRP, median (IQR), mg/dL | 8.2 (4.0–14) | 4.4 (1.3–8.4) | 0.021 |
| ESR, median (IQR), mm/h | 91.0 (59–112.5) | 75.0 (37.0–103.0) | 0.25 |
| LDH, median (IQR), U/L | 197.0 (161–234) | 184.5 (164.0–210.5) | 0.26 |
| MMP-3, median (IQR), ng/mL | 378.5(243.3–662.2) | 251.0 (124.0–555.0) | 0.27 |
| Hb, mean±SD, mg/dL | 10.7 ± 1.8 | 11.5 ± 2.0 | 0.08 |
| Malignancy (within 2 years before and after the diagnosis of RS3PE or seronegative RA), n (%) | 6 (25.0) | 1 (2.1) | 0.005 |
| Patients fulfilling the classification criteria for RA [11,12], n (%) | 7 (29.2) | 48 (100.0) | 0.09 |
| Patients fulfilling the classification criteria for PMR [10], n (%) | 2 (8.3) | 7 (14.6) | 0.71 |
| Patients fulfilling the classification criteria for RA [11,12] + PMR [10], n (%) | 0 (0.0) | 7 (14.6) | 0.09 |
| Characteristics | With Malignancy (n = 14) | Without Malignancy (n = 134) | p Value | ||
|---|---|---|---|---|---|
| Age, median (IQR), years | 79.5 (68.5–82.3) | 69.5 (60.0–79.0) | 0.032 | ||
| Length of follow-up, median (IQR), months | 40.6 (7.9–87.7) | 57.4 (27.4–97.7) | 0.36 | ||
| Male sex, n (%) | 10 (71.4) | 49 (36.6) | 0.011 | ||
| Smoking, n (%) | 5 (35.7) | 23 (17.2) | 0.14 | ||
| Diabetes, n (%) | 4 (28.6) | 16 (11.9) | 0.10 | ||
| Hypertension, n (%) | 5 (35.7) | 48 (35.8) | 1.00 | ||
| Hyperlipidemia, n (%) | 4 (28.6) | 34 (25.4) | 0.76 | ||
| Swollen or/and tender joints, n (%) | |||||
| Shoulders | 5 (35.7) | 70 (52.2) | 0.27 | ||
| Elbows | 5 (35.7) | 50 (37.3) | 1.00 | ||
| Wrists | 11 (78.6) | 106 (79.1) | 1.00 | ||
| Fingers | 13 (92.9) | 126 (94.0) | 1.00 | ||
| Hips | 2 (14.3) | 15 (11.2) | 0.67 | ||
| Knees | 5 (35.7) | 63 (47.0) | 0.56 | ||
| Ankles | 8 (57.1) | 75 (56.0) | 1.00 | ||
| Toes | 4 (28.6) | 39 (29.1) | 1.00 | ||
| Patients with swollen large joints, n (%) | 6 (42.9) | 75 (56.0) | 0.41 | ||
| Patients with swollen small joints, n (%) | 13 (92.9) | 132 (98.0) | 0.26 | ||
| Number of swollen large joints, median (IQR), n | 0.0 (0.0–2.3) | 1.0 (0.0–2.0) | 0.44 | ||
| Number of swollen small joints, median (IQR), n | 12.5 (4.3–18.5) | 8.0 (4.0–13.0) | 0.46 | ||
| 28 swollen joints, median (IQR), n | 9.5 (3.5–16.8) | 8.0 (4.0–12.0) | 0.62 | ||
| 28 tender joints, median (IQR), n | 7.5 (5.8–19.3) | 10.0 (7.0–14.3) | 0.74 | ||
| Patients with erosion, n (%) | 5 (35.7) | 34 (25.4) | 0.52 | ||
| Systemic signs and symptoms, n (%) | |||||
| Temperature ≥ 38 °C | 0 (0.0) | 9 (6.7) | 1.00 | ||
| Malaise or fatigue | 2 (14.3) | 9 (6.7) | 0.28 | ||
| Weight loss | 1 (7.1) | 16 (12.0) | 1.00 | ||
| Morning stiffness (lasting at least 1 h) | 4 (28.6) | 29 (21.7) | 0.55 | ||
| Edema (both hands and feet) | 6 (42.9) | 18 (13.4) | 0.034 | ||
| Edema (only hands) | 0 (0.0) | 1 (0.8) | 1.00 | ||
| Edema (only feet) | 0 (0.0) | 19 (14.2) | 0.22 | ||
| CRP, median (IQR), mg/dL | 6.1 (3.1–11.9) | 3.1 (0.8–7.2) | 0.08 | ||
| ESR, median (IQR), mm/h | |||||
| Men + Women | 46.0 (21.5–112.0) | 59.0 (33.0–91.5) | 0.88 | ||
| Men | 90.0 (35.0–114.0) | 59.0 (31.0–90.5) | 0.53 | ||
| Women | 22.5 (13.8–91.3) | 59.0 (33.5–93.5) | 0.15 | ||
| Alb, median (IQR), g/dL | 3.5 (3.1–4.0) | 3.8 (3.3–4.1) | (n = 109) * | 0.24 | |
| LDH, median (IQR), U/L | 174.5 (166.8–214.8) | 178.0 (155.0–206.3) | 0.79 | ||
| MMP-3, median (IQR), ng/mL | |||||
| Men+Women | 220.0 (43.8–364.8) | (n = 13) * | 181.0 (84.8–428.5) | (n = 118) * | 0.75 |
| Men | 234.7 (133.0–364.8) | (n = 9) * | 213.0 (116.0–426.2) | (n = 43) * | 0.85 |
| Women | 37.7 (28.1–463.8) | (n = 4) * | 162.0 (66.8–465.0) | (n = 75) * | 0.13 |
| Hb, mean ± SD, g/dL | |||||
| Men + Women | 10.9 ± 2.0 | 11.8 ± 1.8 | 0.10 | ||
| Men | 10.3 ± 1.4 | 12.3 ± 1.7 | 0.001 | ||
| Women | 12.7 ± 2.3 | 11.5 ± 1.8 | 0.24 | ||
| Patients diagnosed with RS3PE, n (%) | 6 (42.9) | 18 (13.4) | 0.034 | ||
| Patients diagnosed with RA [11,12], n (%) | 8 (57.1) | 116 (86.6) | 0.034 | ||
| Patients fulfilling the classification criteria for RA [11,12], n (%) | 10 (71.4) | 121 (90.0) | 0.058 | ||
| Patients fulfilling the classification criteria for PMR [10], n (%) | 1 (7.1) | 18 (13.4) | 1.00 | ||
| Characteristics | Odds Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Age | 1.06 | 1.002–1.11 | 0.037 |
| Length of follow–up | 0.999801 | 0.9994–1.0002 | 0.36 |
| Male sex | 4.34 | 1.29–14.57 | 0.007 |
| Smoking | 2.68 | 0.82–8.74 | 0.10 |
| Diabetes | 2.95 | 0.83–10.52 | 0.10 |
| Hypertension | 0.995 | 0.32–3.14 | 0.99 |
| Hyperlipidemia | 1.18 | 0.35–3.997 | 0.79 |
| Swollen or/and tender joints | |||
| Shoulders | 0.51 | 0.16–1.60 | 0.25 |
| Elbows | 0.93 | 0.30–2.94 | 0.91 |
| Wrists | 0.97 | 0.25–3.71 | 0.96 |
| Fingers | 0.83 | 0.10–7.13 | 0.86 |
| Hips | 1.32 | 0.27–6.49 | 0.73 |
| Knees | 0.63 | 0.20–1.97 | 0.42 |
| Ankles | 1.05 | 0.34–3.19 | 0.93 |
| Toes | 0.97 | 0.29–3.29 | 0.97 |
| Patients with swollen large joints | 0.59 | 0.19–1.79 | 0.35 |
| Patients with swollen small joints | 0.20 | 0.02-2.32 | 0.20 |
| Number of swollen large joints | 0.87 | 0.60–1.25 | 0.44 |
| Number of swollen small joints | 1.04 | 0.98–1.10 | 0.22 |
| 28 swollen joints | 1.03 | 0.95–1.11 | 0.50 |
| 28 tender joints | 1.004 | 0.92–1.10 | 0.92 |
| Patients with erosion | 1.63 | 0.51–5.21 | 0.41 |
| Systemic signs and symptoms | |||
| Temperature ≥ 38 °C | 8.20 × 10–7 | 0–>106 | 0.99 |
| Malaise or fatigue | 2.31 | 0.45–11.97 | 0.32 |
| Weight loss | 0.57 | 0.07–4.63 | 0.60 |
| Morning stiffness (lasting at least 1 h) | 1.45 | 0.42–4.96 | 0.56 |
| Edema (both hands and feet) | 4.83 | 1.50–15.56 | 0.034 |
| Edema (only hands) | 6.45 × 10–7 | 0–>105 | 0.99 |
| Edema (only feet) | 2.78 × 10–7 | 0–>106 | 0.99 |
| CRP | 1.08 | 1.18–0.92 | 0.08 |
| ESR | |||
| Men+Women | 0.999905 | 0.98–1.02 | 0.08 |
| Men | 1.006 | 0.988–1.02 | 0.51 |
| Women | 0.98 | 0.95–1.01 | 0.25 |
| Alb | 0.63 | 0.24–1.65 | 0.35 |
| LDH | 1.0009 | 0.99–1.02 | 0.90 |
| MMP–3 | |||
| Men +Women | 1.00009 | 0.9992–1.001 | 0.84 |
| Men | 1.0006 | 0.9993–1.002 | 0.34 |
| Women | 0.9985 | 0.99–1.003 | 0.50 |
| Hb | |||
| Male + Women | 0.77 | 0.57–1.06 | 0.11 |
| Men | 0.51 | 0.33–0.81 | 0.005 |
| Women | 1.47 | 0.80–2.71 | 0.21 |
| Patients with RS3PE | 4.83 | 1.50–15.56 | 0.034 |
| Patients with seronegative RA | 0.21 | 0.06–0.07 | 0.034 |
| Patients fulfilling the classification criteria for RA [11,12] | 0.27 | 0.07–0.98 | 0.046 |
| Patients fulfilling the classification criteria for PMR [10] | 0.50 | 0.06–4.02 | 0.51 |
| Patients fulfilling the classification criteria for RA [11,12] + PMR [10] | 2.82 × 10–7 | 0–>106 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higashida-Konishi, M.; Izumi, K.; Hama, S.; Takei, H.; Oshima, H.; Okano, Y. Comparing the Clinical and Laboratory Features of Remitting Seronegative Symmetrical Synovitis with Pitting Edema and Seronegative Rheumatoid Arthritis. J. Clin. Med. 2021, 10, 1116. https://doi.org/10.3390/jcm10051116
Higashida-Konishi M, Izumi K, Hama S, Takei H, Oshima H, Okano Y. Comparing the Clinical and Laboratory Features of Remitting Seronegative Symmetrical Synovitis with Pitting Edema and Seronegative Rheumatoid Arthritis. Journal of Clinical Medicine. 2021; 10(5):1116. https://doi.org/10.3390/jcm10051116
Chicago/Turabian StyleHigashida-Konishi, Misako, Keisuke Izumi, Satoshi Hama, Hiroshi Takei, Hisaji Oshima, and Yutaka Okano. 2021. "Comparing the Clinical and Laboratory Features of Remitting Seronegative Symmetrical Synovitis with Pitting Edema and Seronegative Rheumatoid Arthritis" Journal of Clinical Medicine 10, no. 5: 1116. https://doi.org/10.3390/jcm10051116
APA StyleHigashida-Konishi, M., Izumi, K., Hama, S., Takei, H., Oshima, H., & Okano, Y. (2021). Comparing the Clinical and Laboratory Features of Remitting Seronegative Symmetrical Synovitis with Pitting Edema and Seronegative Rheumatoid Arthritis. Journal of Clinical Medicine, 10(5), 1116. https://doi.org/10.3390/jcm10051116






