Temporal Trend of Conventional Sperm Parameters in a Sicilian Population in the Decade 2011–2020
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Sperm Analysis
2.3. Statistical Analysis
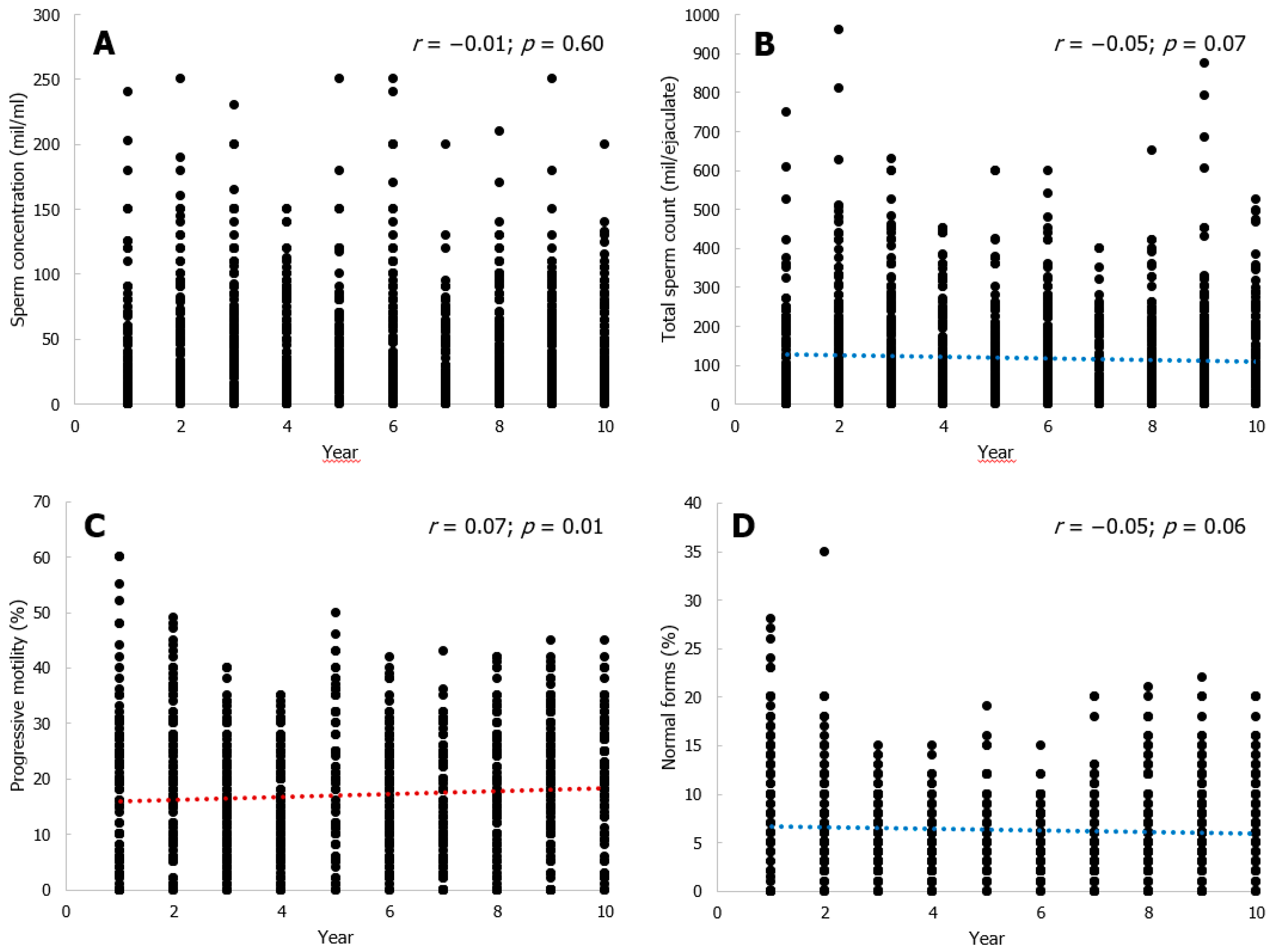
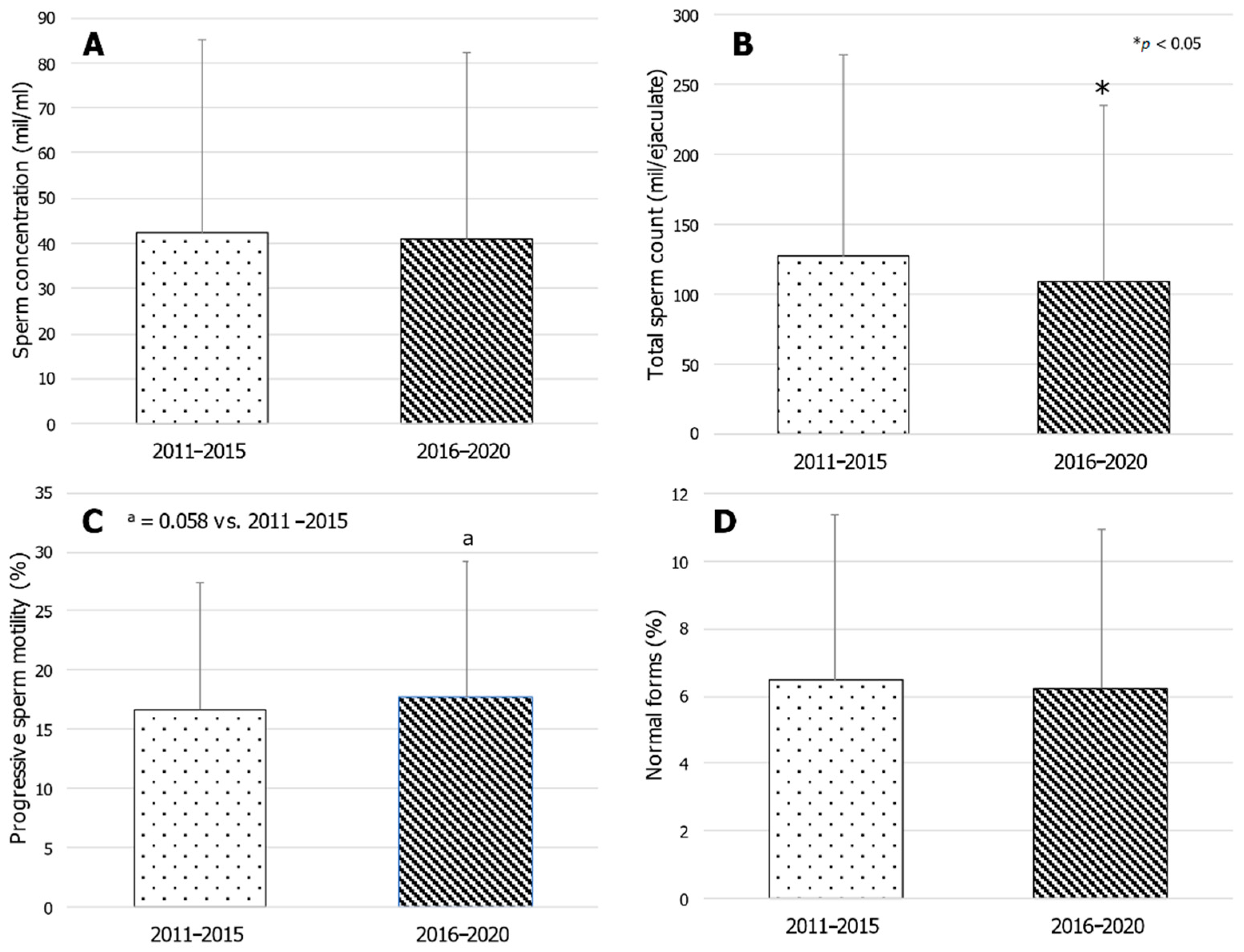
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Auger, J.; Kunstmann, J.M.; Czyglik, F.; Jouannet, P. Decline in Semen Quality among Fertile Men in Paris during the Past 20 Years. N. Engl. J. Med. 1995, 332, 281–285. [Google Scholar] [CrossRef]
- Adamopoulos, D.A.; Pappa, A.; Nicopoulou, S.; Andreou, E.; Karamertzanis, M.; Michopoulos, J.; Deligianni, V.; Simou, M. Andrology: Seminal volume and total sperm number trends in men attending subfertility clinics in the Greater Athens area during the period 1977–1993. Hum. Reprod. 1996, 11, 1936–1941. [Google Scholar] [CrossRef] [PubMed]
- Van Waeleghem, K.; De Clercq, N.; Vermeulen, L.; Schoonjans, F.; Comhaire, F. Deterioration of sperm quality in young healthy Belgian men. Hum. Reprod. 1996, 11, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Swan, S.H.; Elkin, E.P.; Fenster, L. The question of declining sperm density revisited: An analysis of 101 studies published 1934–1996. Environ. Health Perspect. 2000, 108, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Lackner, J.; Schatzl, G.; Waldhör, T.; Resch, K.; Kratzik, C.; Marberger, M. Constant decline in sperm concentration in infertile males in an urban population: Experience over 18 years. Fertil. Steril. 2005, 84, 1657–1661. [Google Scholar] [CrossRef] [PubMed]
- Sripada, S.; Fonseca, S.; Lee, A.; Harrild, K.; Giannaris, D.; Mathers, E.; Bhattacharya, S. Trends in Semen Parameters in the Northeast of Scotland. J. Androl. 2006, 28, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, D.; Varghese, A.C.; Pal, M.; Banerjee, S.K.; Bhattacharyya, A.K.; Sharma, R.K.; Agarwal, A. Semen quality and age-specific changes: A study between two decades on 3729 male partners of couples with normal sperm count and attending an andrology labora-tory for infertility-related problems in an Indian city. Fertil. Steril. 2010, 93, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Geoffroy-Siraudin, C.; Loundou, A.D.; Romain, F.; Achard, V.; Courbiere, B.; Perrard, M.-H.; Durand, P.; Guichaoua, M.-R. Decline of semen quality among 10 932 males consulting for couple infertility over a 20-year period in Marseille, France. Asian J. Androl. 2012, 14, 584–590. [Google Scholar] [CrossRef]
- Rolland, M.; Le Moal, J.; Wagner, V.; Royère, D.; De Mouzon, J. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum. Reprod. 2012, 28, 462–470. [Google Scholar] [CrossRef]
- Mendiola, J.; Jørgensen, N.; Mínguez-Alarcón, L.; Sarabia-Cos, L.; López-Espín, J.J.; Vivero-Salmerón, G.; Ruiz-Ruiz, K.J.; Fernández, M.F.; Olea, N.; Swan, S.H.; et al. Sperm counts may have declined in young university students in Southern Spain. Andrology 2013, 1, 408–413. [Google Scholar] [CrossRef]
- Carlsen, E.; Giwercman, A.; Keiding, N.; Skakkebaek, N.E. Evidence for decreasing quality of semen during past 50 years. BMJ 1992, 305, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, A.; De Neubourg, D.; Punjabi, U. Is there a temporal trend in semen quality in Belgian candidate sperm donors and in sperm donors’ fertility potential from 1995 onwards? Andrology 2020. [Google Scholar] [CrossRef]
- Basnet, P.; Hansen, S.A.; Olaussen, I.K.; Hentemann, M.A.; Acharya, G. Changes in the semen quality among 5739 men seeking infertility treatment in Northern Norway over past 20 years (1993–2012). J. Reprod. Biotechnol. Fertil. 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Mishra, P.; Negi, M.P.S.; Srivastava, M.; Singh, K.; Rajender, S. Decline in seminal quality in Indian men over the last 37 years. Reprod. Biol. Endocrinol. 2018, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Borges, E., Jr.; Setti, A.S.; Braga, D.P.; Figueira Rde, C.; Iaconelli, A., Jr. Decline in semen quality among infertile men in Brazil during the past 10 years. Int. Braz. J. Urol. 2015, 41, 757–763. [Google Scholar] [CrossRef]
- Bujan, L.; Mansat, A.; Pontonnier, F.; Mieusset, R. Time series analysis of sperm concentration in fertile men in Toulouse be-tween 1977 and 1992. BMJ 1996, 312, 471–472. [Google Scholar] [CrossRef]
- Berling, S.; Wölner-Hanssen, P. No evidence of deteriorating semen quality among men in infertile relationships during the last decade: A study of males from Southern Sweden. Hum. Reprod. 1997, 12, 1002–1005. [Google Scholar] [CrossRef]
- Rasmussen, P.E.; Erb, K.; Westergaard, L.G.; Laursen, S.B. No evidence for decreasing semen quality in four birth cohorts of 1055 Danish men born between 1950 and 1970. Fertil. Steril. 1997, 68, 1059–1064. [Google Scholar] [CrossRef]
- Emanuel, E.R.; Goluboff, E.T.; Fisch, H. Macleod Revisited: Sperm Count Distributions in 374 Fertile Men from 1971 to 1994. Urology 1998, 51, 86–88. [Google Scholar] [CrossRef]
- Seo, J.T.; Rha, K.H.; Park, Y.-S.; Lee, M.S. Semen quality over a 10-year period in 22,249 men in Korea. Int. J. Androl. 2000, 23, 194–198. [Google Scholar] [CrossRef]
- Costello, M.F.; Sjöblom, P.; Haddad, Y.; Steigrad, S.J.; Bosch, E.G. No Decline in Semen Quality Among Potential Sperm Donors in Sydney, Australia, between 1983 and 2001. J. Assist. Reprod. Genet. 2002, 19, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, P.; Kapilashrami, M.C.; Misro, M.M.; Singh, G. Evaluation of trend in semen analysis for 11 years in subjects at-tending a fertility clinic in India. Asian J. Androl. 2003, 5, 221–225. [Google Scholar] [PubMed]
- Axelsson, J.; Rylander, L.; Rignell-Hydbom, A.; Giwercman, A. No secular trend over the last decade in sperm counts among Swedish men from the general population. Hum. Reprod. 2011, 26, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Gandini, L.; Lombardo, F.; Culasso, F.; Dondero, F.; Lenzi, A. Myth and reality of the decline in semen quality: An example of the relativity of data interpretation. J. Endocrinol. Investig. 2000, 23, 402–411. [Google Scholar] [CrossRef]
- Vahidi, S.; Moein, M.R.; Yazdinejad, F.; Ghasemi-Esmailabad, S.; Narimani, N. Iranian temporal changes in semen quality during the past 22 years: A report from an infertility center. Int. J. Reprod. Biomed. 2020, 18, 1059–1064. [Google Scholar] [CrossRef]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum. Reprod. Updat. 2017, 23, 646–659. [Google Scholar] [CrossRef]
- Sengupta, P.; Nwagha, U.; Dutta, S.; Krajewska-Kulak, E.; Izuka, E. Evidence for decreasing sperm count in African popula-tion from 1965 to 2015. Afr. Health Sci. 2017, 17, 418–427. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Song, X.H.; Zhang, H.B.; Xu, C.Y.; Chen, Z.J. Decline of semen quality among Chinese sperm bank do-nors within 7 years (2008–2014). Asian J. Androl. 2017, 19, 521–525. [Google Scholar]
- Huang, C.; Li, B.; Xu, K.; Liu, D.; Hu, J.; Yang, Y.; Nie, H.; Fan, L.; Zhu, W. Decline in semen quality among 30,636 young Chinese men from 2001 to 2015. Fertil. Steril. 2017, 107, 83–88.e2. [Google Scholar] [CrossRef]
- Ravanos, K.; Petousis, S.; Margioula-Siarkou, C.; Papatheodorou, A.; Panagiotidis, Y.; Prapas, N.; Prapas, Y. Declining Sperm Counts… or Rather Not? A Mini Review. Obstet. Gynecol. Surv. 2018, 73, 595–605. [Google Scholar] [CrossRef]
- Latif, T.; Jensen, T.K.; Mehlsen, J.; Holmboe, S.A.; Brinth, L.; Pors, K.; Skouby, S.O.; Jørgensen, N.; Lindahl-Jacobsen, R. Semen Quality as a Predictor of Subsequent Morbidity: A Danish Cohort Study of 4712 Men with Long-Term Follow-up. Am. J. Epidemiol. 2017, 186, 910–917. [Google Scholar] [CrossRef]
- Bilotta, P.; Guglielmo, R.; Steffè, M. Analysis of decline in seminal fluid in the Italian population during the past 15 years. Minerva Ginecol. 1999, 51, 223–231. [Google Scholar] [PubMed]
- Vicari, E.; Conticello, A.; Battiato, C.; La Vignera, S. Sperm characteristics in fertile men and healthy men of the south-east Sicily from year 1982 to 1999. Arch. Ital. Urol. Androl. 2003, 75, 28–34. [Google Scholar]
- Elia, J.; Imbrogno, N.; Delfino, M.; Rossi, T.; Mazzilli, R.; Nofroni, I.; Toscano, V.; Mazzilli, F. Comparative study of seminal parameters between samples collected in 1992 and samples collected in 2010. Arch. Ital. Urol. Androl. 2012, 84, 26–31. [Google Scholar] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Chioccarelli, T.; Manfrevola, F.; Migliaccio, M.; Altucci, L.; Porreca, V.; Fasano, S.; Cobellis, G. Fetal-perinatal exposure to bi-sphenol-A affects quality of spermatozoa in adulthood mouse. Int. J. Endocrinol. 2020, 2020, 2750501. [Google Scholar] [CrossRef]
- Barbagallo, F.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Aversa, A.; Calogero, A.E.; La Vignera, S. Effects of Bisphenols on Testicular Steroidogenesis. Front. Endocrinol. 2020, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- Pallotti, F.; Pelloni, M.; Gianfrilli, D.; Lenzi, A.; Lombardo, F.; Paoli, D. Mechanisms of Testicular Disruption from Exposure to Bisphenol A and Phtalates. J. Clin. Med. 2020, 9, 471. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, N.Y.; Park, Y.S.; Lee, S.-W.; Bang, J.S.; Lee, Y.; Ryu, J.-S.; Choi, S.-J.; Lee, S.-H.; Kim, G.S.; et al. Multigenerational effects of maternal cigarette smoke exposure during pregnancy on sperm counts of F1 and F2 male offspring. Reprod. Toxicol. 2018, 78, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Sansone, A.; Pallotti, F.; Ferlin, A.; Pivonello, R.; Isidori, A.M.; Maggi, M.; Jannini, E.A. People smoke for nicotine, but lose sexual and reproductive health for tar: A narrative review on the effect of cigarette smoking on male sexuality and reproduction. J. Endocrinol. Investig. 2020, 43, 1391–1408. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Duca, Y.; Zanghi, G.N.; Calogero, A.E. Nicotine Receptors as a Possible Marker for Smok-ing-related Sperm Damage. Protein Pept. Lett. 2018, 5, 451–454. [Google Scholar] [CrossRef]
- Condorelli, R.; Calogero, A.E.; La Vignera, S. Relationship between Testicular Volume and Conventional or Nonconventional Sperm Parameters. Int. J. Endocrinol. 2013, 2013, 145792. [Google Scholar] [CrossRef] [PubMed]
- Gianfrilli, D.; Ferlin, A.; Isidori, A.M.; Garolla, A.; Maggi, M.; Pivonello, R.; Santi, D.; Sansone, A.; Balercia, G.; Granata, A.R.M.; et al. Risk behaviours and alcohol in adolescence are negatively associated with testicular volume: Results from the Amico-Andrologo survey. Andrology 2019, 7, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.C.; Wang, H.Y.; Wang, J.D. Analysis of change in sperm quality of Chinese fertile men during 1981–1996. J. Reprod. Contracept. 1999, 10, 33–39. [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing: A guideline. Fertil. Steril. 2013, 99, 673–677. [Google Scholar] [CrossRef]
- The ESHRE Guideline Group on RPL; Atik, R.B.; Christiansen, O.B.; Elson, J.; Kolte, A.M.; Lewis, S.; Middeldorp, S.; Nelen, W.; Peramo, B.; Quenby, S.; et al. ESHRE guideline: Recurrent pregnancy loss. Hum. Reprod. Open 2018, 2018, hoy004. [Google Scholar] [CrossRef]
- Giacone, F.; Cannarella, R.; Mongioì, L.M.; Alamo, A.; Condorelli, R.A.; Calogero, A.E.; La Vignera, S. Epigenetics of Male Fertility: Effects on Assisted Reproductive Techniques. World J. Men’s Health 2019, 37, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Condorelli, R.A.; Mongioì, L.M.; La Vignera, S.; Calogero, A.E. Molecular Biology of Spermatogenesis: Novel Targets of Apparently Idiopathic Male Infertility. Int. J. Mol. Sci. 2020, 21, 1728. [Google Scholar] [CrossRef]
- Barbagallo, F.; La Vignera, S.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. Evaluation of Sperm Mitochondrial Function: A Key Organelle for Sperm Motility. J. Clin. Med. 2020, 9, 363. [Google Scholar] [CrossRef]
- Cannarella, R.; Liuzzo, C.; Mongioì, L.M.; Condorelli, R.A.; La Vignera, S.; Bellanca, S.; Calogero, A.E. Decreased total sperm counts in habitants of highly polluted areas of Eastern Sicily, Italy. Environ. Sci. Pollut. Res. 2019, 26, 31368–31373. [Google Scholar] [CrossRef]
- Martenies, S.E.; Perry, M.J. Environmental and occupational pesticide exposure and human sperm parameters: A systematic review. Toxicology 2013, 307, 66–73. [Google Scholar] [CrossRef]
- Grana, M.; Toschi, N.; Vicentini, L.; Pietroiusti, A.; Magrini, A. Exposure to ultrafine particles in different transport modes in the city of Rome. Environ. Pollut. 2017, 228, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J.; Lotti, F.; Baldi, E.; Schlatt, S.; Festin, M.P.; Björndahl, L.; Toskin, I.; Barratt, C.L. Distribution of Semen Examination Results 2020—A follow up of data collated for the WHO semen analysis manual 2010. Andrology 2021. [Google Scholar] [CrossRef] [PubMed]
| Year (n) | Age (Years) | Volume (mL) | pH | Sperm Concentration (×106/mL) | Total Sperm Count (×106/ejaculate) | Progressive Sperm Motility (%) | Sperm Normal Forms (%) | Semen Leukocytes (×106/mL) |
|---|---|---|---|---|---|---|---|---|
| 2011 (135) | 31.81 ± 8.05 | 3.0 (2.0–4.0) | 7.9 (7.6–8.1) | 14.0 (3.0–45.0) | 40.0 (7.0–153.0) | 16.0 (6.0–25.0) | 10.0 (5.0–14.0) | 0.45 (0.07–1.0) |
| 2012 (141) | 34.06 ± 7.15 | 3.2 (2.5–4.5) | 8.0 (7.8–8.1) | 30.0 (10.0–62.0) | 95.0 (27.0–210.0) | 17.0 (10.0–27.0) | 6.0 (3.0–10.0) | 0.5 (0.05–1.0) |
| 2013 (170) | 32.24 ± 9.30 | 3.0 (2.0–4.0) | 8.0 (7.8–8.1) | 35.0 (8.0–69.0) | 75.0 (21.6–220.0) | 15.0 (8.0–21.0) | 5.0 (2.0–8.0) | 0.54 (0.08–1.1) |
| 2014 (146) | 32.24 ± 9.34 | 3.0 (2.0–4.0) | 8.0 (7.9–8.2) | 27.5 (7.0–70.0) | 74.3 (25.5–195.0) | 12.0 (7.0–20.0) | 4.0 (2.0–6.0) | 0.47 (0.06–1.0) |
| 2015 (103) | 32.80 ± 9.34 | 2.5 (2.0–3.8) | 8.0 (7.9–8.2) | 40.0 (14.0–70.0) | 105.6 (39.8–175.0) | 19.0 (10.0–28.0) | 5.0 (3.0–8.0) | 0.72 (0.35–1.4) |
| 2016 (136) | 34.13 ± 8.77 | 2.5 (2.0–3.5) | 8.1 (7.9–8.2) | 40.0 (16.5–80.0) | 103.0 (47.0–200.0) | 15.5 (8.5–24.0) | 5.0 (2.5–8.0) | 0.8 (0.31–1.5) |
| 2017 (127) | 33.89 ± 9.63 | 2.5 (1.7–3.5) | 8.1 (7.9–8.2) | 20.0 (5.0–57.0) | 50.0 (14.0–130.0) | 12.0 (6.0–20.0) | 5.0 (2.0–8.0) | 0.46 (0.18–1.2) |
| 2018 (154) | 32.75 ± 8.59 | 2.5 (1.5–3.5) | 8.1 (8.0–8.3) | 30.0 (6.0–60.0) | 72.5 (15.0–135.0) | 18.0 (10.0–26.0) | 5.0 (3.0–8.0) | 0.53 (0.2–1.2) |
| 2019 (182) | 32 (26–39) | 2.6 (1.5–4.0) | 8.1 (7.9–8.2) | 25.0 (6.0–60.0) | 66.0 (13.5–156.0) | 20.0 (10.0–30.0) | 6.0 (4.0–9.0) | 0.52 (0.13–1.2) |
| 2020 (115) | 31.53 ± 8.64 | 3.0 (2.0–4.0) | 8.1 (7.9–8.1) | 32.0 (11.0–70.0) | 81.0 (27.0–196.0) | 20.0 (14.0–28.0) | 7.0 (4.0–11.0) | 0.6 (0.18–1.1) |
| Parameter | R2 | β | P |
|---|---|---|---|
| Sperm concentration | 0.001 | 0.026 | 0.34 |
| Total sperm count | 0.000 | −0.007 | 0.80 |
| Progressive sperm motility | 0.120 | +0.108 | <0.00 |
| Spermatozoa with normal morphology | 0.001 | −0.240 | 0.39 |
| Semen volume | 0.009 | −0.09 | <0.00 |
| pH | 0.044 | 0.21 | <0.00 |
| Leukocytes | 0.002 | 0.53 | 0.05 |
| Parameter | 2011–2015 | 2016–2020 | ||
|---|---|---|---|---|
| Catania (n = 227) | Other Towns (n = 217) | Catania (n = 259) | Other Towns (n = 249) | |
| Age | 31.0 (25.0–38.0) | 33.0 (27.3–39.0) | 31.0 (26.0–38.0) | 35.0 (28.0–40.0) |
| Sperm concentration (×106/mL) | 42.0 (9.0–75.0) | 30.0 (10.0–65.0) | 35.0 (12.0–70.0) | 30.0 (6.0–62.5) |
| Total sperm count (×106/ejaculate) | 115.0 (24.0–220.0) | 75.0 (27.5.0–210.0) | 81.0 (25.2–175.0) | 81.0 (17.5–168.0) |
| Progressive sperm motility (%) | 15.0 (8.0–24.0) | 15.0 (8.0–25.0) | 18.0 (10.0–26.0) | 18.0 (10.0–25.0) |
| Sperm normal forms (%) | 6.0 (3.0–9.0) | 5.0 (3.0–8.0) | 6.0 (3.0–9.0) | 5.0 (3.0–9.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannarella, R.; Condorelli, R.A.; Gusmano, C.; Barone, N.; Burrello, N.; Aversa, A.; Calogero, A.E.; La Vignera, S. Temporal Trend of Conventional Sperm Parameters in a Sicilian Population in the Decade 2011–2020. J. Clin. Med. 2021, 10, 993. https://doi.org/10.3390/jcm10050993
Cannarella R, Condorelli RA, Gusmano C, Barone N, Burrello N, Aversa A, Calogero AE, La Vignera S. Temporal Trend of Conventional Sperm Parameters in a Sicilian Population in the Decade 2011–2020. Journal of Clinical Medicine. 2021; 10(5):993. https://doi.org/10.3390/jcm10050993
Chicago/Turabian StyleCannarella, Rossella, Rosita A. Condorelli, Carmelo Gusmano, Nunziata Barone, Nunziatina Burrello, Antonio Aversa, Aldo E. Calogero, and Sandro La Vignera. 2021. "Temporal Trend of Conventional Sperm Parameters in a Sicilian Population in the Decade 2011–2020" Journal of Clinical Medicine 10, no. 5: 993. https://doi.org/10.3390/jcm10050993
APA StyleCannarella, R., Condorelli, R. A., Gusmano, C., Barone, N., Burrello, N., Aversa, A., Calogero, A. E., & La Vignera, S. (2021). Temporal Trend of Conventional Sperm Parameters in a Sicilian Population in the Decade 2011–2020. Journal of Clinical Medicine, 10(5), 993. https://doi.org/10.3390/jcm10050993










