Immunohistochemical Detection of Potential Microbial Antigens in Granulomas in the Diagnosis of Sarcoidosis
Abstract
1. Introduction
2. Causative Agents of Granuloma Formation
3. Microorganisms Detected in Sarcoid Tissues
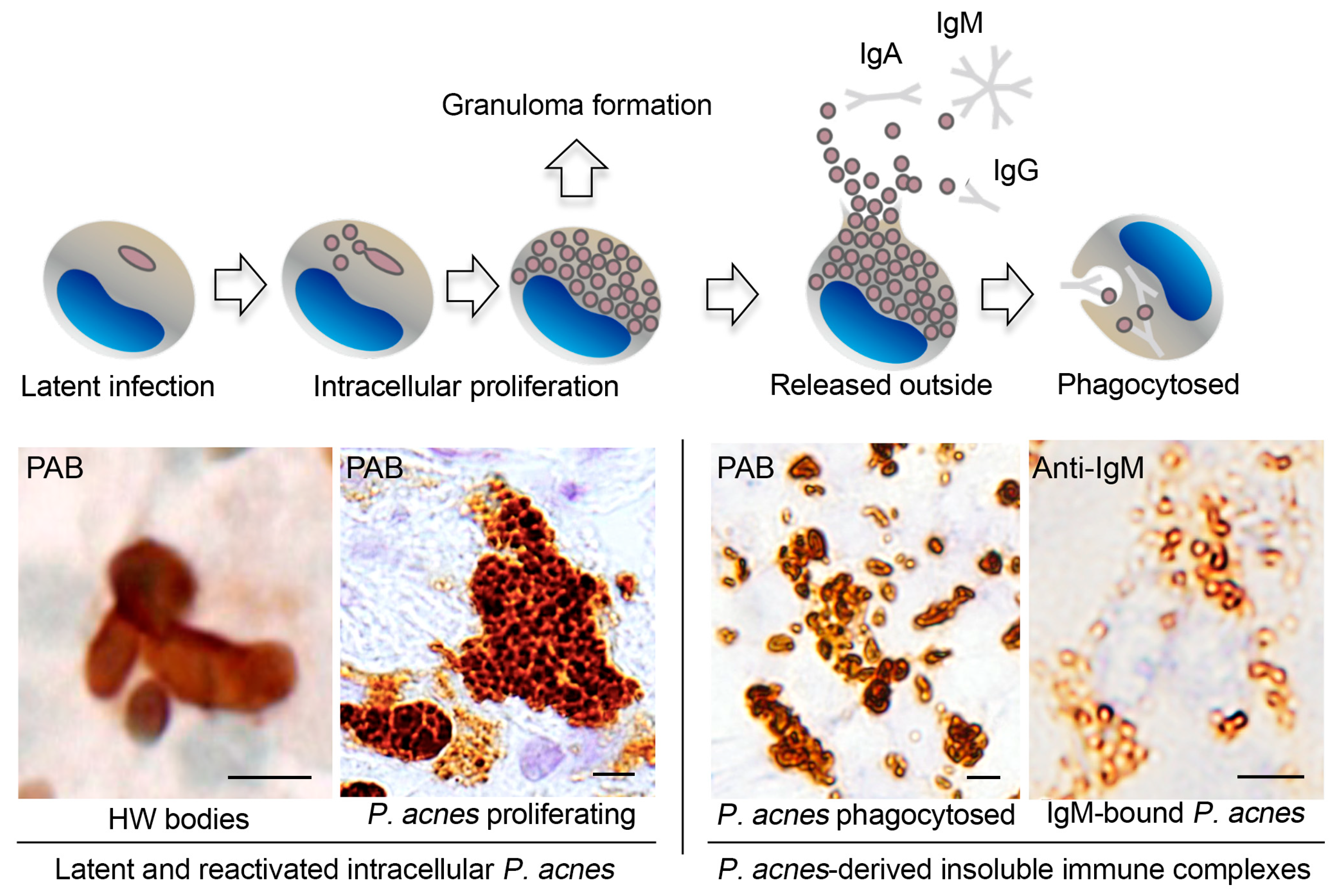
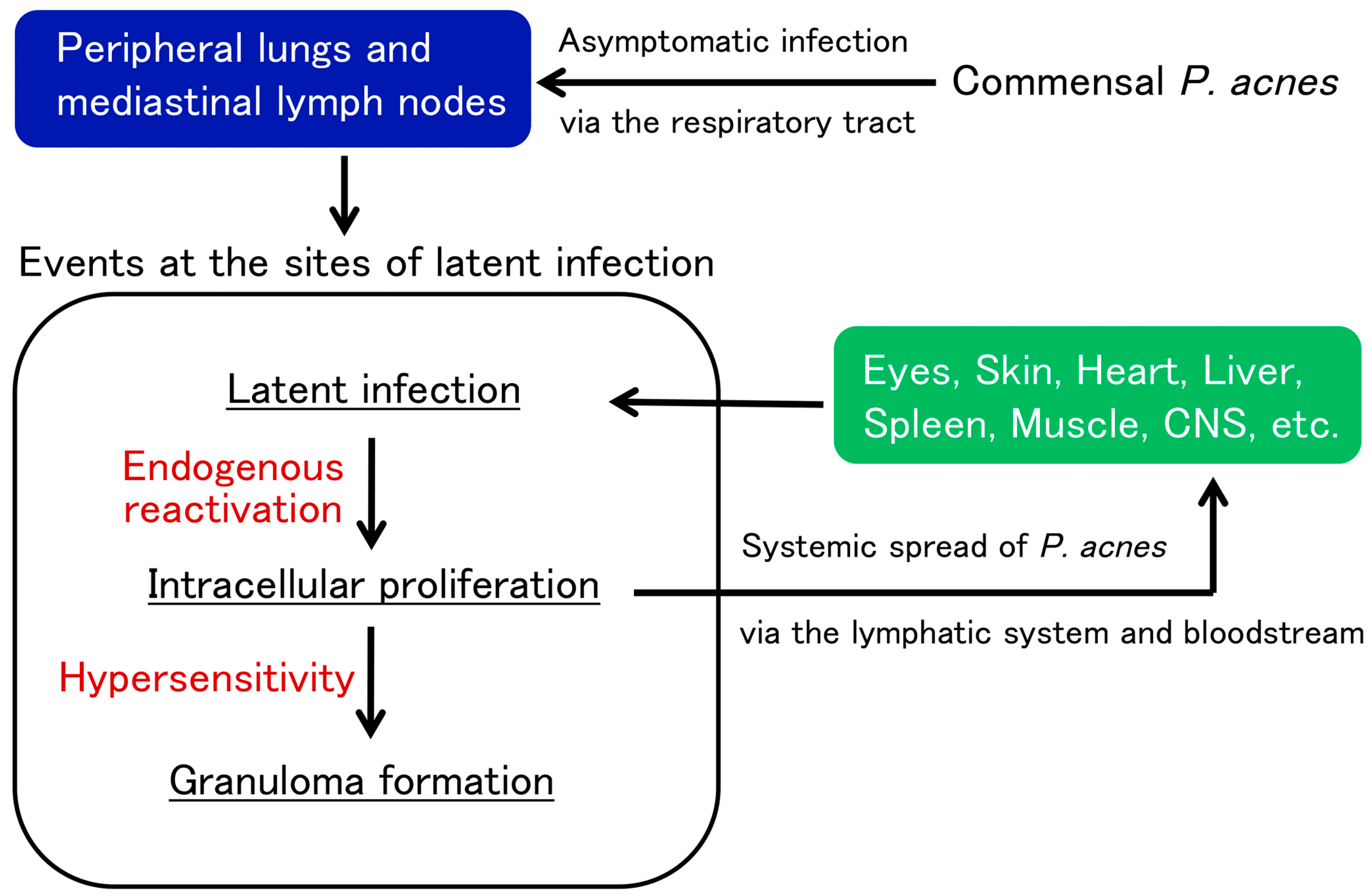
4. Latent Infection and Endogenous Reactivation
5. Immune Responses to M. tuberculosis and P. acnes
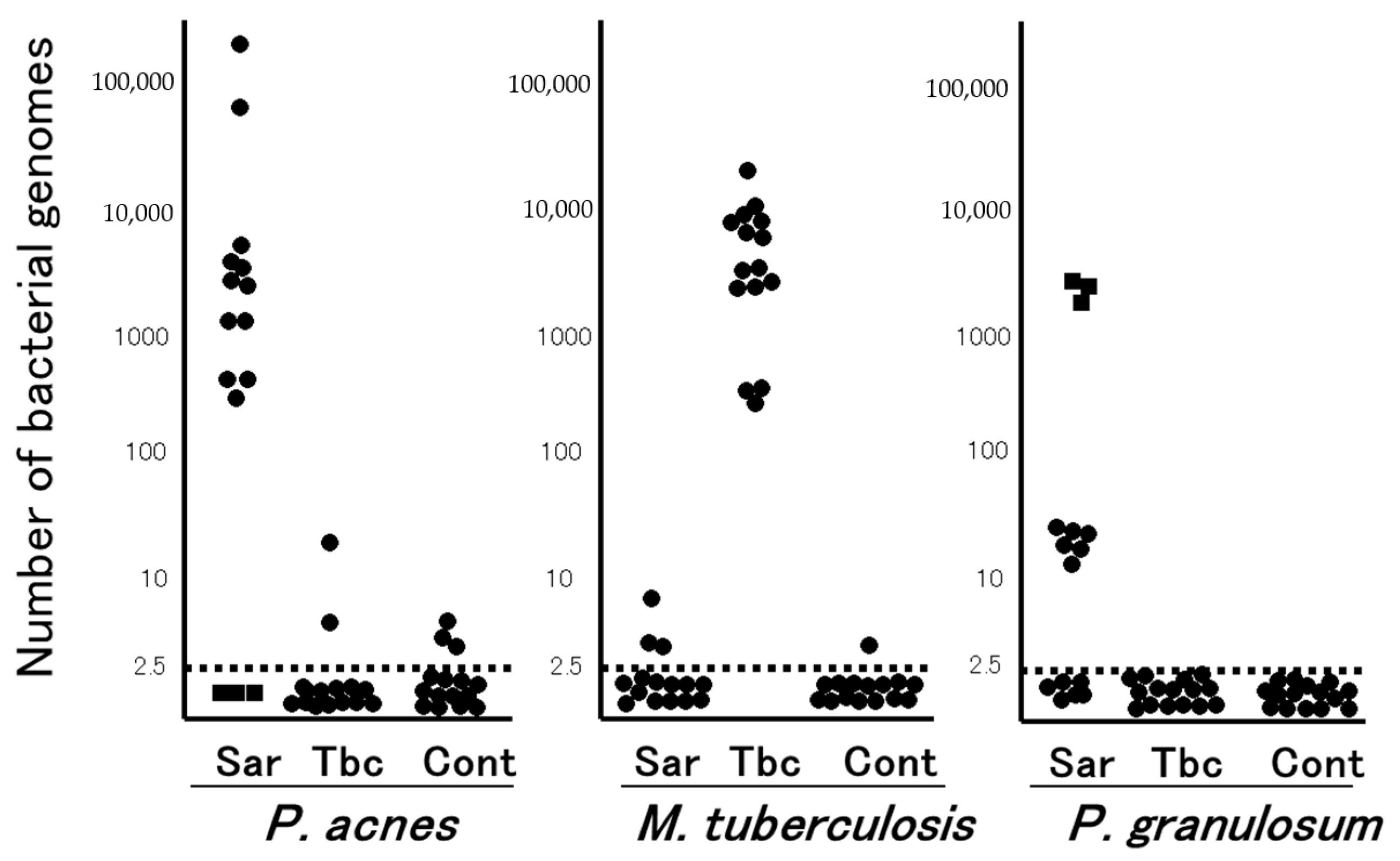
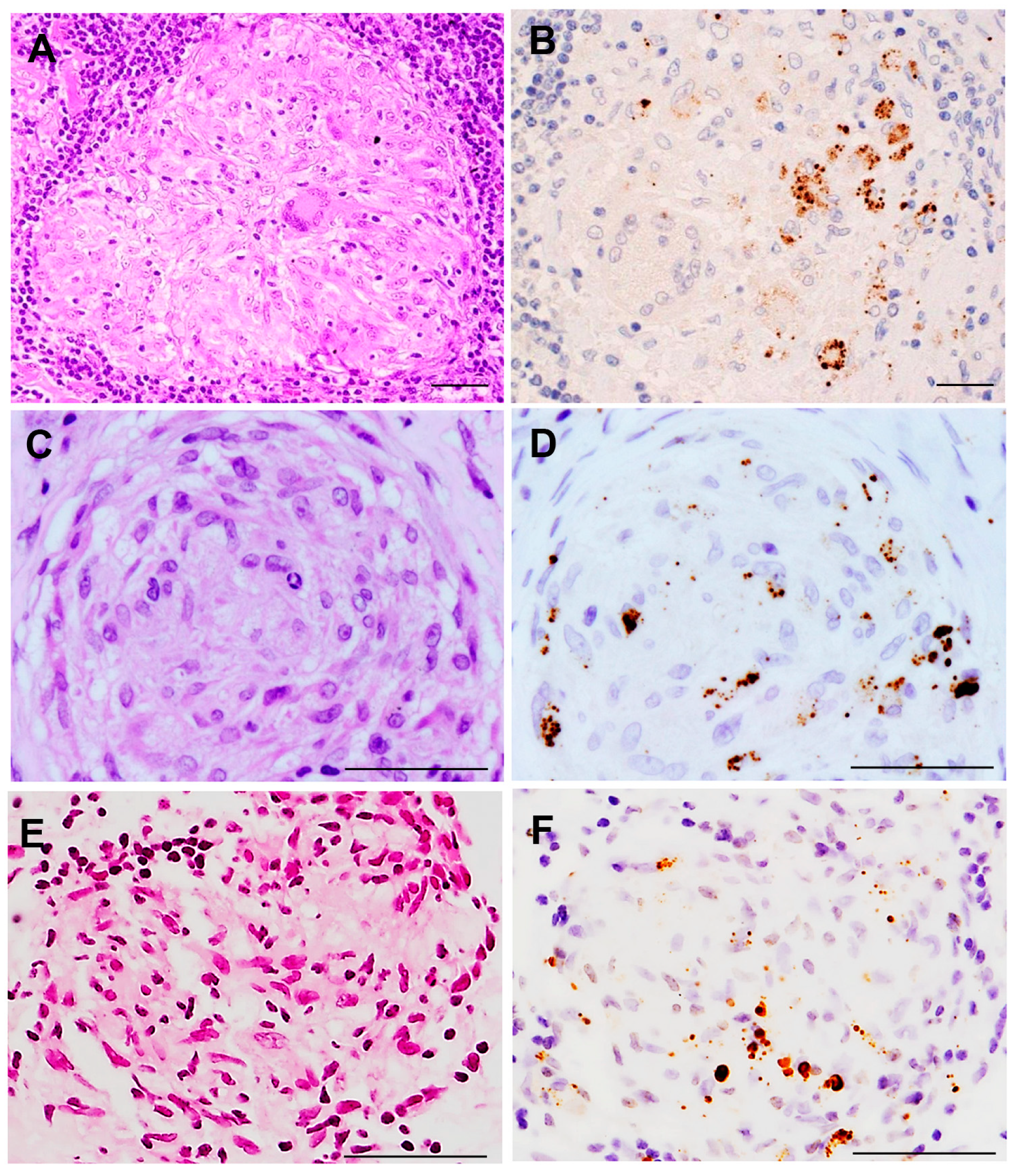
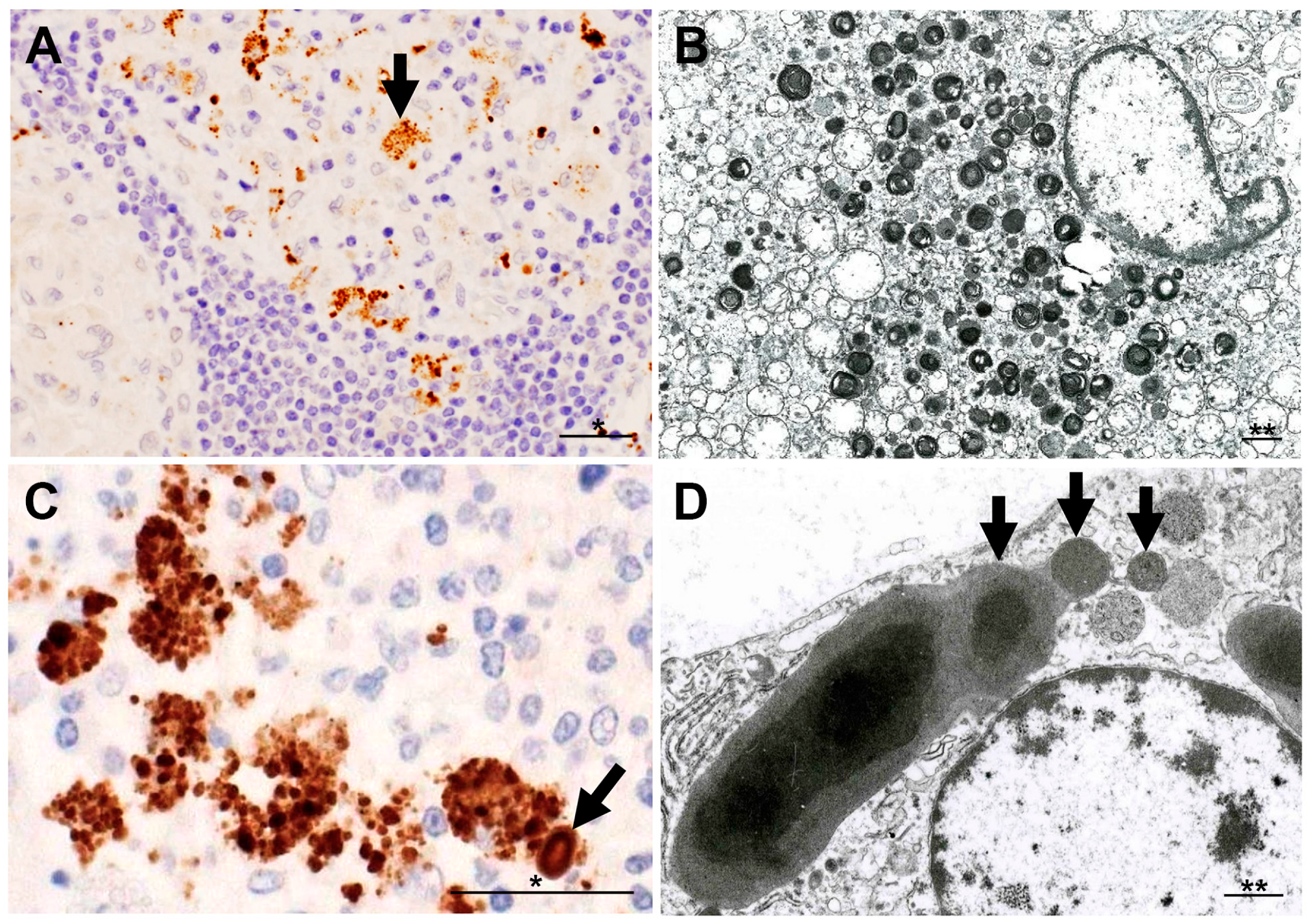
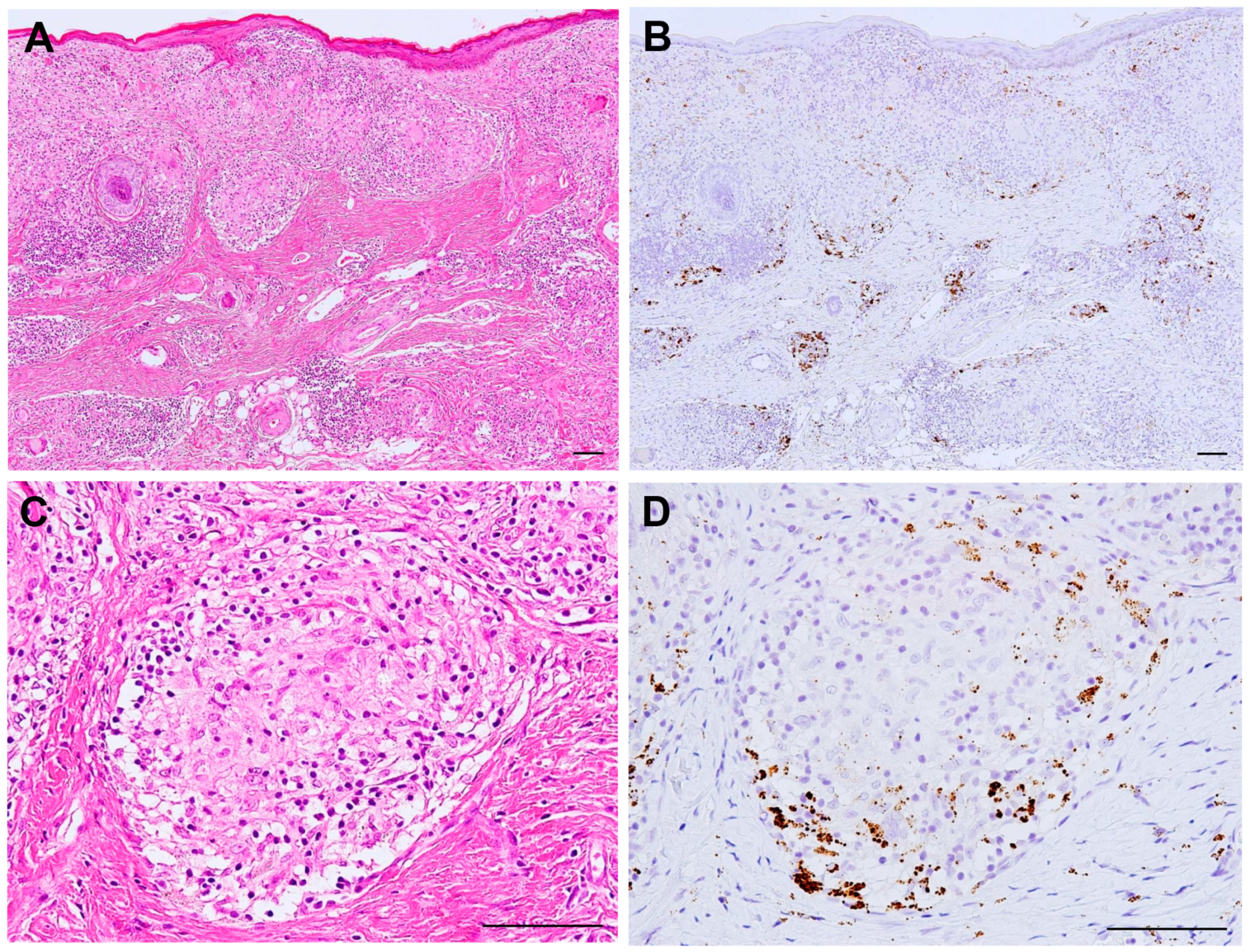
6. M. tuberculosis and P. acnes in Sarcoid Granulomas
7. Potential Pathogenesis of Sarcoidosis
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Valeyre, D.; Prasse, A.; Nunes, H.; Uzunhan, Y.; Brillet, P.-Y.; Müller-Quernheim, J. Sarcoidosis. Lancet 2014, 383, 1155–1167. [Google Scholar] [CrossRef]
- Beijer, E.; Veltkamp, M.; Meek, B.; Moller, D.R. Etiology and Immunopathogenesis of Sarcoidosis: Novel Insights. Semin. Respir. Crit. Care Med. 2017, 38, 404–416. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.A. Host-pathogen interactions: Redefining the basic concepts of virulence and pathogenicity. Infect. Immun. 1999, 67, 3703–3713. [Google Scholar] [CrossRef]
- Alexeyev, O.A.; Dekio, I.; Layton, A.M.; Li, H.; Hughes, H.; Morris, T.; Zouboulis, C.C.; Patrick, S. Why we continue to use the name Propionibacterium acnes. Br. J. Dermatol. 2018, 179, 1227. [Google Scholar] [CrossRef]
- Sell, S. Granulomatous Reactions. In Immunology Immunopathology and Immunity; Elsevier Science Publishing Company, Inc.: Amsterdam, The Netherlands, 1987; pp. 529–544. [Google Scholar]
- Pagán, A.J.; Ramakrishnan, L. The Formation and Function of Granulomas. Annu. Rev. Immunol. 2018, 36, 639–665. [Google Scholar] [CrossRef]
- Zumla, A.; James, D.G. Granulomatous infections: Etiology and classification. Clin. Infect. Dis. 1996, 23, 146–158. [Google Scholar] [CrossRef]
- Carr, I. Sarcoid macrophage giant cells. Ultrastructure and lysozyme content. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1980, 32, 147–155. [Google Scholar] [CrossRef]
- Okabe, T.; Suzuki, A.; Ishikawa, H.; Yotsumoto, H.; Ohsawa, N. Cells originating from sarcoid granulomas in vitro. Am. Rev. Respir. Dis. 1981, 124, 608–612. [Google Scholar] [CrossRef]
- Wilsher, M.L.; Menzies, R.E.; Croxson, M.C. Mycobacterium tuberculosis DNA in tissues affected by sarcoidosis. Thorax 1998, 53, 871–874. [Google Scholar] [CrossRef]
- Saboor, S.A.; Johnson, N.M.; McFadden, J. Detection of mycobacterial DNA in sarcoidosis and tuberculosis with polymerase chain reaction. Lancet 1992, 339, 1012–1015. [Google Scholar] [CrossRef]
- Gupta, D.; Agarwal, R.; Aggarwal, A.N.; Jindal, S.K. Molecular evidence for the role of mycobacteria in sarcoidosis: A meta-analysis. Eur. Respir. J. 2007, 30, 508–516. [Google Scholar] [CrossRef]
- Homma, J.Y.; Abe, C.; Chosa, H.; Ueda, K.; Saegusa, J.; Nakayama, M.; Homma, H.; Washizaki, M.; Okano, H. Bacteriological investigation on biopsy specimens from patients with sarcoidosis. Jpn. J. Exp. Med. 1978, 48, 251–255. [Google Scholar] [PubMed]
- Abe, C.; Iwai, K.; Mikami, R.; Hosoda, Y. Frequent isolation of Propionibacterium acnes from sarcoidosis lymph nodes. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 1984, 256, 541–547. [Google Scholar] [CrossRef]
- Ishige, I.; Usui, Y.; Takemura, T.; Eishi, Y. Quantitative PCR of mycobacterial and propionibacterial DNA in lymph nodes of Japanese patients with sarcoidosis. Lancet 1999, 354, 120–123. [Google Scholar] [CrossRef]
- Eishi, Y.; Suga, M.; Ishige, I.; Kobayashi, D.; Yamada, T.; Takemura, T.; Takizawa, T.; Koike, M.; Kudoh, S.; Costabel, U.; et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J. Clin. Microbiol. 2002, 40, 198–204. [Google Scholar] [CrossRef]
- Esteves, T.; Aparicio, G.; Garcia-Patos, V. Is there any association between Sarcoidosis and infectious agents?: A systematic review and meta-analysis. BMC Pulm. Med. 2016, 16, 165. [Google Scholar] [CrossRef]
- Robinson, L.A.; Smith, P.; Sengupta, D.J.; Prentice, J.L.; Sandin, R.L. Molecular analysis of sarcoidosis lymph nodes for microorganisms: A case-control study with clinical correlates. BMJ Open 2013, 3, e004065. [Google Scholar] [CrossRef]
- Zhao, M.-M.; Du, S.-S.; Li, Q.-H.; Chen, T.; Qiu, H.; Wu, Q.; Chen, S.-S.; Zhou, Y.; Zhang, Y.; Hu, Y.; et al. High throughput 16SrRNA gene sequencing reveals the correlation between Propionibacterium acnes and sarcoidosis. Respir. Res. 2017, 18, 28. [Google Scholar] [CrossRef]
- Iida, T.; Uchida, K.; Lokman, N.; Furukawa, A.; Suzuki, Y.; Kumasaka, T.; Takemura, T.; Kawachi, H.; Akashi, T.; Eishi, Y. Calcified Granulomatous Lung Lesions Contain Abundant Mycobacterium tuberculosis Components. Mycobact. Dis. 2013, 4. [Google Scholar] [CrossRef]
- Hernández-Pando, R.; Jeyanathan, M.; Mengistu, G.; Aguilar, D.; Orozco, H.; Harboe, M.; Rook, G.A.; Bjune, G. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet 2000, 356, 2133–2138. [Google Scholar] [CrossRef]
- McLaughlin, J.; Watterson, S.; Layton, A.M.; Bjourson, A.J.; Barnard, E.; McDowell, A. Propionibacterium acnes and Acne Vulgaris: New Insights from the Integration of Population Genetic, Multi-Omic, Biochemical and Host-Microbe Studies. Microorganisms 2019, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.; Mak, T.N.; Shinohara, D.B.; Sfanos, K.S.; Meyer, T.F.; Brüggemann, H. Deciphering the intracellular fate of Propionibacterium acnes in macrophages. Biomed. Res. Int. 2013, 2013, 603046. [Google Scholar] [CrossRef]
- Nakamura, T.; Furukawa, A.; Uchida, K.; Ogawa, T.; Tamura, T.; Sakonishi, D.; Wada, Y.; Suzuki, Y.; Ishige, Y.; Minami, J.; et al. Autophagy Induced by Intracellular Infection of Propionibacterium acnes. PLoS ONE 2016, 11, e0156298. [Google Scholar] [CrossRef] [PubMed]
- Negi, M.; Takemura, T.; Guzman, J.; Uchida, K.; Furukawa, A.; Suzuki, Y.; Iida, T.; Ishige, I.; Minami, J.; Yamada, T.; et al. Localization of Propionibacterium acnes in granulomas supports a possible etiologic link between sarcoidosis and the bacterium. Mod. Pathol. 2012, 25, 1284–1297. [Google Scholar] [CrossRef]
- Ishige, I.; Eishi, Y.; Takemura, T.; Kobayashi, I.; Nakata, K.; Tanaka, I.; Nagaoka, S.; Iwai, K.; Watanabe, K.; Takizawa, T.; et al. Propionibacterium acnes is the most common bacterium commensal in peripheral lung tissue and mediastinal lymph nodes from subjects without sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2005, 22, 33–42. [Google Scholar]
- Furukawa, A.; Uchida, K.; Ishige, Y.; Ishige, I.; Kobayashi, I.; Takemura, T.; Yokoyama, T.; Iwai, K.; Watanabe, K.; Shimizu, S.; et al. Characterization of Propionibacterium acnes isolates from sarcoid and non-sarcoid tissues with special reference to cell invasiveness, serotype, and trigger factor gene polymorphism. Microb. Pathog. 2009, 46, 80–87. [Google Scholar] [CrossRef]
- Tanabe, T.; Ishige, I.; Suzuki, Y.; Aita, Y.; Furukawa, A.; Ishige, Y.; Uchida, K.; Suzuki, T.; Takemura, T.; Ikushima, S.; et al. Sarcoidosis and NOD1 variation with impaired recognition of intracellular Propionibacterium acnes. Biochim. Biophys. Acta 2006, 1762, 794–801. [Google Scholar] [CrossRef]
- Bae, Y.; Ito, T.; Iida, T.; Uchida, K.; Sekine, M.; Nakajima, Y.; Kumagai, J.; Yokoyama, T.; Kawachi, H.; Akashi, T.; et al. Intracellular Propionibacterium acnes infection in glandular epithelium and stromal macrophages of the prostate with or without cancer. PLoS ONE 2014, 9, e90324. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.P.; Li, Q.H.; Zheng, H.; Zhang, R.X.; Chen, G.; Baughman, R.P. Differentiation of sarcoidosis from tuberculosis using real-time PCR assay for the detection and quantification of Mycobacterium tuberculosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2008, 25, 93–99. [Google Scholar]
- Zhou, Y.; Wei, Y.-R.; Zhang, Y.; Du, S.-S.; Baughman, R.P.; Li, H.-P. Real-time quantitative reverse transcription-polymerase chain reaction to detect propionibacterial ribosomal RNA in the lymph nodes of Chinese patients with sarcoidosis. Clin. Exp. Immunol. 2015, 181, 511–517. [Google Scholar] [CrossRef]
- Rotsinger, J.E.; Celada, L.J.; Polosukhin, V.V.; Atkinson, J.B.; Drake, W.P. Molecular Analysis of Sarcoidosis Granulomas Reveals Antimicrobial Targets. Am. J. Respir. Cell Mol. Biol. 2016, 55, 128–134. [Google Scholar] [CrossRef]
- Fang, C.; Huang, H.; Xu, Z. Immunological Evidence for the Role of Mycobacteria in Sarcoidosis: A Meta-Analysis. PLoS ONE 2016, 11, e0154716. [Google Scholar] [CrossRef]
- Inui, N.; Suda, T.; Chida, K. Use of the QuantiFERON-TB Gold test in Japanese patients with sarcoidosis. Respir. Med. 2008, 102, 313–315. [Google Scholar] [CrossRef][Green Version]
- Milman, N.; Søborg, B.; Svendsen, C.B.; Andersen, A.B. Quantiferon test for tuberculosis screening in sarcoidosis patients. Scand. J. Infect. Dis. 2011, 43, 728–735. [Google Scholar] [CrossRef]
- Piotrowski, W.J.; Adam, B.; Gwadera, Ł.; Kumor-Kisielewska, A.; Fijałkowski, M.; Kurmanowska, Z.; Marczak, J.; Górski, W.; Angowski, W.; Górski, P.; et al. QuantiFERON-TB-GOLD In-Tube in patients with sarcoidosis. Adv. Respir. Med. 2018, 86, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Kumar, S.; Aggarwal, A.N.; Verma, I.; Agarwal, R. Interferon gamma release assay (QuantiFERON-TB Gold In Tube) in patients of sarcoidosis from a population with high prevalence of tuberculosis infection. Sarcoidosis Vasc. Diffus. Lung Dis. 2011, 28, 95–101. [Google Scholar]
- Hörster, R.; Kirsten, D.; Gaede, K.I.; Jafari, C.; Strassburg, A.; Greinert, U.; Kalsdorf, B.; Ernst, M.; Lange, C. Antimycobacterial immune responses in patients with pulmonary sarcoidosis. Clin. Respir. J. 2009, 3, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Hofland, R.W.; Thijsen, S.F.; Bouwman, J.; van der Wel, M.; Bossink, A.W.J. Sarcoidosis and Purified Protein Derivative reactivity. Sarcoidosis Vasc. Diffus. lung Dis. 2014, 31, 142–148. [Google Scholar]
- Furusawa, H.; Suzuki, Y.; Miyazaki, Y.; Inase, N.; Eishi, Y. Th1 and Th17 immune responses to viable Propionibacterium acnes in patients with sarcoidosis. Respir. Investig. 2012, 50, 104–109. [Google Scholar] [CrossRef]
- Ebe, Y.; Ikushima, S.; Yamaguchi, T.; Kohno, K.; Azuma, A.; Sato, K.; Ishige, I.; Usui, Y.; Takemura, T.; Eishi, Y. Proliferative response of peripheral blood mononuclear cells and levels of antibody to recombinant protein from Propionibacterium acnes DNA expression library in Japanese patients with sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2000, 17, 256–265. [Google Scholar]
- Yorozu, P.; Furukawa, A.; Uchida, K.; Akashi, T.; Kakegawa, T.; Ogawa, T.; Minami, J.; Suzuki, Y.; Awano, N.; Furusawa, H.; et al. Propionibacterium acnes catalase induces increased Th1 immune response in sarcoidosis patients. Respir. Investig. 2015, 53, 161–169. [Google Scholar] [CrossRef]
- Schupp, J.C.; Tchaptchet, S.; Lützen, N.; Engelhard, P.; Müller-Quernheim, J.; Freudenberg, M.A.; Prasse, A. Immune response to Propionibacterium acnes in patients with sarcoidosis--in vivo and in vitro. BMC Pulm. Med. 2015, 15, 75. [Google Scholar] [CrossRef] [PubMed]
- Oswald-Richter, K.A.; Beachboard, D.C.; Seeley, E.H.; Abraham, S.; Shepherd, B.E.; Jenkins, C.A.; Culver, D.A.; Caprioli, R.M.; Drake, W.P. Dual analysis for mycobacteria and propionibacteria in sarcoidosis BAL. J. Clin. Immunol. 2012, 32, 1129–1140. [Google Scholar] [CrossRef]
- Song, Z.; Marzilli, L.; Greenlee, B.M.; Chen, E.S.; Silver, R.F.; Askin, F.B.; Teirstein, A.S.; Zhang, Y.; Cotter, R.J.; Moller, D.R. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J. Exp. Med. 2005, 201, 755–767. [Google Scholar] [CrossRef]
- Dubaniewicz, A.; Dubaniewicz-Wybieralska, M.; Sternau, A.; Zwolska, Z.; Izycka-Swieszewska, E.; Augustynowicz-Kopec, E.; Skokowski, J.; Singh, M.; Zimnoch, L. Mycobacterium tuberculosis complex and mycobacterial heat shock proteins in lymph node tissue from patients with pulmonary sarcoidosis. J. Clin. Microbiol. 2006, 44, 3448–3451. [Google Scholar] [CrossRef]
- Yamada, T.; Eishi, Y.; Ikeda, S.; Ishige, I.; Suzuki, T.; Takemura, T.; Takizawa, T.; Koike, M. In situ localization of Propionibacterium acnes DNA in lymph nodes from sarcoidosis patients by signal amplification with catalysed reporter deposition. J. Pathol. 2002, 198, 541–547. [Google Scholar] [CrossRef]
- Arai, T.; Inoue, Y.; Eishi, Y.; Yamamoto, S.; Sakatani, M. Propionibacterium acnes in granulomas of a patient with necrotising sarcoid granulomatosis. Thorax 2008, 63, 90–91. [Google Scholar] [CrossRef][Green Version]
- Eishi, Y. Etiologic aspect of sarcoidosis as an allergic endogenous infection caused by Propionibacterium acnes. Biomed. Res. Int. 2013, 2013, 935289. [Google Scholar] [CrossRef]
- Eishi, Y. Etiologic link between sarcoidosis and Propionibacterium acnes. Respir. Investig. 2013, 51, 56–68. [Google Scholar] [CrossRef]
- Asakawa, N.; Uchida, K.; Sakakibara, M.; Omote, K.; Noguchi, K.; Tokuda, Y.; Kamiya, K.; Hatanaka, K.C.; Matsuno, Y.; Yamada, S.; et al. Immunohistochemical identification of Propionibacterium acnes in granuloma and inflammatory cells of myocardial tissues obtained from cardiac sarcoidosis patients. PLoS ONE 2017, 12, e0179980. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Usui, Y.; Umazume, A.; Uchida, K.; Eishi, Y. Propionibacterium acnes as a possible pathogen of granuloma in patients with ocular sarcoidosis. Br. J. Ophthalmol. 2017, 101, 1510–1513. [Google Scholar] [CrossRef]
- Nagata, K.; Eishi, Y.; Uchida, K.; Yoneda, K.; Hatanaka, H.; Yasuhara, T.; Nagata, M.; Sotozono, C.; Kinoshita, S. Immunohistochemical Detection of Propionibacterium acnes in the Retinal Granulomas in Patients with Ocular Sarcoidosis. Sci. Rep. 2017, 7, 15226. [Google Scholar] [CrossRef]
- Suzuki, T.; Fujita, A. Implication of Immunohistochemistry for Propionibacterium acnes in Differential Diagnosis of Necrotizing Granuloma. J. Pulm. Respir. Med. 2016, 6. [Google Scholar] [CrossRef]
- Isshiki, T.; Matsuyama, H.; Sakamoto, S.; Honma, N.; Mikami, T.; Shibuya, K.; Eishi, Y.; Homma, S. Development of Propionibacterium acnes-associated Sarcoidosis During Etanercept Therapy. Intern. Med. 2019, 58, 1473–1477. [Google Scholar] [CrossRef]
- Asahina, A.; Miura, K.; Saito, I.; Oshikata, C.; Ishii, N.; Eishi, Y. Cutaneous sarcoidosis with livedoid lesions: Evidence of the involvement of Propionibacterium acnes. J. Dermatol. 2013, 40, 501–502. [Google Scholar] [CrossRef] [PubMed]
- Takama, H.; Yanagishista, T.; Muto, J.; Ohshima, Y.; Takahashi, E.; Tsuzuki, T.; Uchida, K.; Eishi, Y.; Akiyama, M.; Watanabe, D. Granulomatous pigmented purpuric dermatosis containing Propionibacterium acnes. Eur. J. Dermatol. 2018, 28, 540–542. [Google Scholar] [CrossRef]
- Noda, S.; Maeda, A.; Komiya, Y.; Soejima, M. A Patient with Necrotizing Vasculitis Related to Sarcoidosis, which Was Diagnosed via Immunohistochemical Methods Using Propionibacterium acnes-specific Monoclonal Antibodies. Intern. Med. 2020, 59, 2423–2425. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Teraki, Y. Association of Propionibacterium acnes with the efficacy of minocycline therapy for cutaneous sarcoidosis. Int. J. Dermatol. 2020, 59, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Takama, H.; Ohshima, Y.; Ando, Y.; Yanagishita, T.; Takahashi, E.; Tsuzuki, T.; Uchida, K.; Eishi, Y.; Akiyama, M.; Watanabe, D. Annular Sarcoidosis with Geographic Appearance in a Patient with Systemic Sarcoidosis. Acta Derm. Venereol. 2020, 100, adv00182. [Google Scholar] [CrossRef]
- Sasaki, S.; Kato, M.; Nakamura, K.; Namba, Y.; Nagashima, O.; Takahashi, K. Management of skin sarcoidosis with minocycline monotherapy. Respirol. Case Rep. 2019, 7, e00413. [Google Scholar] [CrossRef]
- Ishikawa, M.; Ohashi, T.; Eishi, Y.; Yamamoto, T. Palmoplantar pustulosis in a patient with sarcoidosis. Eur. J. Dermatol. 2020, 30, 325–326. [Google Scholar] [CrossRef]
- Shimamura, S.; Yokogawa, N.; Murata, K.; Yamaguchi, T.; Uchida, K.; Eishi, Y. Saddle nose with sarcoidosis: A great imitator of relapsing polychondritis. Mod. Rheumatol. 2018, 28, 1053–1057. [Google Scholar] [CrossRef]
- Yang, G.; Eishi, Y.; Raza, A.; Rojas, H.; Achiriloaie, A.; De Los Reyes, K.; Raghavan, R. Propionibacterium acnes-associated neurosarcoidosis: A case report with review of the literature. Neuropathology 2018, 38, 159–164. [Google Scholar] [CrossRef]
- Akimoto, J.; Nagai, K.; Ogasawara, D.; Tanaka, Y.; Izawa, H.; Kohno, M.; Uchida, K.; Eishi, Y. Solitary tentorial sarcoid granuloma associated with Propionibacterium acnes infection: Case report. J. Neurosurg. 2017, 127, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Takemori, N.; Nakamura, M.; Kojima, M.; Eishi, Y. Successful treatment in a case of Propionibacterium acnes-associated sarcoidosis with clarithromycin administration: A case report. J. Med. Case Rep. 2014, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Sawahata, M.; Fujiki, Y.; Nakano, N.; Ohtsuki, M.; Yamaguchi, T.; Uchida, K.; Eishi, Y.; Suzuki, T.; Hagiwara, K.; Bando, M. Propionibacterium acnes-associated Sarcoidosis Possibly Initially Triggered by Interferon-alpha Therapy. Intern. Med. 2020, 30. [Google Scholar] [CrossRef]
- Sawahata, M.; Sakamoto, N.; Yamasawa, H.; Iijima, Y.; Kawata, H.; Yamaguchi, T.; Uchida, K.; Eishi, Y.; Bando, M.; Hagiwara, K. Propionibacterium acnes-associated sarcoidosis complicated by acute bird-related hypersensitivity pneumonitis. BMC Pulm. Med. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Moller, D.R. Potential etiologic agents in sarcoidosis. Proc. Am. Thorac. Soc. 2007, 4, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.S.; Moller, D.R. Etiologies of Sarcoidosis. Clin. Rev. Allergy Immunol. 2015, 49, 6–18. [Google Scholar] [CrossRef]
- Kinloch, A.J.; Kaiser, Y.; Wolfgeher, D.; Ai, J.; Eklund, A.; Clark, M.R.; Grunewald, J. In Situ Humoral Immunity to Vimentin in HLA-DRB1*03+ Patients with Pulmonary Sarcoidosis. Front. Immunol. 2018, 9, 1516. [Google Scholar] [CrossRef]
- Thiboutot, D.M.; Layton, A.M.; Anne Eady, E. IL-17: A key player in the P. acnes inflammatory cascade? J. Investig. Dermatol. 2014, 134, 307–310. [Google Scholar] [CrossRef]
- Kistowska, M.; Meier, B.; Proust, T.; Feldmeyer, L.; Cozzio, A.; Kuendig, T.; Contassot, E.; French, L.E. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J. Investig. Dermatol. 2015, 135, 110–118. [Google Scholar] [CrossRef]
- Calender, A.; Weichhart, T.; Valeyre, D.; Pacheco, Y. Current Insights in Genetics of Sarcoidosis: Functional and Clinical Impacts. J. Clin. Med. 2020, 9, 2633. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, Y.; Lim, C.X.; Weichhart, T.; Valeyre, D.; Bentaher, A.; Calender, A. Sarcoidosis and the mTOR, Rac1, and Autophagy Triad. Trends Immunol. 2020, 41, 286–299. [Google Scholar] [CrossRef]
- Calender, A.; Lim, C.X.; Weichhart, T.; Buisson, A.; Besnard, V.; Rollat-Farnier, P.A.; Bardel, C.; Roy, P.; Cottin, V.; Devouassoux, G.; et al. Exome sequencing and pathogenicity-network analysis of five French families implicate mTOR signalling and autophagy in familial sarcoidosis. Eur. Respir. J. 2019, 54. [Google Scholar] [CrossRef] [PubMed]
- Scharschmidt, T.C.; Vasquez, K.S.; Truong, H.-A.; Gearty, S.V.; Pauli, M.L.; Nosbaum, A.; Gratz, I.K.; Otto, M.; Moon, J.J.; Liese, J.; et al. A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes. Immunity 2015, 43, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Senaldi, G.; Yin, S.; Shaklee, C.L.; Piguet, P.F.; Mak, T.W.; Ulich, T.R. Corynebacterium parvum- and Mycobacterium bovis bacillus Calmette-Guerin-induced granuloma formation is inhibited in TNF receptor I (TNF-RI) knockout mice and by treatment with soluble TNF-RI. J. Immunol. 1996, 157, 5022–5026. [Google Scholar]
- Yamane, H.; Tachibana, I.; Takeda, Y.; Saito, Y.; Tamura, Y.; He, P.; Suzuki, M.; Shima, Y.; Yoneda, T.; Hoshino, S.; et al. Propionibacterium acnes-induced hepatic granuloma formation is impaired in mice lacking tetraspanin CD9. J. Pathol. 2005, 206, 486–492. [Google Scholar] [CrossRef]
- Gabrilovich, M.I.; Walrath, J.; van Lunteren, J.; Nethery, D.; Seifu, M.; Kern, J.A.; Harding, C.V.; Tuscano, L.; Lee, H.; Williams, S.D.; et al. Disordered Toll-like receptor 2 responses in the pathogenesis of pulmonary sarcoidosis. Clin. Exp. Immunol. 2013, 173, 512–522. [Google Scholar] [CrossRef]
- Werner, J.L.; Escolero, S.G.; Hewlett, J.T.; Mak, T.N.; Williams, B.P.; Eishi, Y.; Núñez, G. Induction of Pulmonary Granuloma Formation by Propionibacterium acnes Is Regulated by MyD88 and Nox2. Am. J. Respir. Cell Mol. Biol. 2017, 56, 121–130. [Google Scholar] [CrossRef]
- Nishiwaki, T.; Yoneyama, H.; Eishi, Y.; Matsuo, N.; Tatsumi, K.; Kimura, H.; Kuriyama, T.; Matsushima, K. Indigenous pulmonary Propionibacterium acnes primes the host in the development of sarcoid-like pulmonary granulomatosis in mice. Am. J. Pathol. 2004, 165, 631–639. [Google Scholar] [CrossRef][Green Version]
- Minami, J.; Eishi, Y.; Ishige, Y.; Kobayashi, I.; Ishige, I.; Kobayashi, D.; Ando, N.; Uchida, K.; Ikeda, S.; Sorimachi, N.; et al. Pulmonary granulomas caused experimentally in mice by a recombinant trigger-factor protein of Propionibacterium acnes. J. Med. Dent. Sci. 2003, 50, 265–274. [Google Scholar]
- Suzuki, Y.; Uchida, K.; Takemura, T.; Sekine, M.; Tamura, T.; Furukawa, A.; Hebisawa, A.; Sakakibara, Y.; Awano, N.; Amano, T.; et al. Propionibacterium acnes-derived insoluble immune complexes in sinus macrophages of lymph nodes affected by sarcoidosis. PLoS ONE 2018, 13, e0192408. [Google Scholar] [CrossRef]
- Yamada, Y.; Tatsumi, K.; Yamaguchi, T.; Tanabe, N.; Takiguchi, Y.; Kuriyama, T.; Mikami, R. Influence of stressful life events on the onset of sarcoidosis. Respirology 2003, 8, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.; Harpaz, R.; Zhang, J.; Wollan, P.C.; Bialek, S.R.; Yawn, B.P. Risk Factors for Herpes Zoster Among Adults. Open Forum Infect. Dis. 2016, 3, ofw119. [Google Scholar] [CrossRef] [PubMed]
- Keane, J. TNF-blocking agents and tuberculosis: New drugs illuminate an old topic. Rheumatology 2005, 44, 714–720. [Google Scholar] [CrossRef]
- Chopra, A.; Nautiyal, A.; Kalkanis, A.; Judson, M.A. Drug-Induced Sarcoidosis-Like Reactions. Chest 2018, 154, 664–677. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, T.; Costabel, U.; McDowell, A.; Guzman, J.; Uchida, K.; Ohashi, K.; Eishi, Y. Immunohistochemical Detection of Potential Microbial Antigens in Granulomas in the Diagnosis of Sarcoidosis. J. Clin. Med. 2021, 10, 983. https://doi.org/10.3390/jcm10050983
Yamaguchi T, Costabel U, McDowell A, Guzman J, Uchida K, Ohashi K, Eishi Y. Immunohistochemical Detection of Potential Microbial Antigens in Granulomas in the Diagnosis of Sarcoidosis. Journal of Clinical Medicine. 2021; 10(5):983. https://doi.org/10.3390/jcm10050983
Chicago/Turabian StyleYamaguchi, Tetsuo, Ulrich Costabel, Andrew McDowell, Josune Guzman, Keisuke Uchida, Kenichi Ohashi, and Yoshinobu Eishi. 2021. "Immunohistochemical Detection of Potential Microbial Antigens in Granulomas in the Diagnosis of Sarcoidosis" Journal of Clinical Medicine 10, no. 5: 983. https://doi.org/10.3390/jcm10050983
APA StyleYamaguchi, T., Costabel, U., McDowell, A., Guzman, J., Uchida, K., Ohashi, K., & Eishi, Y. (2021). Immunohistochemical Detection of Potential Microbial Antigens in Granulomas in the Diagnosis of Sarcoidosis. Journal of Clinical Medicine, 10(5), 983. https://doi.org/10.3390/jcm10050983







