Risk of Metabolic Syndrome in Kidney Stone Formers: A Comparative Cohort Study with a Median Follow-Up of 19 Years
Abstract
1. Introduction
2. Methods
2.1. Definitions
2.2. Study Population
2.3. Comparator Population
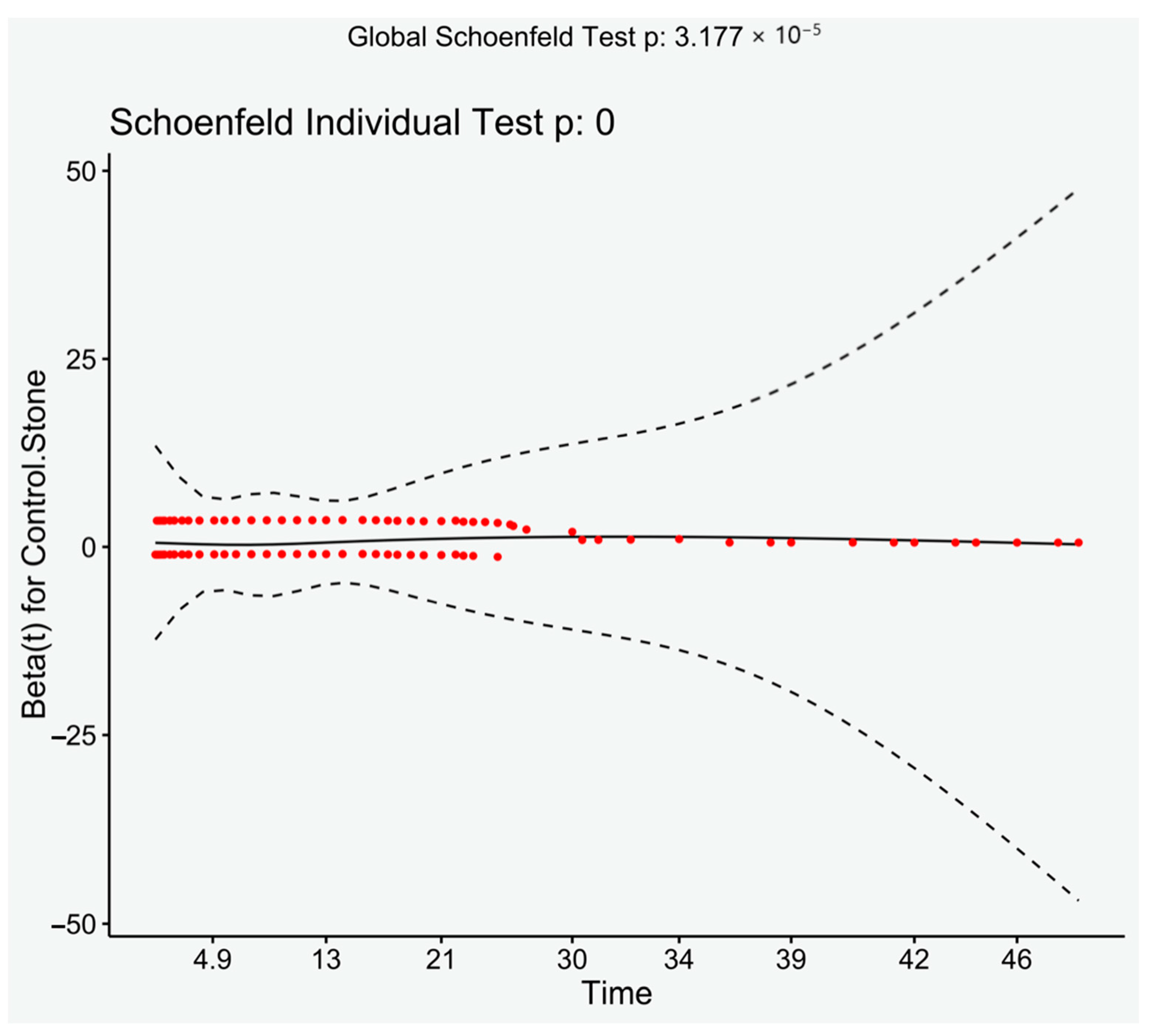
2.4. Statistical Methods
2.5. Sample Size Calculation
2.6. Ethical Approval
3. Results
3.1. Demographics
3.2. Risk of Metabolic Syndrome in Stone Formers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Geraghty, R.M.; Cook, P.; Walker, V.; Somani, B.K. Evaluation of the economic burden of kidney stone disease in the UK: A retro-spective cohort study with a mean follow-up of 19 years. BJU Int. 2020, 125, 586–594. [Google Scholar] [CrossRef]
- Chewcharat, A.; Curhan, G. Trends in the prevalence of kidney stones in the United States from 2007 to 2016. Urolithiasis 2021, 49, 27–39. [Google Scholar] [CrossRef]
- Geraghty, R.; Abdi, A.; Somani, B.; Cook, P.; Roderick, P. Does chronic hyperglycaemia increase the risk of kidney stone disease? results from a systematic review and meta-analysis. BMJ Open 2020, 10, e032094. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, D.; Reaven, G.M.; Cobin, R.H.; Ford, E.; Ganda, O.P.; Handelsman, Y.; Hellman, R.; Jellinger, P.S.; Kendall, D.; Krauss, R.M.; et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr. Pract. 2003, 9, 237–252. [Google Scholar] [CrossRef]
- Abate, N.; Chandalia, M.; Cabo-Chan, A.V.; Moe, O.W.; Sakhaee, K. The metabolic syndrome and uric acid nephrolithiasis: Novel features of renal manifestation of insulin resistance. Kidney Int. 2004, 65, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Kadlec, A.O.; Greco, K.; Fridirici, Z.C.; Hart, S.T.; Vellos, T.; Turk, T.M. Metabolic Syndrome and Urinary Stone Composition: What Factors Matter Most? Urology 2012, 80, 805–810. [Google Scholar] [CrossRef]
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev. Chronic Dis. 2017, 14, E24. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Stampfer, M.J.; Curhan, G.C. Obesity, weight gain, and the risk of kidney stones. JAMA 2005, 293, 455–462. [Google Scholar] [CrossRef]
- Madore, F.; Stampfer, M.J.; Rimm, E.B.; Curhan, G.C. Nephrolithiasis and Risk of Hypertension. Am. J. Hypertens. 1998, 11, 46–53. [Google Scholar] [CrossRef]
- Akoudad, S.; Szklo, M.; McAdams, M.A.; Fulop, T.; Anderson, C.A.; Coresh, J.; Köttgen, A. Correlates of kidney stone disease differ by race in a multi-ethnic middle-aged population: The ARIC study. Prev. Med. 2010, 51, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, F.C.M.; De, S.K.; Gebreselassie, S.; Li, I.; Sarkissian, C.; Monga, M. Urolithiasis/Endourology Dyslipidemia and Kidney Stone Risk. J. Urol. 2014, 191, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, J. Lipotoxicity. Kidney Int. 2006, 70, 1560–1566. [Google Scholar] [CrossRef]
- Chung, S.-D.; Chen, Y.-K.; Lin, H.-C. Increased Risk of Diabetes in Patients with Urinary Calculi: A 5-Year Followup Study. J. Urol. 2011, 186, 1888–1893. [Google Scholar] [CrossRef] [PubMed]
- Kittanamongkolchai, W.; Mara, K.C.; Mehta, R.A.; Vaughan, L.E.; Denic, A.; Knoedler, J.J.; Enders, F.T.; Lieske, J.C.; Rule, A.D. Risk of Hypertension among First-Time Symptomatic Kidney Stone Formers. Clin. J. Am. Soc. Nephrol. 2017, 12, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E. The metabolic syndrome and cardiovascular disease. Ann. Med. 2006, 38, 64–80. [Google Scholar] [CrossRef]
- Walker, V.; Stansbridge, E.M.; Griffin, D.G. Demography and biochemistry of 2800 patients from a renal stones clinic. Ann. Clin. Biochem. Int. J. Lab. Med. 2013, 50, 127–139. [Google Scholar] [CrossRef]
- Thiru, K.; Hassey, A.; Sullivan, F. Systematic review of scope and quality of electronic patient record data in primary care. BMJ 2003, 326, 1070. [Google Scholar] [CrossRef]
- Ljunghall, S.; Danielson, B.G. A Prospective Study of Renal Stone Recurrences. Br. J. Urol. 1984, 56, 122–124. [Google Scholar] [CrossRef]
- Daudon, M.; Lacour, B.; Jungers, P. Influence of body size on urinary stone composition in men and women. Urol. Res. 2006, 34, 193–199. [Google Scholar] [CrossRef]
- Halbritter, J.; Baum, M.; Hynes, A.M.; Rice, S.J.; Thwaites, D.T.; Gucev, Z.S.; Fisher, B.; Spaneas, L.; Porath, J.D.; Braun, D.A.; et al. Fourteen Monogenic Genes Account for 15% of Nephrolithiasis/Nephrocalcinosis. J. Am. Soc. Nephrol. 2014, 26, 543–551. [Google Scholar] [CrossRef]
- Bobulescu, I.A.; Dubree, M.; Zhang, J.; McLeroy, P.; Moe, O.W. Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and am-monium secretion. Am. J. Physiol. Ren. Physiol. 2008, 294, F1315–F1322. [Google Scholar] [CrossRef]
- Maalouf, N.M.; Cameron, M.A.; Moe, O.W.; Sakhaee, K. Metabolic Basis for Low Urine pH in Type 2 Diabetes. Clin. J. Am. Soc. Nephrol. 2010, 5, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Wiederkehr, M.R.; Moe, O.W. Uric Acid Nephrolithiasis: A Systemic Metabolic Disorder. Clin. Rev. Bone Miner. Metab. 2011, 9, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Hartman, C.; Friedlander, J.I.; Moreira, D.M.; Leavitt, D.A.; Hoenig, D.M.; Smith, A.D.; Okeke, Z. Does hypertension impact 24-hour urine parameters in patients with nephro-lithiasis? Urology 2015, 85, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Howles, S.A.; Wiberg, A.; Goldsworthy, M.; Bayliss, A.L.; Gluck, A.K.; Ng, M.; Grout, E.; Tanikawa, C.; Kamatani, Y.; Terao, C.; et al. Genetic variants of calcium and vitamin D metabolism in kidney stone disease. Nat. Commun. 2019, 10, 5175. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, C.; Kamatani, Y.; Terao, C.; Usami, M.; Takahashi, A.; Momozawa, Y.; Suzuki, K.; Ogishima, S.; Shimizu, A.; Satoh, M.; et al. Novel Risk Loci Identified in a Genome-Wide Association Study of Urolithiasis in a Japanese Population. J. Am. Soc. Nephrol. 2019, 30, 855–864. [Google Scholar] [CrossRef]
- Oh, S.-W.; Lee, J.-E.; Shin, E.; Kwon, H.; Choe, E.K.; Choi, S.-Y.; Rhee, H.; Choi, S.H. Genome-wide association study of metabolic syndrome in Korean populations. PLoS ONE 2020, 15, e0227357. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, M.; Lin, S.; Jian, R.; Li, X.; Chan, J.; Dong, G.; Fang, H.; Robinson, A.E.; Snyder, M.P.; et al. A Quantitative Proteome Map of the Human Body. Cell 2020, 183, 269–283. [Google Scholar] [CrossRef]
- Vaxillaire, M.; Cavalcanti-Proença, C.; Dechaume, A.; Tichet, J.; Marre, M.; Balkau, B.; Froguel, P. For the DESIR study group The Common P446L Polymorphism in GCKR Inversely Modulates Fasting Glucose and Triglyceride Levels and Reduces Type 2 Diabetes Risk in the DESIR Prospective General French Population. Diabetes 2008, 57, 2253–2257. [Google Scholar] [CrossRef]
- Sagesaka, H.; Sato, Y.; Someya, Y.; Tamura, Y.; Shimodaira, M.; Miyakoshi, T.; Hirabayashi, K.; Koike, H.; Yamashita, K.; Watada, H.; et al. Type 2 Diabetes: When Does It Start? J. Endocr. Soc. 2018, 2, 476–484. [Google Scholar] [CrossRef]
- Gamage, K.N.; Jamnadass, E.; Sulaiman, S.K.; Pietropaolo, A.; Aboumarzouk, O.; Somani, B.K. The role of fluid intake in the prevention of kidney stone disease: A systematic review over the last two decades. Turk. J. Urol. 2020, 46 (Suppl. 1), S92. [Google Scholar] [CrossRef] [PubMed]
- New, F.; Somani, B.K. A complete world literature review on quality of life (QOL) in patients with kidney stone disease (KSD). Curr. Urol. Rep. 2016, 17, 88. [Google Scholar] [CrossRef] [PubMed]


| Metabolic Syndrome (Modified AACE Criteria) | |
|---|---|
| Fasting Plasma Glucose | >6.1 mmol/L or Hypoglycaemic treatment or Physician diagnosis of Impaired Glucose Tolerance or Diabetes Mellitus |
| Body Mass Index | ≥25 kg/m2 or Physician diagnosis of Obesity |
| Blood Pressure | ≥130/≥85 mmHg or Antihypertensive treatment or Physician diagnosis of hypertension |
| Triglycerides | >1.7 mmol/L |
| High-Density Lipoprotein | M: <1.04 mmol/L; F: <1.29 mmol/L |
| Controls | Stone Formers | HR (95% CI) | p | ||
|---|---|---|---|---|---|
| Age at Presentation (Years), Mean ± SD | 49 ± 14 | 49 ± 14 | |||
| Sex, n (%) | Female | 723 (29.1%) | 241 (29.1%) | ||
| Male | 1761 (70.9%) | 587 (70.9%) | |||
| Follow-Up (Years), Median (IQR) | 22 (17–27) | 22 (17–27) | |||
| Metabolic Syndrome, n (%) | 617 (24.8%) | 361 (43.6%) | 1.77 (1.55–2.03) | <0.001 | |
| Metabolic Syndrome Components Developed, n (%) | 0 | 478 (19.2%) | 114 (13.8%) | ||
| 1 | 793 (31.9%) | 146 (17.6%) | |||
| 2 | 596 (24.0%) | 172 (20.8%) | |||
| 3 | 399 (16.1%) | 170 (20.5%) | |||
| 4 | 182 (7.3%) | 134 (16.2%) | |||
| 5 | 36 (1.4%) | 83 (1.0%) | |||
| Primary Stone Composition, n (%) | Ca Ox | - | 425 (51.3%) | 1.82 (1.53–2.16) | <0.001 |
| Urate | - | 21 (2.5%) | 3.87 (2.23–6.72) | <0.001 | |
| Ca Po | - | 17 (2.1%) | 0.89 (0.33–2.38) | 0.82 | |
| Struvite | - | 5 (0.6%) | 0.78 (0.11–5.54) | 0.80 | |
| Unclear | - | 360 (43.5%) | 1.71 (1.43–2.05) | <0.001 | |
| Component | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Impaired Glucose Tolerance | 1.19 (0.97–1.46) | 0.09 | 1.17 (0.95–1.43) | 0.13 |
| Hypertension | 1.56 (1.41–1.81) | <0.001 | 1.51 (1.33–1.71) | <0.001 |
| BMI > 25 | 1.41 (1.03–1.26) | 0.01 | 1.11 (1.01–1.24) | 0.04 |
| TGL > 1.70 | 1.58 (1.37–1.83) | <0.001 | 1.50 (1.30–1.74) | <0.001 |
| HDL < 1.04 for women; <1.29 for men | 1.26 (1.09–1.45) | <0.001 | 1.25 (1.09–1.44) | 0.002 |
| Metabolic Syndrome | 1.78 (1.56–2.03) | <0.001 | 1.77 (1.55–2.03) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geraghty, R.M.; Cook, P.; Roderick, P.; Somani, B. Risk of Metabolic Syndrome in Kidney Stone Formers: A Comparative Cohort Study with a Median Follow-Up of 19 Years. J. Clin. Med. 2021, 10, 978. https://doi.org/10.3390/jcm10050978
Geraghty RM, Cook P, Roderick P, Somani B. Risk of Metabolic Syndrome in Kidney Stone Formers: A Comparative Cohort Study with a Median Follow-Up of 19 Years. Journal of Clinical Medicine. 2021; 10(5):978. https://doi.org/10.3390/jcm10050978
Chicago/Turabian StyleGeraghty, Robert M., Paul Cook, Paul Roderick, and Bhaskar Somani. 2021. "Risk of Metabolic Syndrome in Kidney Stone Formers: A Comparative Cohort Study with a Median Follow-Up of 19 Years" Journal of Clinical Medicine 10, no. 5: 978. https://doi.org/10.3390/jcm10050978
APA StyleGeraghty, R. M., Cook, P., Roderick, P., & Somani, B. (2021). Risk of Metabolic Syndrome in Kidney Stone Formers: A Comparative Cohort Study with a Median Follow-Up of 19 Years. Journal of Clinical Medicine, 10(5), 978. https://doi.org/10.3390/jcm10050978






