Disposition Decision Support by Laboratory Based Outcome Prediction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
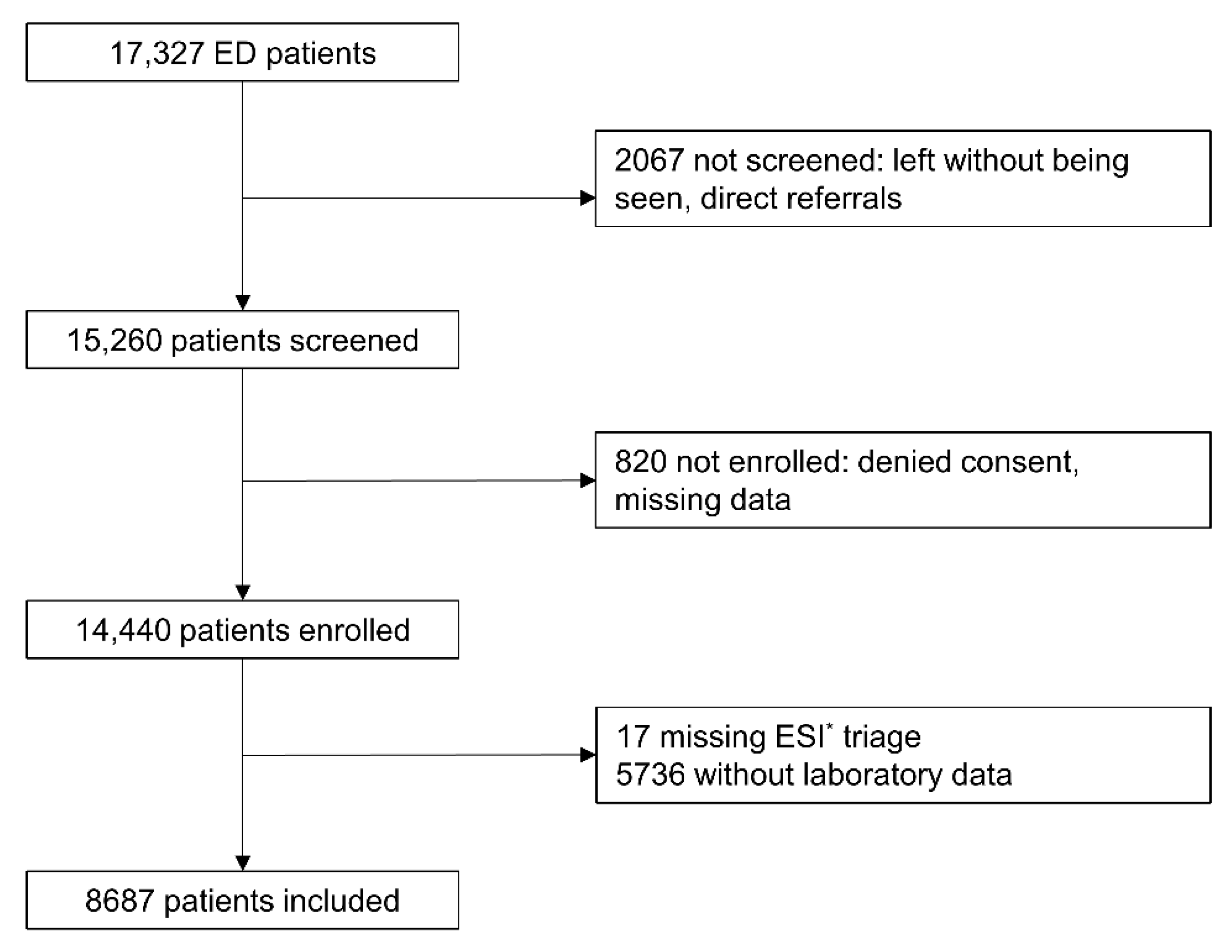
2.2. Selection of Participants
2.3. Data Collection
2.3.1. Triage
2.3.2. Laboratory Data
2.4. Outcomes
2.5. Data Analysis
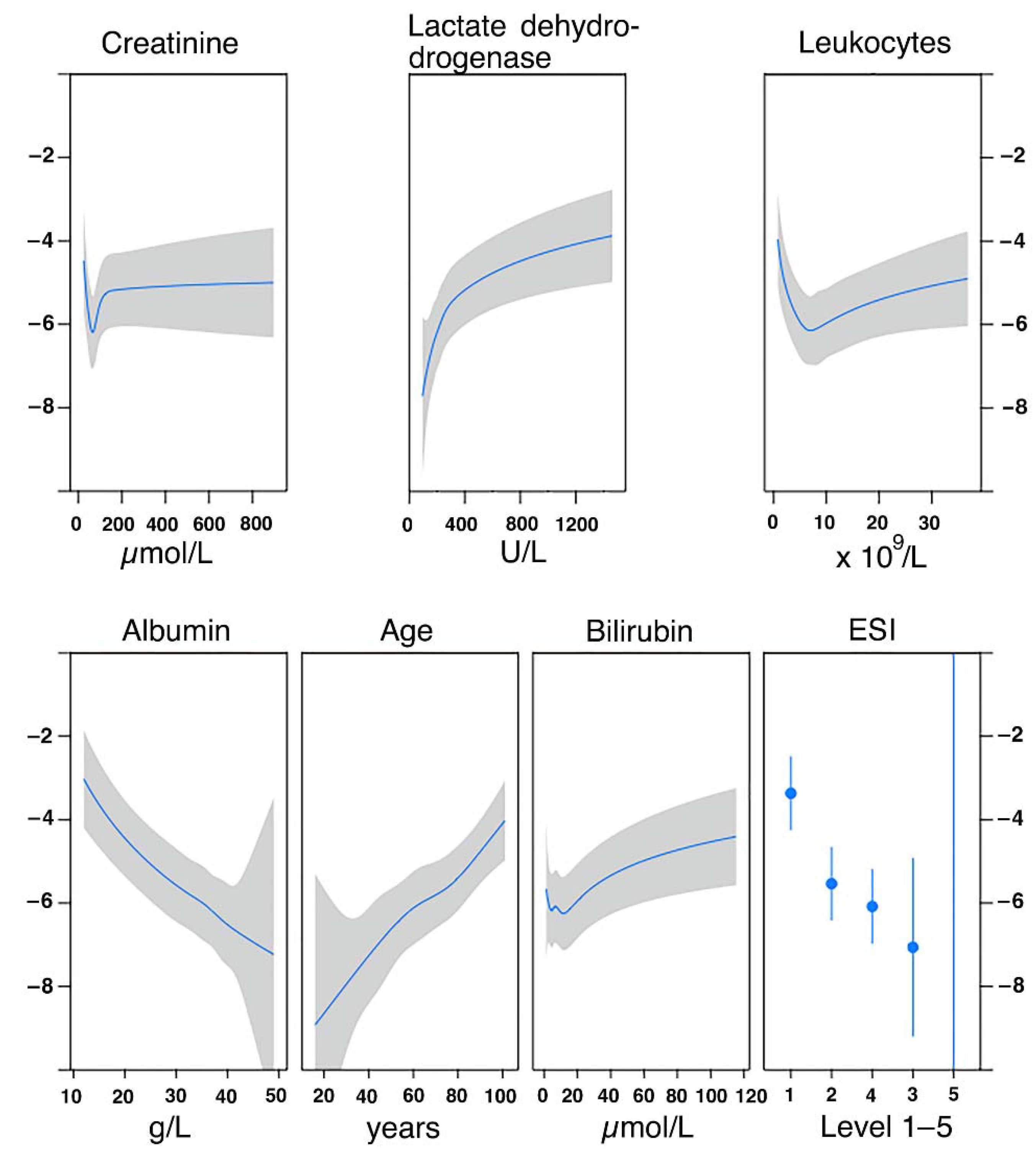
2.5.1. Derivation of Predictive Models
2.5.2. Development of the TRIAL Score
3. Results
3.1. Characteristics
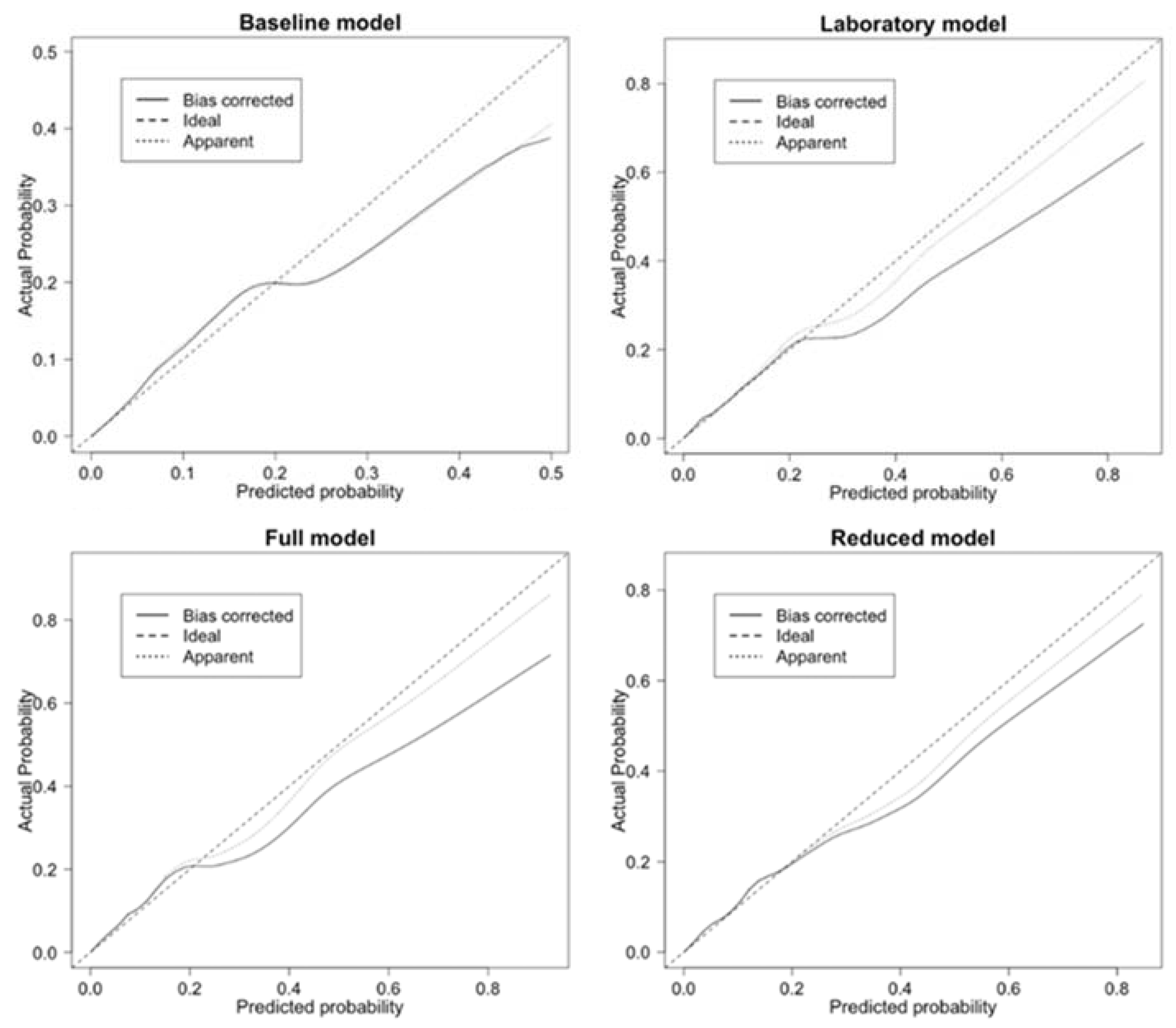
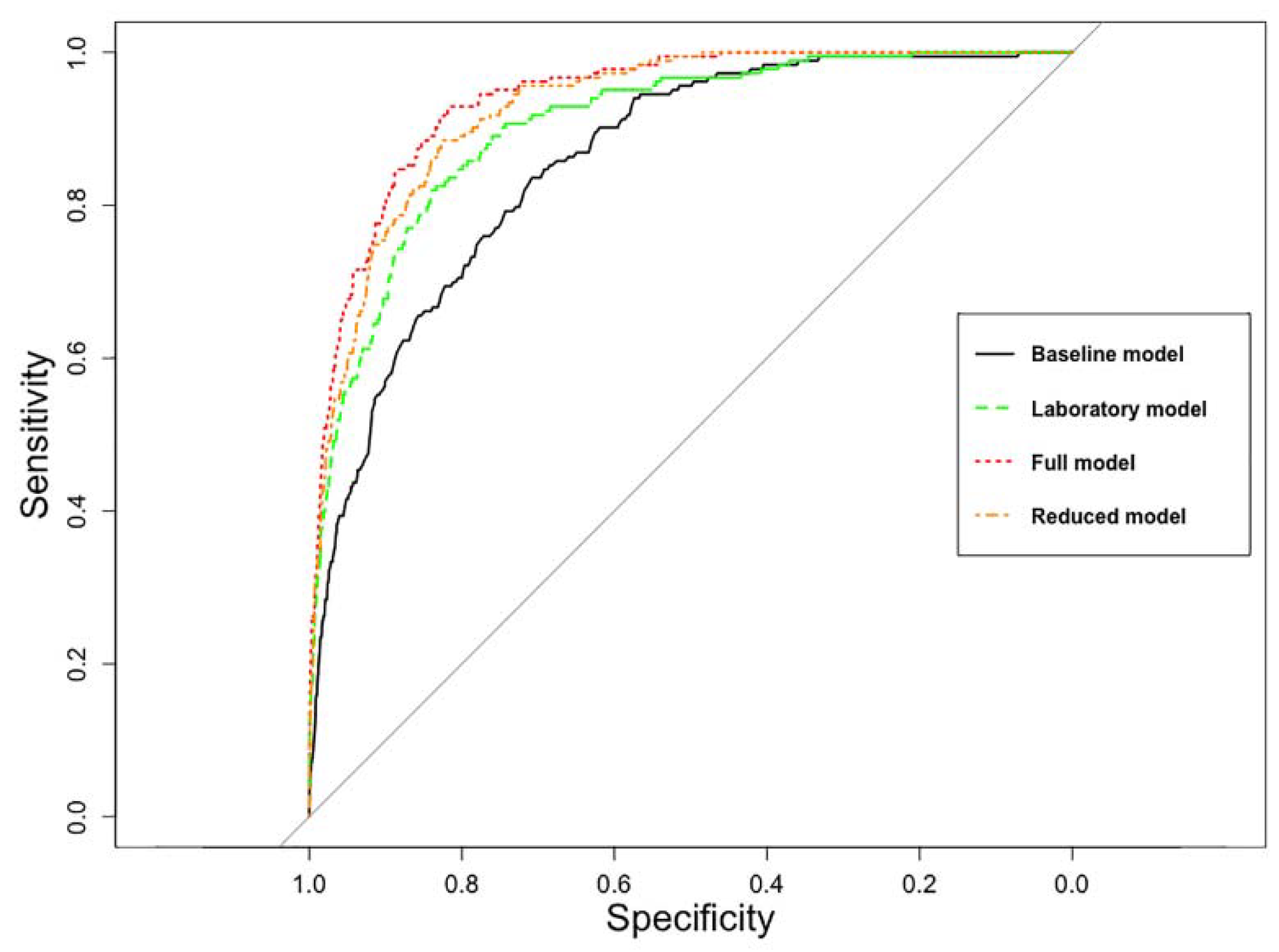
3.2. Performance of Predictive Models
3.3. TRIAL Risk Score
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Cause of Death | Reason for Missing Laboratory | Age | Gender | ESI * |
|---|---|---|---|---|
| Gastrointestinal bleeding with cardiac arrest | Stop of resuscitation in consultation with the patient’s family | 84 | w | 1 |
| 3rd degree burns (90% of body surface) in suicidal intent | Not possible to intubate or to perform a tracheotomy, no resuscitation performed at cardiac arrest | 61 | m | 1 |
| Cardiac arrest | Unobserved cardiac arrest outside the hospital, arrival in resuscitation facility after 90 min of resuscitation | 62 | m | 1 |
| Cardiogenic shock | No resuscitation in accordance with the patient’s family | 97 | w | 2 |
| ST-segment elevation myocardial infarction | Resuscitation was stopped in accordance with the living will | 76 | w | 1 |
| Cardiac arrest (10 days after presentation) | Analysis of glucose was missing. The patient was transferred to our ED † and hospitalized for suspicion of pleural empyema | 49 | m | 3 |
| Death by unknown causes | Analysis of glucose was missing. Patient was admitted with a fracture of the humerus | 82 | m | 3 |
| Septic shock due to hospital-acquired pneumonia (5 weeks after admission) | Analysis of glucose was missing. Patient was transferred from another hospital to our ED and hospitalized for surgery the next day | 65 | m | 3 |
| ST-segment elevation myocardial infarction | Analysis of AST ‡ and LDH § impossible for hemolysis | 55 | w | 3 |
| Acute abdomen with retroperitoneal and gastrointestinal bleeding, sudden worsening of condition. No resuscitation performed according to the living will. | Analysis of AST and LDH impossible for hemolysis | 77 | m | 3 |
| Cardiac arrest | Analysis of AST and LDH impossible for hemolysis | 57 | m | 1 |
| Mass hemorrhage in the left hemisphere | Analysis of AST and LDH impossible for hemolysis | 81 | m | 1 |
| Aortic dissection type A | Analysis of AST and LDH impossible for hemolysis | 69 | m | 1 |
| Sepsis | Analysis of AST and LDH impossible for hemolysis | 82 | m | 1 |
| Presentation at Triage | Final Diagnosis | Age | Gender | ESI † |
|---|---|---|---|---|
| Fall without signs of concussion | Acute-on-chronic subdural hematoma | 86 | w | 4 ‡ |
| Abdominal pain | Appendicitis | 47 | m | 5 |
| Infection of the finger | Flexor tendon infection, in-hospital acute coronary syndrome | 79 | m | 4 |
| Change of behavior | Shunt dysfunction (shunt of 18 years for hydrocephalus) | 64 | w | 4 |
| Necrotic fingertip | Multiple cerebral ischemic infarctions | 63 | w | 4 |
| Loss of sensation in the left foot in the last months, worse since a few days | Glioblastoma multiforme | 78 | m | 4 |
| Fall with multiple contusions | Fractures and shoulder injury | 67 | w | 4 |
| Infection of unknown origin | Cerebrovascular infarction due to embolism | 42 | m | 4 |
| Transfer from another hospital (suspicion of mediastinal problem) | Acute osteomyelitis | 71 | w | 4 |
| Dyspnea of unknown origin | Acute heart failure | 72 | m | 4 |
| First Model to Be Compared | Second Model to Be Compared | p-Value |
|---|---|---|
| Baseline model | Laboratory model | 0.005 |
| Baseline model | Full model | <0.001 |
| Baseline model | Reduced model | <0.001 |
| Laboratory model | Full model | <0.001 |
| Laboratory model | Reduced model | 0.003 |
| Full model | Reduced model | 0.002 |
| Cause of Death | Gender | Age | ESI * |
|---|---|---|---|
| Exacerbation of COPD † | w | 61 | 2 |
| Aortic dissection | m | 39 | 2 |
| Aortic dissection | m | 56 | 2 |
| Committed suicide during hospital stay | w | 69 | 3 |
| Aortic dissection | w | 84 | 3 |
| Pneumonia (possibly due to aspiration) | m | 70 | 3 |
| Acute on chronic subdural hematoma | w | 86 | 4 |
| Outcome | Area under the ROC* Curve | Area under the ROC Curve Adjusted with Bootstrapping |
|---|---|---|
| Hospitalization | 0.80 | 0.80 |
| Admission to an intensive care unit | 0.81 | 0.81 |
References
- Bingisser, R.; Nickel, C.H. The last decade of symptom-oriented research in emergency medicine: Triage, work-up, and disposition. Swiss Med. Wkly. 2019, 149, w20141. [Google Scholar] [CrossRef]
- Obermeyer, Z.; Cohn, B.; Wilson, M.; Jena, A.B.; Cutler, D.M. Early death after discharge from emergency departments: Analysis of national US insurance claims data. BMJ 2017, 356, j239. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Heng, B.H.; Tay, S.Y.; Seow, E. Predicting hospital admissions at emergency department triage using routine administrative data. Acad. Emerg. Med. 2011, 18, 844–850. [Google Scholar] [CrossRef]
- Moore, C.C.; Hazard, R.; Saulters, K.J.; Ainsworth, J.; Adakun, S.A.; Amir, A.; Andrews, B.; Auma, M.; Baker, T.; Banura, P.; et al. Derivation and validation of a universal vital assessment (UVA) score: A tool for predicting mortality in adult hospitalised patients in sub-Saharan Africa. BMJ Glob. Health 2017, 2, e000344. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, I.; Price, C.; Sitch, A.; Banda, P.; Kellett, J.; Nyirenda, M.; Rylance, J. Early warning scores generated in developed healthcare settings are not sufficient at predicting early mortality in Blantyre, Malawi: A prospective cohort study. PLoS ONE 2013, 8, e59830. [Google Scholar] [CrossRef] [PubMed]
- Nabayigga, B.; Kellett, J.; Brabrand, M.; Opio, M.O. The mortality of acutely ill medical patients for up to 60days after admission to a resource poor hospital in sub-Saharan Africa compared with patients of similar illness severity admitted to a Danish Regional Teaching Hospital—An exploratory observational study. Eur. J. Intern. Med. 2016, 27, 24–30. [Google Scholar]
- Kellett, J.; Kim, A. Validation of an abbreviated Vitalpac™ Early Warning Score (ViEWS) in 75,419 consecutive admissions to a Canadian regional hospital. Resuscitation 2012, 83, 297–302. [Google Scholar] [CrossRef]
- Misch, F.; Messmer, A.S.; Nickel, C.H.; Gujan, M.; Graber, A.; Blume, K.; Bingisser, R. Impact of observation on disposition of elderly patients presenting to emergency departments with non-specific complaints. PLoS ONE 2014, 9, e98097. [Google Scholar] [CrossRef] [PubMed]
- Raita, Y.; Goto, T.; Faridi, M.K.; Brown, D.F.M.; Camargo, C.A.; Hasegawa, K., Jr. Emergency department triage prediction of clinical outcomes using machine learning models. Crit. Care 2019, 23, 64. [Google Scholar] [CrossRef]
- Brabrand, M.; Lassen, A.T.; Knudsen, T.; Hallas, J. Seven-day mortality can be predicted in medical patients by blood pressure, age, respiratory rate, loss of independence, and peripheral oxygen saturation (the PARIS score): A prospective cohort study with external validation. PLoS ONE 2015, 10, e0122480. [Google Scholar] [CrossRef]
- Loekito, E.; Bailey, J.; Bellomo, R.; Hart, G.K.; Hegarty, C.; Davey, P.; Bain, C.; Pilcher, D.; Schneider, H. Common laboratory tests predict imminent medical emergency team calls, intensive care unit admission or death in emergency department patients. Emerg. Med. Australas. 2013, 25, 132–139. [Google Scholar] [CrossRef]
- Asadollahi, K.; Hastings, I.M.; Gill, G.V.; Beeching, N.J. Prediction of hospital mortality from admission laboratory data and patient age: A simple model. Emerg. Med. Australas. 2011, 23, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Prytherch, D.R.; Sirl, J.S.; Schmidt, P.; Featherstone, P.I.; Weaver, P.C.; Smith, G.B. The use of routine laboratory data to predict in-hospital death in medical admissions. Resuscitation 2005, 66, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Froom, P.; Shimoni, Z. Prediction of hospital mortality rates by admission laboratory tests. Clin. Chem. 2006, 52, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Redfern, O.C.; Pimentel, M.A.F.; Prytherch, D.; Meredith, P.; Clifton, D.A.; Tarassenko, L.; Smith, G.B.; Watkinson, P.J. Predicting in-hospital mortality and unanticipated admissions to the intensive care unit using routinely collected blood tests and vital signs: Development and validation of a multivariable model. Resuscitation 2018, 133, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Riordan, J.P.; Dell, W.L.; Patrie, J.T. Can Patient Variables Measured on Arrival to the Emergency Department Predict Disposition in Medium-acuity Patients? J. Emerg. Med. 2017, 52, 769–779. [Google Scholar] [CrossRef]
- Nickel, C.H.; Messmer, A.S.; Geigy, N.; Misch, F.; Mueller, B.; Dusemund, F.; Hertel, S.; Hartmann, O.; Giersdorf, S.; Bingisser, R. Stress markers predict mortality in patients with nonspecific complaints presenting to the emergency department and may be a useful risk stratification tool to support disposition planning. Acad. Emerg. Med. 2013, 20, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Dinh, M.M.; Russell, S.B.; Bein, K.J.; Rogers, K.; Muscatello, D.; Paoloni, R.; Hayman, J.; Chalkley, D.R.; Ivers, R. The Sydney Triage to Admission Risk Tool (START) to predict Emergency Department Disposition: A derivation and internal validation study using retrospective state-wide data from New South Wales, Australia. BMC Emerg Med. 2016, 16, 46. [Google Scholar] [CrossRef]
- Kellett, J.; Nickel, C.H.; Skyttberg, N.; Brabrand, M. Is it possible to quickly identify acutely unwell patients who can be safely managed as outpatients? The need for a “Universal Safe to Discharge Score”. Eur. J. Intern. Med. 2019, 67, e13–e15. [Google Scholar] [CrossRef]
- Grossmann, F.F.; Nickel, C.H.; Christ, M.; Schneider, K.; Spirig, R.; Bingisser, R. Transporting clinical tools to new settings: Cultural adaptation and validation of the Emergency Severity Index in German. Ann. Emerg. Med. 2011, 57, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.A.; Liu, N.; Wu, S.X.; Shen, Y.; Lam, S.S.W.; Ong, M.E.H. Predicting hospital admission at the emergency department triage: A novel prediction model. Am. J. Emerg. Med. 2019, 37, 1498–1504. [Google Scholar] [CrossRef]
- Bingisser, R.; Baerlocher, S.M.; Kuster, T.; Nieves Ortega, R.; Nickel, C.H. Physicians’ Disease Severity Ratings are Non-Inferior to the Emergency Severity Index. J. Clin. Med. 2020, 9, 762. [Google Scholar] [CrossRef]
- Gilboy, N.; Tanabe, P.; Travers, D.A.; Rosenau, A.M.; Eitel, D.R. Emergency Severity Index, Version 4, Implementation Handbook; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2005; pp. 1–72. [Google Scholar]
- Silke, B.; Kellett, J.; Rooney, T.; Bennett, K.; O’Riordan, D. An improved medical admissions risk system using multivariable fractional polynomial logistic regression modelling. QJM 2010, 103, 23–32. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Lawless, J.F.; Singhal, K. Efficient Screening of Nonnormal Regression Models. Biometrics 1978, 34, 318–327. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.A. Public hospital bed crisis: Too few or too misused? Aust. Health Rev. 2010, 34, 317–324. [Google Scholar] [CrossRef]
- Philp, I.; Mills, K.A.; Thanvi, B.; Ghosh, K.; Long, J.F. Reducing hospital bed use by frail older people: Results from a systematic review of the literature. Int. J. Integr. Care 2013, 13, e048. [Google Scholar] [CrossRef] [PubMed]
- Darehed, D.; Norrving, B.; Stegmayr, B.; Zingmark, K.; Blom, M.C. Patients with acute stroke are less likely to be admitted directly to a stroke unit when hospital beds are scarce: A Swedish multicenter register study. Eur. Stroke J. 2017, 2, 178–186. [Google Scholar] [CrossRef]
- Weingarten, S.; Riedinger, M.S.; Sandhu, M.; Bowers, C.; Ellrodt, A.G.; Nunn, C.; Hobson, P.; Greengold, N. Can practice guidelines safely reduce hospital length of stay? Results from a multicenter interventional study. Am. J. Med. 1998, 105, 33–40. [Google Scholar] [CrossRef]
- Vidán, M.; Serra, J.A.; Moreno, C.; Riquelme, G.; Ortiz, J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: A randomized, controlled trial. J. Am. Geriatr. Soc. 2005, 53, 1476–1482. [Google Scholar] [CrossRef]
- Hardy, C.; Whitwell, D.; Sarsfield, B.; Maimaris, C. Admission avoidance and early discharge of acute hospital admissions: An accident and emergency based scheme. Emerg. Med. J. 2001, 18, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Purdy, S.; Griffin, T. Reducing hospital admissions. BMJ 2008, 336, 4–5. [Google Scholar] [CrossRef]
- Tanabe, P.; Gimbel, R.; Yarnold, P.R.; Kyriacou, D.N.; Adams, J.G. Reliability and validity of scores on The Emergency Severity Index version 3. Acad. Emerg. Med. 2004, 11, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lyons, O.; Whelan, B.; Bennett, K.; O’Riordan, D.; Silke, B. Serum albumin as an outcome predictor in hospital emergency medical admissions. Eur. J. Intern. Med. 2010, 21, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Iversen, A.K.S.; Gerds, T.A.; Østervig, R.; Linnet, J.D.; Barfod, C.; Lange, K.H.W.; Sölétormos, G.; Forberg, J.L.; Eugen-Olsen, J. Routine blood tests are associated with short term mortality and can improve emergency department triage: A cohort study of >12,000 patients. Scand. J. Trauma, Resusc. Emerg. Med. 2017, 25, 115. [Google Scholar] [CrossRef]
- Jarvis, S.W.; Kovacs, C.; Badriyah, T.; Briggs, J.; Mohammed, M.A.; Meredith, P.; Schmidt, P.E.; Featherstone, P.I.; Prytherch, D.R.; Smith, G.B. Development and validation of a decision tree early warning score based on routine laboratory test results for the discrimination of hospital mortality in emergency medical admissions. Resuscitation 2013, 84, 1494–1499. [Google Scholar] [CrossRef]
- Udy, A.A.; Scheinkestel, C.; Pilcher, D.; Bailey, M. The Association Between Low Admission Peak Plasma Creatinine Concentration and In-Hospital Mortality in Patients Admitted to Intensive Care in Australia and New Zealand. Crit. Care Med. 2016, 44, 73–82. [Google Scholar] [CrossRef]
- Cartin-Ceba, R.; Afessa, B.; Gajic, O. Low baseline serum creatinine concentration predicts mortality in critically ill patients independent of body mass index. Crit. Care Med. 2007, 35, 2420–2423. [Google Scholar] [CrossRef] [PubMed]
- Brabrand, M.; Knudsen, T.; Hallas, J. Identifying admitted patients at risk of dying: A prospective observational validation of four biochemical scoring systems. BMJ Open 2013, 3, e002890. [Google Scholar] [CrossRef][Green Version]
- Kim, S.W.; Li, J.Y.; Hakendorf, P.; Teubner, D.J.; Ben-Tovim, D.I.; Thompson, C.H. Predicting admission of patients by their presentation to the emergency department. Emerg. Med. Australas. 2014, 26, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Rudge, G.; Wood, G.; Smith, G.; Nangalia, V.; Prytherch, D.; Holder, R.; Briggs, J. Which is more useful in predicting hospital mortality—Dichotomised blood test results or actual test values? A retrospective study in two hospitals. PLoS ONE 2012, 7, e46860. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n = 14,423) | Patients Included (n = 8687) | Patients without Laboratory Data (n = 5736) | |
|---|---|---|---|
| Age, median (IQR *) | 52 (34–72) | 61 (42–78) § | 40 (29–56) § |
| Male gender, n (%) | 7547 (52.3) | 4471 (51.5) ‖ | 3076 (53.6) ‖ |
| ESI † level | § | § | |
| ESI 1, n (%) | 339 (2.4) | 320 (3.7) | 19 (0.3) |
| ESI 2, n (%) | 3562 (24.7) | 3244 (37.3) | 318 (5.5) |
| ESI 3, n (%) | 5939 (41.2) | 4492 (51.7) | 1447 (25.2) |
| ESI 4, n (%) | 4243 (29.4) | 623 (7.2) | 3620 (63.1) |
| ESI 5, n (%) | 340 (2.4) | 8 (0.1) | 332 (5.8) |
| In-hospital mortality, n (%) | 197 (1.4) | 183 (2.1) § | 14 (0.2) § |
| Hospitalization, n (%) | 4986 (34.6) | 4623 (53.2) § | 363 (6.3) § |
| ICU ‡ admsission, n (%) | 791 (5.5) | 758 (8.7) § | 33 (0.6) § |
| Age, Median (IQR *) | Male Gender n (%) | Hospitalization n (%) | ICU †-Admission n (%) | Mortality n (%) | |
|---|---|---|---|---|---|
| ESI 1 ‡ | 69 (55–79) | 194 (60.6) | 295 (92.2) | 180 (56.3) | 61 (19.1) |
| ESI 2 | 62 (45–78) | 1758 (54.2) | 1999 (61.6) | 391 (12.1) | 67 (2.1) |
| ESI 3 | 61 (40–79) | 2192 (48.8) | 2166 (48.2) | 177 (3.9) | 54 (1.2) |
| ESI 4 | 45 (32–66) | 321 (51.5) | 160 (25.7) | 9 (1.4) | 1 (0.2) |
| ESI 5 | 41 (28–72) | 6 (75.0) | 3 (37.5) | 1 (12.5) | 0 (0.0) |
| Age (years) | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | ||||
| Points | 0 | 9 | 18 | 27 | 35 | 42 | 46 | 51 | 59 | 68 | ||||
| ESI * (levels) | 5 | 4 | 3 | 2 | 1 | |||||||||
| Points | 0 | 52 | 65 | 72 | 100 | |||||||||
| Creatinine (μmol/L) | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | 500 | |||
| Points | 44 | 26 | 16 | 9 | 4 | 0 | 0 | 3 | 6 | 9 | 14 | |||
| LDH † (U/L) | 200 | 300 | 400 | 500 | 600 | 700 | 800 | 900 | 1000 | 1100 | 1200 | |||
| Points | 0 | 9 | 13 | 16 | 18 | 21 | 22 | 24 | 25 | 26 | 28 | |||
| Albumin (g/L) | 50 | 45 | 40 | 35 | 30 | 25 | 20 | 15 | ||||||
| Points | 0 | 5 | 10 | 17 | 22 | 29 | 37 | 47 | ||||||
| Leukocytes (×109/L) | 1 | 2 | 3 | 4 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 50 |
| Points | 23 | 13 | 7 | 3 | 0 | 0 | 4 | 7 | 10 | 12 | 13 | 15 | 16 | 17 |
| Bilirubin (μmol/L) | 2 | 5 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | 110 | 120 |
| Points | 5 | 1 | 0 | 4 | 8 | 11 | 14 | 16 | 18 | 19 | 21 | 22 | 23 | 24 |
| Total points | 145 | 176 | ||||||||||||
| Risk of mortality | 1% | 10% |
| Total, n (%) | Non-Survivors, n (%) | Survivors, n (%) | ||||
|---|---|---|---|---|---|---|
| All | 8687 | (100.0) | ||||
| High risk | 344 | (4.0) | 90 | (26.2) | 254 | (73.8) |
| Intermediate risk | 2478 | (28.5) | 86 | (3.5) | 2392 | (96.5) |
| Low risk | 5865 | (67.5) | 7 | (0.1) | 5858 | (99.9) |
| ESI * 1 | 320 | (100.0) | ||||
| High risk | 173 | (54.1) | 53 | (30.6) | 120 | (69.4) |
| Intermediate risk | 127 | (39.7) | 8 | (6.3) | 119 | (93.7) |
| Low risk | 20 | (6.3) | 0 | (0.0) | 20 | (100.0) |
| ESI 2 | 3244 | (100.0) | ||||
| High risk | 111 | (3.4) | 23 | (20.7) | 88 | (79.3) |
| Intermediate risk | 1147 | (35.4) | 41 | (3.6) | 1106 | (96.4) |
| Low risk | 1986 | (61.2) | 3 | (0.2) | 1983 | (99.8) |
| ESI 3 | 4492 | (100.0) | ||||
| High risk | 60 | (1.3) | 14 | (23.3) | 46 | (76.7) |
| Intermediate risk | 1184 | (26.4) | 37 | (3.1) | 1147 | (96.9) |
| Low risk | 3248 | (72.3) | 3 | (0.1) | 3245 | (99.9) |
| ESI 4 | 623 | (100.0) | ||||
| High risk | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Intermediate risk | 20 | (3.2) | 0 | (0.0) | 20 | (100.0) |
| Low risk | 603 | (96.8) | 1 | (0.2) | 602 | (99.8) |
| ESI 5 | 8 | (100.0) | ||||
| High risk | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Intermediate risk | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Low risk | 8 | (100.0) | 0 | (0.0) | 8 | (100.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueller, O.S.; Rentsch, K.M.; Nickel, C.H.; Bingisser, R. Disposition Decision Support by Laboratory Based Outcome Prediction. J. Clin. Med. 2021, 10, 939. https://doi.org/10.3390/jcm10050939
Mueller OS, Rentsch KM, Nickel CH, Bingisser R. Disposition Decision Support by Laboratory Based Outcome Prediction. Journal of Clinical Medicine. 2021; 10(5):939. https://doi.org/10.3390/jcm10050939
Chicago/Turabian StyleMueller, Oliver S., Katharina M. Rentsch, Christian H. Nickel, and Roland Bingisser. 2021. "Disposition Decision Support by Laboratory Based Outcome Prediction" Journal of Clinical Medicine 10, no. 5: 939. https://doi.org/10.3390/jcm10050939
APA StyleMueller, O. S., Rentsch, K. M., Nickel, C. H., & Bingisser, R. (2021). Disposition Decision Support by Laboratory Based Outcome Prediction. Journal of Clinical Medicine, 10(5), 939. https://doi.org/10.3390/jcm10050939





