The Efficacy of S-1 as Adjuvant Chemotherapy for Resected Biliary Tract Carcinoma: A Propensity Score-Matching Analysis
Abstract
1. Introduction
2. Materials and Methods
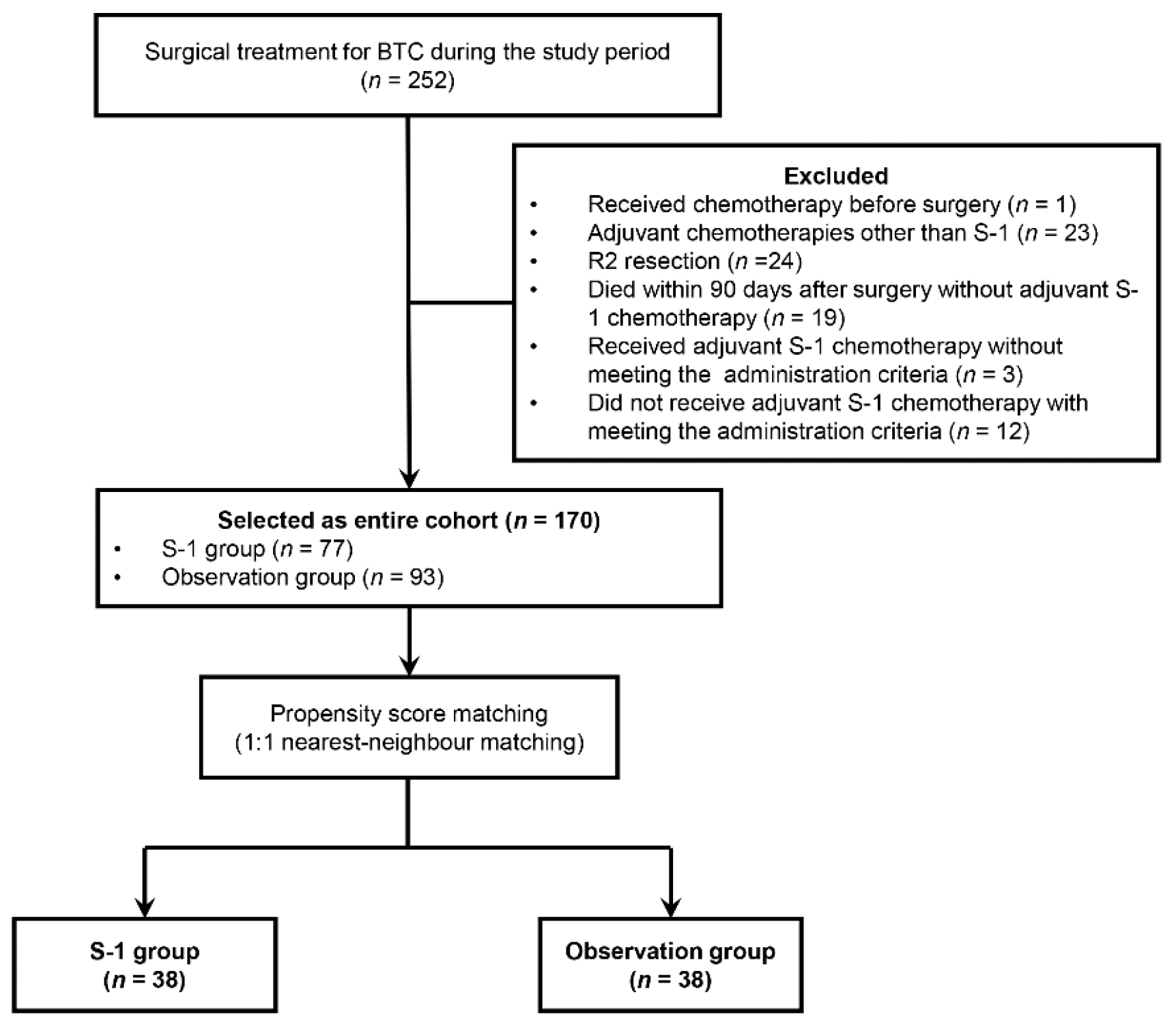
2.1. Patient Selection
2.2. Treatment Strategy and Follow Up
2.3. Administration Criteria of Adjuvant S-1 Chemotherapy
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics in the Entire Cohort
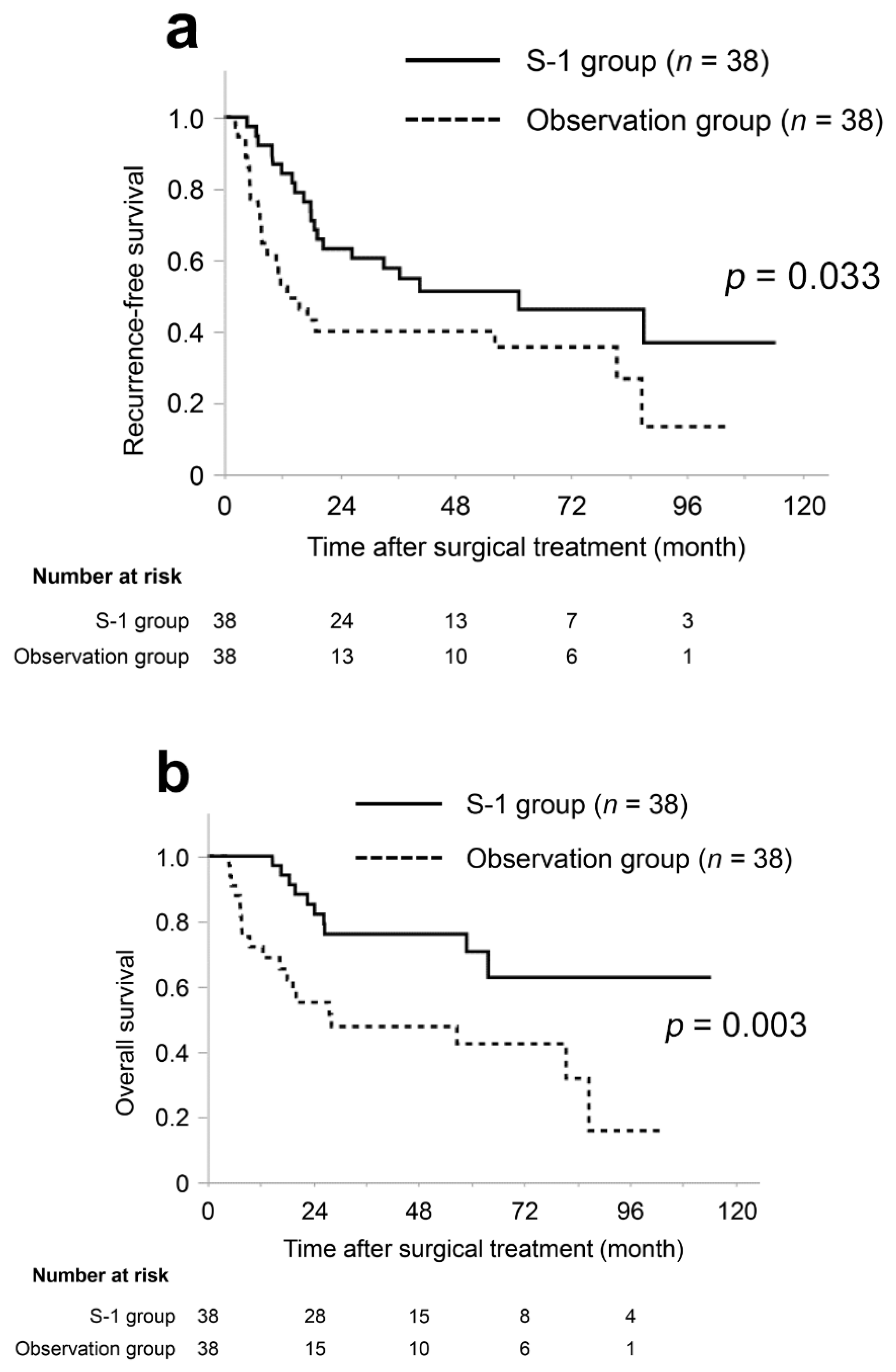
3.2. Patient Characteristics and Survival in the Matched Cohort
3.3. Univariate and Multivariate Analysis of OS in the Matched Cohort
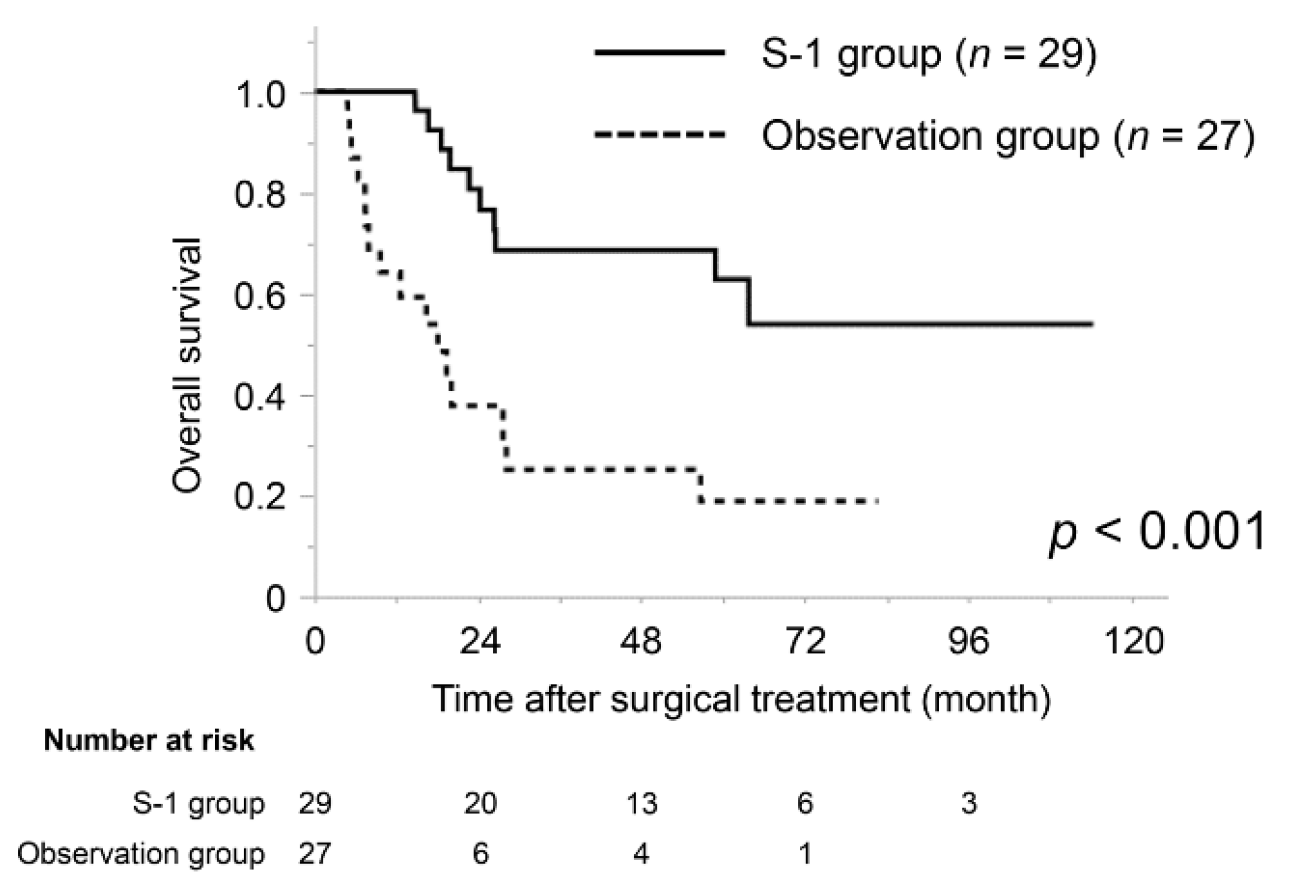
3.4. Subgroup Analysis of the Prognostic Impact of S-1 Adjuvant Chemotherapy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. WHO Classification of Tumours of the Digestive System, 4th ed.; IARC: Lyon, France, 2010. [Google Scholar]
- Miyazaki, Y.; Kokudo, T.; Amikura, K.; Kageyama, Y.; Takahashi, A.; Ohkohchi, N.; Sakamoto, H. Survival of surgery for recurrent biliary tract cancer: A single-center experience and systematic review of literature. Jpn. J. Clin. Oncol. 2017, 47, 206–212. [Google Scholar] [CrossRef]
- Sakuramoto, S.; Sasako, M.; Yamaguchi, T.; Kinoshita, T.; Fujii, M.; Nashimoto, A.; Furukawa, H.; Nakajima, T.; Ohashi, Y.; Imamura, H.; et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N. Engl. J. Med. 2007, 357, 1810–1820. [Google Scholar] [CrossRef]
- Bang, Y.J.; Kim, Y.W.; Yang, H.K.; Chung, H.C.; Park, Y.K.; Lee, K.H.; Lee, K.W.; Kim, Y.H.; Noh, S.I.; Cho, J.Y.; et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet 2012, 379, 315–321. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017, 20, 1–19. [Google Scholar] [CrossRef]
- NIH Consensus Conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA 1990, 264, 1444–1450. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef]
- Uesaka, K.; Boku, N.; Fukutomi, A.; Okamura, Y.; Konishi, M.; Matsumoto, I.; Kaneoka, Y.; Shimizu, Y.; Nakamori, S.; Sakamoto, H.; et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: A phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016, 388, 248–257. [Google Scholar] [CrossRef]
- Edeline, J.; Benabdelghani, M.; Bertaut, A.; Watelet, J.; Hammel, P.; Joly, J.P.; Boudjema, K.; Fartoux, L.; Bouhier-Leporrier, K.; Jouve, J.L.; et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J. Clin. Oncol. 2019, 37, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Ebata, T.; Hirano, S.; Konishi, M.; Uesaka, K.; Tsuchiya, Y.; Ohtsuka, M.; Kaneoka, Y.; Yamamoto, M.; Ambo, Y.; Shimizu, Y.; et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br. J. Surg. 2018, 105, 192–202. [Google Scholar] [CrossRef]
- Shirasaka, T.; Shimamato, Y.; Ohshimo, H.; Yamaguchi, M.; Kato, T.; Yonekura, K.; Fukushima, M. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs 1996, 7, 548–557. [Google Scholar] [CrossRef]
- Shirasaka, T. Development history and concept of an oral anticancer agent S-1 (TS-1): Its clinical usefulness and future vistas. Jpn. J. Clin. Oncol. 2009, 39, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Terashima, T.; Shiba, S.; Yoshida, Y.; Yamada, I.; Iwadou, S.; Horiguchi, S.; Takahashi, H.; Suzuki, E.; Moriguchi, M.; et al. Multicenter retrospective analysis of systemic chemotherapy for unresectable combined hepatocellular and cholangiocarcinoma. Cancer Sci. 2018, 109, 2549–2557. [Google Scholar] [CrossRef]
- Okabayashi, T.; Shima, Y.; Iwata, J.; Morita, S.; Sumiyoshi, T.; Sui, K.; Shimada, Y.; Iiyama, T. Characterization of Prognostic Factors and the Efficacy of Adjuvant S-1 Chemotherapy in Patients with Post-surgery Extrahepatic Bile Duct Cancer. Anticancer Res. 2017, 37, 7049–7056. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. International Union Against Cancer (UICC): TNM Classification of Malignant Tumors; Wiley: New York, NY, USA, 2017. [Google Scholar]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Bergeat, D.; Turrini, O.; Courtin-Tanguy, L.; Truant, S.; Darnis, B.; Delpero, J.R.; Mabrut, J.Y.; Regenet, N.; Sulpice, L. Impact of adjuvant chemotherapy after pancreaticoduodenectomy for distal cholangiocarcinoma: A propensity score analysis from a French multicentric cohort. Langenbecks Arch. Surg. 2018, 403, 701–709. [Google Scholar] [CrossRef]
- Murakami, Y.; Uemura, K.; Sudo, T.; Hayashidani, Y.; Hashimoto, Y.; Nakamura, H.; Nakashima, A.; Sueda, T. Adjuvant gemcitabine plus S-1 chemotherapy improves survival after aggressive surgical resection for advanced biliary carcinoma. Ann. Surg. 2009, 250, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Uemura, K.; Sudo, T.; Hayashidani, Y.; Hashimoto, Y.; Nakamura, H.; Nakashima, A.; Sueda, T. Gemcitabine-based adjuvant chemotherapy improves survival after aggressive surgery for hilar cholangiocarcinoma. J. Gastrointest. Surg. 2009, 13, 1470–1479. [Google Scholar] [CrossRef]
- Kim, Y.S.; Jeong, C.Y.; Song, H.N.; Kim, T.H.; Kim, H.J.; Lee, Y.J.; Hong, S.C. The efficacy of fluoropyrimidine-based adjuvant chemotherapy on biliary tract cancer after R0 resection. Chin. J. Cancer 2017, 36, 9. [Google Scholar] [CrossRef]
- Yin, L.; Xu, Q.; Li, J.; Wei, Q.; Ying, J. The efficiency and regimen choice of adjuvant chemotherapy in biliary tract cancer: A STROBE-compliant retrospective cohort study. Medicine 2018, 97, e13570. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Moore, M.J.; Cox, T.F.; Valle, J.W.; Palmer, D.H.; McDonald, A.C.; Carter, R.; Tebbutt, N.C.; Dervenis, C.; Smith, D.; et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: The ESPAC-3 periampullary cancer randomized trial. JAMA 2012, 308, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Nassour, I.; Mokdad, A.A.; Porembka, M.R.; Choti, M.A.; Polanco, P.M.; Mansour, J.C.; Minter, R.M.; Wang, S.C.; Yopp, A.C. Adjuvant Therapy Is Associated with Improved Survival in Resected Perihilar Cholangiocarcinoma: A Propensity Matched Study. Ann. Surg. Oncol. 2018, 25, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Kemp Bohan, P.M.; Kirby, D.T.; Chick, R.C.; Bader, J.O.; Clifton, G.T.; Vreeland, T.J.; Nelson, D.W. Adjuvant Chemotherapy in Resectable Gallbladder Cancer is Underutilized Despite Benefits in Node-Positive Patients. Ann. Surg. Oncol. 2020. [Google Scholar] [CrossRef]
- Chuah, B.; Goh, B.C.; Lee, S.C.; Soong, R.; Lau, F.; Mulay, M.; Dinolfo, M.; Lim, S.E.; Soo, R.; Furuie, T.; et al. Comparison of the pharmacokinetics and pharmacodynamics of S-1 between Caucasian and East Asian patients. Cancer Sci. 2011, 102, 478–483. [Google Scholar] [CrossRef]
- Matsukuma, S.; Tokumitsu, Y.; Shindo, Y.; Matsui, H.; Nagano, H. Essential updates to the surgical treatment of biliary tract cancer. Ann. Gastroenterol. Surg. 2019, 3, 378–389. [Google Scholar] [CrossRef]
- Beetz, O.; Klein, M.; Schrem, H.; Gwiasda, J.; Vondran, F.W.R.; Oldhafer, F.; Cammann, S.; Klempnauer, J.; Oldhafer, K.J.; Kleine, M. Relevant prognostic factors influencing outcome of patients after surgical resection of distal cholangiocarcinoma. BMC Surg. 2018, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Petrova, E.; Ruckert, F.; Zach, S.; Shen, Y.; Weitz, J.; Grutzmann, R.; Wittel, U.A.; Makowiec, F.; Hopt, U.T.; Bronsert, P.; et al. Survival outcome and prognostic factors after pancreatoduodenectomy for distal bile duct carcinoma: A retrospective multicenter study. Langenbecks Arch. Surg. 2017, 402, 831–840. [Google Scholar] [CrossRef]
- Bhuiya, M.R.; Nimura, Y.; Kamiya, J.; Kondo, S.; Fukata, S.; Hayakawa, N.; Shionoya, S. Clinicopathologic studies on perineural invasion of bile duct carcinoma. Ann. Surg. 1992, 215, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, R.; Nagino, M.; Oda, K.; Kamiya, J.; Uesaka, K.; Nimura, Y. Perineural invasion has a negative impact on survival of patients with gallbladder carcinoma. Br. J. Surg. 2002, 89, 1130–1136. [Google Scholar] [CrossRef]
- Nakachi, K.; Konishi, M.; Ikeda, M.; Mizusawa, J.; Eba, J.; Okusaka, T.; Ishii, H.; Fukuda, H.; Furuse, J. A randomized Phase III trial of adjuvant S-1 therapy vs. observation alone in resected biliary tract cancer: Japan Clinical Oncology Group Study (JCOG1202, ASCOT). Jpn. J. Clin. Oncol. 2018, 48, 392–395. [Google Scholar] [CrossRef] [PubMed]
| S-1 Group n = 77 | Observation Group n = 93 | p Value | |
|---|---|---|---|
| Age [y] | 70 (44–87) | 75 (42–90) | <0.001 * |
| Gender, male | 48 (62) | 58 (62) | 0.997 |
| Diagnosis | |||
| Hilar cholangiocarcinoma | 20 (26) | 29 (31) | 0.018 * |
| Distal cholangiocarcinoma | 39 (51) | 28 (30) | |
| Gallbladder carcinoma | 18 (23) | 36 (39) | |
| Serum CA19-9 [U/mL] | 91 (1–33,564) | 53 (1–2524) | 0.133 |
| Hepatectomy | 23 (30) | 28 (30) | 0.973 |
| Clavien-Dindo classification, III–V | 35 (45) | 31 (33) | 0.107 |
| Pathological findings | |||
| Tumor differentiation, well | 25 (32) | 37 (40) | 0.230 |
| Lymphatic invasion | 50 (65) | 37 (40) | 0.001 * |
| Venous invasion | 57 (74) | 45 (48) | 0.001 * |
| Perineural invasion | 61 (79) | 52 (56) | 0.005 * |
| T status, T3 and T4 | 46 (60) | 29 (31) | <0.001 * |
| N status, N1 | 48 (62) | 23 (25) | <0.001 * |
| R status, R0 | 53 (69) | 69 (74) | 0.255 |
| S-1 Group n = 38 | Observation Group n = 38 | p Value | |
|---|---|---|---|
| Age [y] | 72 (52–82) | 74 (42–85) | 0.640 |
| Gender, male | 25 (66) | 22 (58) | 0.479 |
| Diagnosis | |||
| Hilar cholangiocarcinoma | 11 (29) | 10 (26) | 0.871 |
| Distal cholangiocarcinoma | 18 (47) | 17 (45) | |
| Gallbladder carcinoma | 9 (24) | 11 (29) | |
| Serum CA19-9 [U/mL] | 64 (1–1807) | 68 (1–2524) | 0.593 |
| Hepatectomy | 11 (29) | 13 (34) | 0.622 |
| Clavien-Dindo classification, III–V | 16 (42) | 17 (45) | 0.817 |
| Pathological findings | |||
| Tumor differentiation, well | 14 (37) | 14 (37) | 1.000 |
| Lymphatic invasion | 25 (66) | 23 (61) | 0.634 |
| Venous invasion | 25 (66) | 26 (68) | 0.807 |
| Perineural invasion | 29 (76) | 27 (71) | 0.602 |
| T status, T3 and T4 | 20 (53) | 21 (55) | 0.818 |
| N status, N1 | 17 (45) | 16 (42) | 0.817 |
| R status, R0 | 29 (76) | 28 (74) | 0.791 |
| Variable | n | Median (Months) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|---|
| p Value † | HR | 95% CI | p Value ‡ | ||||
| Age [y] | <65 | 16 | 86.7 | 0.274 | |||
| ≥65 | 60 | 63.8 | |||||
| Preoperative CA19-9 [U/mL] | <37 | 23 | 86.7 | 0.317 | |||
| ≥37 | 53 | 58.9 | |||||
| Clavien-Dindo classification | I–II | 43 | 86.7 | 0.666 | |||
| III–V | 33 | 63.8 | |||||
| Differentiation | well | 28 | 86.7 | 0.134 | |||
| not well | 48 | 58.9 | |||||
| Lymphatic invasion | no | 28 | 86.7 | 0.341 | |||
| yes | 51 | 81.5 | |||||
| Venous invasion | no | 25 | 86.7 | 0.024 * | |||
| yes | 51 | 56.7 | 1.342 | 0.510–4.102 | 0.568 | ||
| Perineural invasion | no | 20 | 86.7 | 0.007 * | |||
| yes | 56 | 56.7 | 6.038 | 1.709–29.153 | 0.004 * | ||
| T status | T0–T2 | 35 | 86.7 | 0.053 | |||
| T3 and T4 | 41 | 58.9 | |||||
| N status | N0 | 43 | 86.7 | 0.110 | |||
| N1 | 33 | 81.5 | |||||
| R status | R0 | 57 | 81.5 | 0.569 | |||
| R1 | 19 | 28.2 | |||||
| Adjuvant S-1 | yes | 42 | NA | 0.003 * | |||
| no | 42 | 28.2 | 4.370 | 1.989–10.298 | <0.001 * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyata, Y.; Kogure, R.; Nakazawa, A.; Nagata, R.; Mitsui, T.; Ninomiya, R.; Komagome, M.; Maki, A.; Kawarabayashi, N.; Beck, Y. The Efficacy of S-1 as Adjuvant Chemotherapy for Resected Biliary Tract Carcinoma: A Propensity Score-Matching Analysis. J. Clin. Med. 2021, 10, 925. https://doi.org/10.3390/jcm10050925
Miyata Y, Kogure R, Nakazawa A, Nagata R, Mitsui T, Ninomiya R, Komagome M, Maki A, Kawarabayashi N, Beck Y. The Efficacy of S-1 as Adjuvant Chemotherapy for Resected Biliary Tract Carcinoma: A Propensity Score-Matching Analysis. Journal of Clinical Medicine. 2021; 10(5):925. https://doi.org/10.3390/jcm10050925
Chicago/Turabian StyleMiyata, Yoichi, Ryota Kogure, Akiko Nakazawa, Rihito Nagata, Tetsuya Mitsui, Riki Ninomiya, Masahiko Komagome, Akira Maki, Nobuaki Kawarabayashi, and Yoshifumi Beck. 2021. "The Efficacy of S-1 as Adjuvant Chemotherapy for Resected Biliary Tract Carcinoma: A Propensity Score-Matching Analysis" Journal of Clinical Medicine 10, no. 5: 925. https://doi.org/10.3390/jcm10050925
APA StyleMiyata, Y., Kogure, R., Nakazawa, A., Nagata, R., Mitsui, T., Ninomiya, R., Komagome, M., Maki, A., Kawarabayashi, N., & Beck, Y. (2021). The Efficacy of S-1 as Adjuvant Chemotherapy for Resected Biliary Tract Carcinoma: A Propensity Score-Matching Analysis. Journal of Clinical Medicine, 10(5), 925. https://doi.org/10.3390/jcm10050925






