Abstract
Platelets are active key players in haemostasis. Qualitative platelet dysfunctions result in thrombocytopathies variously characterized by defects of their adhesive and procoagulant activation endpoints. In this review, we summarize the traditional platelet defects in adhesion, secretion, and aggregation. In addition, we review the current knowledge about procoagulant platelets, focusing on their role in bleeding or thrombotic pathologies and their pharmaceutical modulation. Procoagulant activity is an important feature of platelet activation, which should be specifically evaluated during the investigation of a suspected thrombocytopathy.
1. Introduction
Platelets or thrombocytes are small (2–5 µm) discoid anucleated cells produced by megakaryocytes. They are released in the blood stream where they circulate for 7–10 days to be eventually cleared by the spleen and the liver [1]. Platelets are responsible for maintaining the integrity of the vascular system, are active key players of primary haemostasis and enhance coagulation. Consequently, platelet disorders cause defective clot formation that may induce a bleeding or thrombotic diathesis.
Platelet disorders can be either inherited or acquired and are characterized by (i) quantitative defects, with an abnormal number of circulating platelets, either high (thrombocytosis) or low (thrombocytopenia); and/or (ii) qualitative platelet dysfunctions (thrombocytopathies) [2].
Thrombocytopathies may be induced either by extrinsic (e.g., systemic disease or medication) or by intrinsic factors [3,4]. In this review, we summarize intrinsic platelet anomalies resulting in defects of the various traditional activation endpoints, such as adhesion and aggregation (See Section 2), and we offer an in-depth and complete overview of the accumulating evidence for the physiological and clinical role of procoagulant platelets as an alternative, increasingly recognized critical endpoint of platelet function (see Section 3 and Section 4).
2. Platelet Activation End-Points and Related Defects
At the site of vascular damage, platelets interact with exposed adhesive agonists such as von Willebrand factor (VWF) and collagen. VWF binds to the platelet glycoprotein (GP) Ib-IX-V complex tethering platelets at the site of vessel wall injury. Collagen interacts with integrin α2β1 (also named GPIa/IIa) for adhesion and GPVI to initiate platelet activation. Soluble agonists, such as thromboxane A2 and adenosine diphosphate (ADP) subsequently amplify activation. Endpoints following platelet activation are characterized by: (1) shape change, (2) secretion of soluble agonists and granule content enhancing the activation process, (3) change of GPIIb/IIIa conformation to bind fibrinogen, which sustains platelet aggregation, and/or (4) externalization of negatively charged amino-phospholipids contributing to platelet procoagulant activity (Figure 1) [5,6,7]. Because of the three-dimensional configuration of the growing thrombus, platelets are differently exposed to agonists resulting in heterogeneous activation profiles [8]. Common examples of the pathophysiology are described below for each activation endpoint.
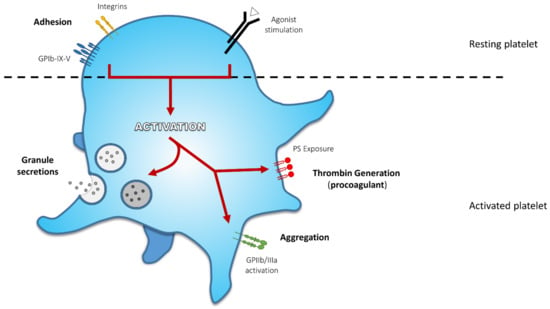
Figure 1.
Principal Activation Endpoints During Platelet Activation. At first, platelet receptors interact with adhesive agonists exposed at the site of lesion: von Willebrand factor (VWF) binds to glycoprotein (GP) Ib-IX-V complex and collagen interacts with integrin α2β1 for adhesion and GPVI to mediate platelet activation. These first interactions initiate platelet response. Soluble agonists released by either activated platelets or injured tissue amplify platelet response and activation. These agonists induce proper receptor activation and their signalling converge to activate a core set of intracellular signalling pathways leading to various activation endpoints, such as shape change and formation of pseudopodia, secretion of α-granule and dense granule content, activation of GPIIb/IIIa sustaining platelet aggregation, and externalization of negatively charged amino-phospholipids, contributing to platelet procoagulant activity (thrombin generation).
2.1. Adhesion
Under normal physiological conditions, the endothelium does not provide an adhesive surface for platelets. However, in the presence of vascular damage, the sub-endothelial matrix and/or layer(s) become exposed, revealing collagen and tissue factor, which are powerful haemostatic activators. The main function of platelet receptor GPIb-IX-V is to mediate the initial adhesion of circulating platelets to VWF adhered on the exposed collagen [9]. Four subunits compose the GPIb-IX-V complex: GPIbα, GPIbβ, GPIX, and GPV (encoded by four different genes GPIBA, GPIBB, GP9, and GP5) [10,11]. The N-terminal domain of GPIbα subunit has a binding site for VWF, which acts as a bridge between platelets and the fibrils of collagen in the sub-endothelial matrix and/or layer(s). This interaction is particularly important in the presence of high shear stress, in order to: i) slow down platelets in the blood stream, ii) recruit them to the site of the injury and iii) initiate the signalling cascades that will lead to platelet activation [12]. In addition to VWF, the same N-terminal domain of GPIα offers a binding site for multiple ligands, which are critical for normal or pathological haemostasis. For instance, the GPIb-IX-V complex binds to P-selectin [13] (which is present on activated platelets and endothelial cells) and to leukocyte integrin aMB2 [14], thus regulating both the recruitment of leukocytes at the site of vascular injury [15] and the complex interactions between platelets and leukocytes in thrombosis and response to inflammation [16]. In addition, the GPIb-IX-V receptor has procoagulant functions, since it mediates platelet dependent coagulation through the binding of α-thrombin, coagulation factors XI (FXI) and XII (FXII), and high molecular weight kininogen [17]. Finally, The GPIb-IX-V complex is anchored to the actin filaments of the platelets’ cytoskeleton through the binding of GPIbα cytoplasmic tail to filamin A [18]. This interaction is important for maintaining platelet shape and stability [19,20]. Defects and/or dysfunctions of this multitasker receptor have major consequences in platelet functions.
2.1.1. Bernard-Soulier Syndrome
Bernard Soulier Syndrome (BSS) is an inherited bleeding disorders characterized by bleeding tendency, macro-thrombocytopenia, and defective ristocetin-induced platelet agglutination [21,22,23].
Clinical features of patients with BSS are non-specific and characterized by epistaxis, mucocutaneous and post trauma bleedings, and severe menorrhagia in females [17,24].
In most patients, BSS has an autosomal recessive pattern of inheritance, but rare forms with autosomal dominant pattern are also known [25,26]. A large number of mutations (missense, nonsense or deletions) in genes GPIBA, GPIBB, and GP9 (but not in GP5 [27]) have been mapped and found to be causative of BSS [17,24]. In fact, these genes (GPIBA, GPIBB, and GP9) are required to express efficiently the functional GPIb-IX-V complex at the platelets’ surface. In BSS platelets, the GPIb-IX-V complex is either low, absent or dysfunctional (i.e., unable to bind VWF). Thus, in BSS platelets, the normal interaction of GPIbα with VWF is abolished and platelets’ adhesion to the sub-endothelium is impaired [28]. In addition, BSS platelets show other characteristics, such as an increased membrane deformability, a poor response to low doses of thrombin, and a decreased ability to support thrombin generation [29,30,31]. All these features can be related to the absence/dysfunction of GPIb-IX-V complex [17].
The clinical suspicion of BSS has to be confirmed by different laboratory investigations. A variable degree of thrombocytopenia (platelet count range: <30 × 109/L to normal [22,24]) might be observed, with a blood smear revealing abnormally large or irregularly shaped platelets (even in patients with normal platelet count) [32,33]. The closure time measured by the platelet function analyser (PFA-100/200) is increased and the bleeding time prolonged [34,35]. However, the sensitivity of PFA-100/200 assay depends on the severity of the defect [15,36], which implies further investigations (aggregometry and/or flow cytometry) to establish an accurate diagnosis. The VWF-dependent agglutination measured in the presence of ristocetin by light transmission aggregometry (LTA) is defective in homozygous BSS platelets (but normal in heterozygous form) [33]. Of note, this defect will not be rescued by the addition of normal plasma, which distinguishes BSS from von Willebrand disease (VWD) [35,37]. In vitro aggregation of BSS platelets in response to epinephrine, ADP, collagen, and arachidonic acid is normal, but a slow response is observed with low doses of thrombin [33]. The expression of GPIb-IX-V complex at the platelet surface can be assessed by flow cytometry. The specific antibody anti-CD42b directed against GPIbα is reduced or absent in BSS platelets, while the expression of CD41 (GPIIb) and CD61 (GPIIIa)–the two components of the fibrinogen receptor (also named integrin αIIbβ3)–is normal [32,38]. Finally, in BSS platelets, the expression of GPIb-IX-V could apparently be normal because of the enlarged surface of the platelets, but the ratio between GPI-IX-V and GPIIb-IIIa will always be decreased compared to normal platelets [33].
2.1.2. Platelet Type von Willebrand’s Disease
Platelet type pseudo-von Willebrand’s disease (PT-VWD) is a rare autosomal dominant disorder with a mild to moderate bleeding phenotype, intermittent thrombocytopenia, and enlarged platelets.
PT-VWD is characterized by mutations in GP1BA [39], which enhance the affinity of the surface glycoprotein GPIbα for the VWF multimers. As a result, spontaneous binding of high molecular weight VWF to platelets occurs in vivo, leading to platelet clumping and increasing platelet clearance [40]. This causes thrombocytopenia and removal from plasma of the largest VWF multimers (which have the greatest haemostatic capacity), leading to an increased bleeding risk.
At laboratory work-up, patients with PT-VWD often have a prolonged bleeding time and platelet clumping can be observed on blood smears. The response of PT-VWD platelets to low doses of ristocetin is enhanced and the VWF multimers analysis (which assesses concentration and distribution of VWF multimers in plasma) shows loss/reduction of the largest VWF forms. PT-VWD phenotype is very similar to type 2B VWD. However, in type 2B VWD, the defect lies in the VWF molecules, which have an increased affinity for platelets. Differential diagnosis is fundamental for the correct therapy of PT-VWF or VWD 2B patients. The two conditions can be distinguished by (i) ristocetin induced platelets agglutination (RIPA) mixing experiments, (ii) cryoprecipitate challenge, and iii) flow cytometry. In the RIPA assay, washed or gel-filtered platelets from the patient are mixed with normal plasma and vice versa (i.e., normal platelets are mixed with patient plasma) in presence of low dose ristocetin. Washed/gel-filtered platelets from PT-VWD patients (but not VWD 2B platelets) will agglutinate in normal plasma (because of the abnormal GPIbα avidity for VWF characteristic of PT-VWD) and washed/gel-filtered normal platelets will aggregate in the presence of VWD 2B plasma (containing the hyper-adhesive VWF) [41,42]. Of note, in the negative control (washed/gel-filtered platelets + plasma from a healthy donor) there is no aggregation at low doses of ristocetin. The cryoprecipitate challenge [43] consists in the addition of high concentrate normal VWF to platelets, which causes PT-VWF spontaneous aggregation, but not for VWD 2B platelets; however, false positive results have been observed among VWD 2B patients [44] and this test is not included in the diagnostic algorithm proposed for PT-VWD diagnostic work-up [45]. A flow cytometry method able to highlight the increased affinity of VWF for GPIbα and to discriminate between PT-VWD and VWD 2B through mixing tests has also been proposed [46]. Finally, the identification of mutations in the GPIBA gene will confirm the diagnosis of PT-VWD [45].
2.2. Secretion
The secretion of bioactive molecules is one of the characteristics of platelet activation. Once a platelet agonist has engaged its corresponding platelet surface receptor, a signal transduction takes place, leading to a short-time increase of intracellular calcium, which promotes platelet shape change, fusion of platelet granules with the plasma membrane and consequent release of platelet contents [47]. Platelets contain three major types of granules, which are in order of abundance, α-granules (50–80/platelet), dense-granules (3–8/platelet), and lysosomes (1–3/platelet) [48]. α- and dense-granules seem to derive, like lysosomes, from multivesicular precursors [49,50,51]. The content of α-granules consists of a large variety of proteins, such as adhesive molecules (e.g., fibrinogen, VWF, fibronectin, P-selectin), coagulation factors (e.g., FV, FIX, FXIII), anticoagulants (e.g., antithrombin), fibrinolytic proteins (e.g., plasminogen), and growth factors, immune mediators, and integral membrane proteins (e.g., αIIbβ3, P-selectin) [52,53]. Thus, α-granule proteins can be involved in a large spectrum of physiological functions, such as normal and pathological haemostasis, inflammation, wound healing, antimicrobial response, and cancer metastasis [54,55]. Dense-granules contain small non-protein molecules, such as nucleotides (ADP/ATP), serotonin, histamine, calcium ions (which give the dense appearance on electron microscopy), inorganic polyphosphates, membrane proteins [such as granulophysin (CD63), lysosomal-associated membrane protein 2 (LAMP-2)], [55]); plasma membrane adhesive receptors GPIb and αIIbβ3 have also been identified on dense-granules by immune-histochemical studies [56]. The main function of dense-granules content is to amplify platelet activation and to sustain platelet aggregation [57]. Lysosomes store digestive enzyme involved in the degradation of proteins, carbohydrates and lipids. Their role in haemostasis and thrombosis is still unknown [55].
Platelet storage pool deficiencies (SPD) refer to a group of inherited heterogeneous disorders in which the number and/or the content of α-granules, dense-granules, or both are reduced and cannot be adequately released during platelet activation [58,59]; as a consequence, a defect mostly in ADP release from activated platelet and in secretion-dependent aggregation is observed [60]. According to the type of granule pool deficiency, the clinical syndrome is called α-SPD, δ-SPD or αδ-SPD [58,61] These anomalies are to be distinguished from secretion defects, in which granules are normally present, but abnormally secreted due to defective signal transduction or granule trafficking defects [62].
2.2.1. α-Storage Pool Disease or Gray Platelet Syndrome
The Gray platelet syndrome (GPS) is a very rare disease characterized by a quantitative and qualitative deficiency of α-granules [63,64]. Patients with GPS have a mild to moderate bleeding diathesis, mild but progressive thrombocytopenia, and presence of larger and vacuolated platelets [65]. The associated phenotype is the presence of bone marrow fibrosis (due to the release of megakaryocytes in the bone marrow environment) and of splenomegaly (due to extramedullary haematopoiesis) [65,66,67]. Other features of GPS have been linked to immune dysregulation and autoimmune defects [68].
GPS megakaryocytes show a defect in α-granule production and are unable to correctly pack and store endogenous and exogenous proteins into α-granule precursors [69]. The lack of soluble proteins within α-granules, whose content is fundamental for normal haemostasis, leads to a small and unstable platelet plug [70]. The classical GPS is inherited with an autosomal-recessive pattern and it is associated with mutations or splicing alterations in NBEAL2 gene, involved in granule trafficking [71] and retention of cargo proteins in maturing α-granules [72]. Other GPS forms with autosomal-dominant or X-linked inheritance have been reported (reviewed in [73]).
The absence of α-granules in the cytoplasm of affected platelets results in a characteristic pale or gray appearance, opposite to the purple staining of granules in normal platelets on Wright-Giemsa stained blood smears. Platelet aggregation analysis by LTA is variable: in most of the GPS patients, the responses to ADP, epinephrine, and acid arachidonic are normal, while responses to thrombin and collagen are decreased [65]. Of note, content and surface expression of P-selectin are variable and their assessment is inadequate for diagnostic purposes [65,74,75,76,77]. The diagnosis is confirmed by the lack of α-granules observed by electron microscopy and by the absence of α-granule proteins [78,79].
2.2.2. δ-Storage Pool Disease
δ-Storage pool disease (δ-SPD) is a congenital abnormality characterized by a deficiency of dense-granules in megakaryocytes and platelets [79]. δ-SPD can be associated with disorders of others lysosome related organelles leading to syndromic forms, known as Hermansky-Pudlack, Chediak-Higashi, and Grisicelli syndromes, in which albinism and immune deficiency are associated with platelet function defects [80]. Patients with non-syndromic δ-SPD have a mild to moderate bleeding diathesis, mainly mucocutaneous; however life-threatening bleedings can occur after surgery or trauma [81]. Clinical presentation of δ-SPD is highly variable and so far there are no validated recommendations concerning the decisional algorithm to reach an accurate diagnosis [81], nor for δ-SPD management [82].
Patients with δ-SPD usually have normal platelet counts with a prolonged bleeding time [83]. The lack of dense-granules (and thus of ADP/ATP and serotonin) will be reflected by an impaired aggregation response to different agonists in vitro. Typically, LTA curves performed with citrated platelet rich plasma (PRP) are characterized by the absence of a second wave in ADP induced platelet aggregation and a diminished response to collagen induced aggregation (at low concentrations) [79,81]. However, a study reported that δ-SPD patients (23% of the cohort studied) had normal aggregation response [84]. Thus, further specialized tests are sometimes needed to confirm the diagnosis. In particular, whole mount transmission electron microscopy can be used to highlight the absence/reduction of dense-granules [85], while flow cytometry, by the mepacrine test uptake, is useful to evaluate the dense-granule content and secretion capacity of platelets. The mepacrine test is based on the fact that mepacrine binds to adenine nucleotides and accumulates rapidly in dense-granules. The mepacrine taken up by dense-granules is then released after platelet stimulation and fluorescence can be quantified before and after platelet activation [86,87]. δ-SPD platelets will have reduced dense-granules and low uptake and release of mepacrine [79,88,89]. Platelet content of adenine nucleotides and serotonin can be evaluated by chemiluminescence aggregometry and radio-labelled or chemical methods, respectively (reviewed in [59,81]). δ-SPD platelets will be characterized by reduced adenine nucleotides and serotonin content, with an elevated ATP/ADP ratio [90].
2.3. Aggregation
Platelet aggregation is mediated by the GPIIb/IIIa (integrin αIIbβ3), a major receptor of the platelet surface, whose activated form binds to fibrinogen. Surface expression of GPIIb/IIIa increases after platelet activation. Upon agonist induced platelet activation, a signalling process (“inside-out” signalling) leads to conformational changes of the GPIIb/IIIa receptor, which increases its affinity for fibrinogen. The binding of fibrinogen with platelet GPIIb/IIIa receptors allows platelet aggregation (leading to the primary platelet plug), providing primary haemostasis. Binding of fibrinogen to the GPIIb/IIIa receptor initiates further intracellular signalling (“outside-in”) which induces additional granule secretion, platelet spreading, and contraction of the fibrin mesh. This signalling pathway culminates in a stable and irreversible aggregation of platelets [47,91].
Glanzmann Thrombasthenia
Glanzmann thrombasthenia (GT) is a rare autosomal inherited bleeding disorder, characterized by a quantitative or qualitative defect in integrin αIIbβ3, also known as glycoprotein GPIIb/IIIa, which is essential for platelet aggregation and normal haemostasis.
GT is caused by mutations in the genes ITGA2B and ITGB3, which encode for subunits αIIb (GPIIb, CD41) and β3 (GPIIIa, CD61), respectively, of integrin αIIbβ3. Mutations in these genes compromise the normal function of the GPIIb/IIIa receptor, impairing platelet aggregation and interaction with its adhesive ligands and thus leading to inefficient clot formation/consolidation and to GT bleeding phenotype.
Bleeding tendency in patients with GT is highly variable and poorly correlated with the underlying genetic mutations or αIIbβ3 expression level [92]. It ranges from a mild to severe haemorrhagic condition [93,94]. Typical bleeding manifestations are purpura, gum bleeding and menorrhagia, while gastrointestinal or central nervous system bleeding are less frequently reported [95]; bleeding after trauma or surgery might be severe [93,96,97]. Most patients are diagnosed in childhood, but heterozygous patients can reach adulthood being asymptomatic [93]; in general, the bleeding tendency in GT decreases with age [98].
GT is divided in three subtypes [93,99] according to the GPIIb/IIIa expression (determined by flow cytometry [100]) on the platelet membrane:
- -
- ߓType I, the most severe form of GT: the expression of GPIIb/IIIa is absent (<5% of normal); platelet fibrinogen and clot retraction are also absent;
- -
- ߓType II, a moderate form of the disease: surface GPIIb/IIIa is reduced with a level of expression varying between 10–20% of normal; reduced fibrinogen content and clot retraction;
- -
- ߓType III, a variant form: the expression of GPIIb/IIIa is near normal or normal (between 50–100%), but the receptor is dysfunctional; variable platelet fibrinogen content and clot retraction.
GT platelets adhere normally to the sub-endothelium, but spreading is abnormal [101,102,103]. GT platelets have decreased or absent aggregation to physiological agonists, but agglutination in response to ristocetin is normal (because it is mediated by GPIb-IX-V via VWF). Since a functional GPIIb/IIIa is required for efficient dense-granules release, in GT platelets an abnormal release might also be observed [104,105]. Laboratory findings include a normal platelet count, size and granularity, but a prolonged bleeding time [35,98]. PFA-100/200 assay shows a very prolonged closure time (>300 s), which is compatible with GT, but not specific [36,98]. LTA is considered the gold standard method for the clinical diagnosis of GT [98]. GT PRP is analysed before and after the addition of different agonists, such as arachidonic acid, ADP, collagen, and epinephrine. The absence or marked reduced aggregation in response to low or high concentrations of multiple agonists, along with a maintained response to ristocetin, indicates a defect in GPIIb/IIIa and is highly indicative of GT [36,98]. Due to variability of platelet aggregation results, it is recommended that the analysis be confirmed with a second sample [98,106,107] and to use a second round of testing with a larger spectrum of agonists [106,107]. Flow cytometry can be used to assess the quantitative deficiency of GPIIb/IIIa (GT type I and II) in the membrane of resting platelets through the use of fluorescent probes recognizing αIIb (CD41) and/or β3 (CD61) subunits. The GT variant form, (GPIIb/IIIa expressed but not functional) can be investigated by flow cytometry using the monoclonal antibody PAC-1, which recognizes the activated form of the GPIIb/IIIa receptor after platelets stimulation. GT activated platelets will not bind with the PAC-1 monoclonal antibody, due to the dysfunctional GPIIb/IIIa receptor [107,108,109]. Finally, the identification of the specific mutation variants in ITGA2B and ITGB3 genes is the key to a complete diagnosis of GT [98,108].
2.4. Procoagulant Activity
Following strong activation, platelets expose negatively charged phospholipids on their outer membrane. This is essential in order to achieve an efficient haemostatic response by generating high amounts of thrombin and subsequent clot stabilization by fibrin. This peculiar platelet feature and its clinical role and relevance will be extensively described in the second part of this review.
3. Expression of Negatively Charged Phospholipids and Their Role in Coagulation
At resting state, the phospholipids of the cell membrane are asymmetrically distributed, thanks to flippase/floppase activity [110]. Neutral phospholipids (e.g., phosphatidylcholine, sphingomyelin, and sugar-linked sphingolipids) are located on the external leaflet of the membrane, while negatively charged phospholipids (phosphatidylserine (PS) and phosphatidylethanolamine) are within the inner surface of the membrane.
Under specific circumstances, such as apoptosis or strong platelet activation, this distribution is altered. During platelet activation, scramblases (such as TMEM16F, also known as anoctamin 6) shuffles the phospholipids between the two layers, resulting in the expression of PS on the external leaflet [110]. Despite similar endpoints, apoptotic-induced and agonist-induced PS exposure are two distinct pathways, both resulting in PS exposure (reviewed in [111]).
Apoptosis is a slow process (taking hours) that results with platelet aging and is mediated through the activation of caspases, pro-apoptotic Bak/Bax-mediated mitochondrial collapse, and PS exposure (mostly TMEM16F-independent) [112]. This slow process leads to platelet clearance.
Strong platelet activation induces a rapid (one–two minutes) necrotic-like phenotype via elevated and sustained cytosolic calcium concentration, mitochondrial depolarization, calpain activation, and TMEM16F-dependent PS exposure [113,114]. Plasma membranes form a small “cap” area enriched in exposed PS [115]. Such micro-domains concentrate blood coagulation factors and accelerate enzymatic reactions.
Indeed, in synchrony with platelet activation and aggregation, fibrin deposition is an important process for the stabilization of the haemostatic clot [116]. This is achieved by thrombin cleaving fibrinogen into fibrin as a consequence of a series of sequential reactions engaging activated coagulation factors, in which calcium and negatively charged phospholipids are critical mediators [117].
Some coagulation factors (factors II, VII, IX, X) experience vitamin-K dependent posttranslational ɣ-carboxylation of C-terminal glutamic acid residues [118,119]. These highly negative domains confer to factors high-affinity binding for calcium, which facilitates their interaction with negatively charged phospholipids. In fact, activated coagulation factors interact poorly with each other in solution. Calcium binding is instrumental for supporting binding of coagulation factors to a membrane of negatively-charge phospholipids, such as the surface of procoagulant platelets [120,121].
In addition to rapid phospholipid membrane remodelling and PS externalization, platelet procoagulant response is accompanied by the release of microparticles from the membrane surface of activated platelets [122,123]. The mechanisms underlying the formation of platelet derived microparticles (PMPs) involve the increase of cytoplasmic calcium affecting the activity of intracellular enzymes, the phospholipid transient mass imbalance between the two leaflets of the membrane, and the proteolytic action of calpain on the cytoskeleton [124]. PMPs shed from activated platelets provide a source of supplementary negatively charged surface on which blood coagulation factors can assemble, thereby enhancing the procoagulant response [122]. Dale et al. [125] showed that the number of PMPs produced by procoagulant platelet was higher than the number of PMPs produced by aggregating platelets but 5.4 times lower than PMPs originating from A23187 calcium ionophore activated platelets. Sinauridze et al. [126] studied the procoagulant properties of A23187-calcium ionophore activated platelets and PMPs. The authors showed that the surface of PMPs originated after A23187 activation is 50- to 100-fold more procoagulant than the surface of activated procoagulant platelets. This stronger procoagulant activity was related to a higher density of procoagulant phospholipids on PMPs’ membrane. From a physiological point of view, the observation that procoagulant collagen-and-thrombin (COAT) platelets produce less PMPs than ionophore does [125,127], might indicate that COAT platelet dependent thrombin generation (TG) should be contained at the site of vascular injury to avoid an unnecessary and dangerous systemic spread.
Taken together, the phospholipid surfaces enhance the enzymatic function of coagulation factors [128]. Membrane binding and surface diffusion facilitate and accelerate the encounter of coagulation partners (e.g., the assembly of tenase and prothrombinase complexes) [128]. This facilitates the rate of activation of prothrombin by several orders of magnitude. Therefore, the platelet contribution has a considerable impact on the procoagulant response, by localizing and enhancing thrombin generation directly at the site of vascular wall damage.
4. Procoagulant Platelets
Following strong activation, a fraction of platelets expresses PS on their surface and become highly efficient in sustaining thrombin generation.
Since the first descriptions in the late 1990s, several synonyms have been used (extensively described in recent reviews [129,130]) such as collagen-and-thrombin (COAT)-activated platelets [87,127,131], COATed platelets [132,133], ballooned and procoagulant platelets (BAPS) [134], sustained calcium-induced platelet morphology (SCIP) platelets [135], super-activated platelets [136], super platelets [137] and even zombie platelets [138,139]. Despite this diverse classification, they all share the very same characteristics of necrotic-like mechanisms [111,140], leading to procoagulant activity through expression of PS [130].
In particular, after strong activation, all platelets display an initial cytosolic calcium increase and GPIIb/IIIa activation [131]. However, after a certain delay (1–2 min), while aggregating, platelets decrease their calcium level, and procoagulant platelets reach higher cytosolic calcium concentration [131,141,142]. In addition to calcium mobilization from intracellular stores and store-operated calcium entry, calcium influx mediated by sodium-calcium exchanger (NCX) reverse mode is critical for achieving the high calcium level required to trigger the formation of the mitochondrial permeability transition pore (mPTP), leading to cyclophilin D-dependent mitochondrial depolarization [141,142,143]. This results in very high and sustained cytoplasmic calcium, gradual inactivation of GPIIb/IIIa receptors [131,144], activation of TMEM16F [113], and PS externalization [114,134], which eventually induces the procoagulant activity of platelets together with microparticle generation [47,127,134,145].
In addition to the procoagulant activity mediated through PS exposure, procoagulant platelets gain pro-haemostatic function by retaining α-granule proteins on their membranes, such as coagulation factor V/Va, fibrinogen, VWF, thrombospondin, fibronectin, and α2-antiplasmin in a serotonin- and transglutaminase-dependent mechanism [146].
4.1. Clinical Features of Procoagulant Platelets
The potential generation of procoagulant platelets is on average ca. 30% in healthy donors, with a wide range from 15–57% described in the literature [87,132,147,148]. In our laboratory, we have a mean of 38.9% (SD 8.3; range 21.9–59.1%, n = 73) ([149] and Adler et al., manuscript in preparation). However, despite a wide inter-person variability, the individual values are stable over time [132].
Clinical interest in procoagulant platelet potential has largely increased during the last two decades. Especially, stratification of this wide range could associate extreme values to clinically relevant medical situations, such as in haemostatic imbalances (bleeding or thrombotic events) or even in non-haemostatic circumstances.
4.1.1. Low Level of Procoagulant Platelets Is Associated with Impaired Platelet Function and Bleeding Diathesis
The Scott syndrome was the first clinically relevant bleeding disorder associated with impaired platelet procoagulant activity [150]. In this very rare congenital bleeding disorder, patients have impaired phospholipid scrambling and do not expose PS at the membrane surface even after treatment with calcium ionophores [151,152]. Besides this complete absence of PS exposure, a reduced ability to generate procoagulant platelets has been shown to increase bleeding risk. Of note, low levels of procoagulant platelets (<20%) were detectable in about 15% of patients with a clinically relevant bleeding diathesis and an unrevealing standard work-up, including LTA and secretion assays ([87,153] and Adler et al., manuscript in preparation).
Moreover, patients with spontaneous intracerebral haemorrhage have a significantly lower percentage of procoagulant platelets compared to controls (24.8 ± 9.7% vs. 32.9 ± 12.6%) [154]. In a similar cohort of patients, those who generated the lowest levels of procoagulant platelets encountered more severe haemorrhages with increased bleed volumes [155] and, in another study, patients with procoagulant platelet levels lower than 27% had a poor outcome and increased mortality at 30 days [156]. Similarly, patients with subarachnoid haemorrhage that generate procoagulant platelets in the lowest range of the cohort (<36.7%) faced an increased mortality rate after one month [157]. However, these patients had on average a higher level of procoagulant platelets compared to controls (41.8 ± 11.4% vs. 30.7 ± 12.2%). As discussed by the authors, this antithetical observation could be related to the presence of a chronic inflammation in this pathology (but whether inflammatory state amplifies the procoagulant activity or the other way around is difficult to clarify; see below).
Interestingly, even in some cerebral thrombotic pathologies, patients who generated procoagulant platelets in the lowest range of the cohort presented increased bleeding phenotypes, with more microbleeds [158] or early secondary bleeding into the ischemic brain area compared to the other patients from the same cohort [159].
Discordant observations were reported regarding platelet procoagulant potential in two cohorts of haemophilia A patients. Both studies reported a reduced potency in generating procoagulant platelets compared to controls [160,161]. However, while Saxena et al. [160] observed a significant difference of procoagulant platelet levels in relationship to the phenotype severity, this was not replicated by Lastrapes et al. [161].
A single study also reported an impaired ability to generate procoagulant platelet in patients with essential thrombocythemia compared to controls and this was rescued by hydroxyurea treatment [162].
4.1.2. High Level of Procoagulant Platelets Worsens Thrombotic Events
In contrast to the findings in bleeding phenotypes, it was demonstrated that patients with prothrombotic states had a higher potential to generate procoagulant platelets.
Mean levels of procoagulant platelets were elevated in patients with cortical strokes [163] or transient ischemic attack (TIA) [164]. Moreover, the stratification of procoagulant platelet levels increased their prognostic value. Higher levels of procoagulant platelets at the time of the cortical strokes (>34%) or TIA (>51%) were associated in both conditions with an increased incidence of stroke recurrences [165,166]. In patients with symptomatic large-artery disease, procoagulant platelet levels in the highest range of the cohort (≥50%) were associated with a higher risk for early ischemic events [167]. Similarly, for patients with asymptomatic carotid stenosis, higher levels of procoagulant platelets (≥45%) predicted a risk for stroke or TIA [168].
Contrary to the other brain ischemic situations, data showed lower mean levels of procoagulant platelets following lacunar stroke compared to non-lacunar or control levels [163]. Nevertheless, patients with higher procoagulant platelet levels (≥42.6%) experienced more recurrent ischemic events following lacunar stroke [169].
In addition to brain infarction, a high level of procoagulant platelets was also observed in coronary artery disease and heart failures [170,171,172].
Monitoring of procoagulant platelet potential, following an acute event, may also predict severe outcomes. A significant rise of procoagulant platelet generation after aneurysmal subarachnoid haemorrhage predicted delayed cerebral ischemia and worsening of cognitive and physical outcomes [173,174].
Higher mean levels of procoagulant platelets were also measured in cigarette smokers compared to non-smokers [147,169,175]. This is of particular interest as smoking is widely associated with an increased risk factor for cardiovascular diseases. Interestingly, smoking cessation was observed to lower the procoagulant platelet levels for individuals who quit smoking after a stroke in comparison to those who continued smoking [176].
4.1.3. Procoagulant Platelets in Non-Haemostatic Pathologies
Massive haemorrhage in trauma is a leading cause of morbidity and mortality. Interestingly, it was recently reported that these patients experienced an increase in circulating procoagulant (balloon-like) platelets, which is in line with an increased ability to generate thrombin and a reduction of platelet aggregation [177]. This work highlights that trauma contributes to the increase of the procoagulant phenotype by the release of histone H4 from injured tissues, and, very interestingly, the authors could identify a platelet procoagulant phenotype that is already present in vivo, in contrast to other studies where the procoagulant ability of platelets is usually appreciated with ex vivo stimulations.
Interestingly, procoagulant platelets are also able to retain full-length amyloid precursor protein on their surface [178]. Further studies related levels of procoagulant platelets with Alzheimer disease severity and progression. Higher levels of procoagulant platelets were measured in early stages of the disease [179], among patients with the most severe decline [180], and among amnesic subtypes of patients with mild cognitive impairment with a progression to Alzheimer disease [181,182].
High levels of procoagulant platelets were observed in patients with end-stage renal failure [183]. Authors associated this with an increased inflammation state, but the role of procoagulant platelets as marker or trigger of thrombosis in this situation needs further investigations. Moreover, the direct influence of inflammation on procoagulant platelets (or vice versa) is not fully understood and dissecting this clearly remains challenging. Of note, inflammation is able to directly activate the haemostatic system [184] and some authors reported a relationship between high levels of procoagulant platelets and inflammation or immune system activation [132,147,183]. However, necrotic-like phenotypes, such as in procoagulant platelets, are also known to activate inflammation and immune cells [111,185].
In transfusion medicine, a low level of procoagulant platelets was observed in platelet concentrates from apheresis (16%) [186], buffy-coat (8%) [187], or cryopreserved platelet concentrates (17%) [188].
4.2. Pharmacological Modulation of Procoagulant Platelets
Platelets play a very important role in arterial thrombosis. Various antiplatelet therapies have been developed to prevent thrombotic events. However, these drugs aim at inhibiting platelet aggregation and, thus far, poor attention has been paid to platelet procoagulant activity.
On the other hand, different clinically relevant pharmacologic molecules have already been shown to modulate generation of procoagulant platelets.
4.2.1. Antiplatelet Drugs
Aspirin (acetyl-salicylic acid) is universally used as a standard for secondary prevention of recurrent arterial ischemic events. It irreversibly acetylates the active site of cyclooxygenase-1 (COX-1), required for the production of the soluble platelet agonist thromboxane A2. Chronic use of aspirin reduces the levels of procoagulant platelets in individuals [140,147,176]. However, intermittent or short-term uses do not relevantly impact potency in generating procoagulant platelets. While long-term use of aspirin appears to have an effect on megakaryocyte physiology inducing impaired platelet function, the direct interference with thromboxane A2 signalling does not seem to have a direct impact on the generation of procoagulant platelets [189].
ADP is able to augment the procoagulant potential induced by combined platelet activation with strong agonists, such as collagen and thrombin [187,189,190]. Accordingly, inhibition of P2Y12 (but not P2Y1) with clopidogrel [176,190] and cangrelor [191] reduces the generation of procoagulant platelets [189]. A similar effect was observed in vitro with the active metabolite of prasugrel [192].
Some of the data is sparse on the in vitro use of antagonists of the GPIIb/IIIa and the effect on procoagulant platelets. One study demonstrated that pre-treatment with either eptifibatide, tirofiban, or abciximab augmented the potential to generate procoagulant platelets [193]. This could explain the failure of long-term use of oral GPIIb/IIIa-antagonists observed in the early 2000s [194]. However, the procoagulant potentiation obtained with GPIIb/IIIa-antagonists was not corroborated by others [149,195,196,197]. These discordant data were all obtained with in vitro pre-treatment. Directly investigating the ability to generate procoagulant platelets in patients under treatment with GPIIb/IIIa-antagonists would help to clarify these discrepancies.
4.2.2. Off-Target Procoagulant Platelet Modulation
Desmopressin (1-deamino-8-D-arginine vasopressin (DDAVP)), a synthetic analogue of vasopressin initially used to treat diabetes insipidus and enuresis nocturna, improves the haemostatic status of patients by raising plasma levels of VWF and coagulation factor VIII [198]. In addition, it has also been demonstrated in vitro that DDAVP is a weak inducer of procoagulant response of platelets [199] as well as arginine vasopressin [200]. This was corroborated with in vivo treatment of patients with mild platelet disorders [201]. In this study, DDAVP was able to increase generation of procoagulant platelets by enhancing calcium and sodium mobilization. A similar observation was made in cardiac surgery patients receiving DDAVP because of postoperative excessive bleeding [202].
Auranofin, a thioredoxin reductase inhibitor used to treat rheumatoid arthritis was reported to induce calcium overload and increased oxidative stress in platelets, which would contribute to a necrotic PS exposure [203].
Patients using selective serotonin reuptake inhibitors (SSRI) had significantly lower procoagulant platelet levels compared to individuals not taking SSRI [147]. Furthermore, citalopram, a SSRI, was demonstrated to impair GPVI-mediated platelet function [204]. This is supported by the importance of serotonin for the formation of procoagulant platelets [146,205] and the mild bleeding diathesis reported in patients under SSRI treatment [206].
Inhibition of the procoagulant response of platelets was also observed with tyrosine kinase inhibitors used in oncology [207,208,209,210]. These pharmaceuticals reduce formation of procoagulant platelets by inhibiting tyrosine signalling downstream of GPVI activation.
4.3. Laboratory Work-Up for Investigating Procoagulant Platelets
Procoagulant platelets can be easily detected and characterized in vitro with fluorescence labelling and therefore by using microscopy or flow cytometry. Flow cytometry assays allow quantification of the ability to generate procoagulant platelets (see above, Section 4.1 and Section 4.2) and to analyse phenotypically different platelet subpopulations. Moreover, flow cytometry is an accessible, easy, and rapid diagnostic tool for haematological diagnostic laboratories. Procoagulant activity can be appreciated as well with other assays, such as ex vivo platelet-dependent thrombin generation and flow chambers. However, these latter techniques are for now experimental methods and their diagnostic utility still needs more investigations. Finally, in vivo assays with animal models are also of high interest to study the thrombus distribution of procoagulant platelets and to understand better physiological and pathophysiological thrombus formation.
4.3.1. Quantification and Characterization of Procoagulant Platelets
Table 1 summarizes the main procoagulant activation endpoints and the markers used to detect and to discriminate the procoagulant platelet subpopulation, commonly used for flow cytometry. Surface expression of PS is the major standard activation endpoint widely recognized for procoagulant platelets. The gold standard assay to detect this event resides in the ability of the platelets to bind Annexin V [87,127] or lactadherin [211,212,213]. Another necrotic-like event that occurs in procoagulant platelets is the loss of the mitochondrial potential. This cytoplasmic event can be detected with mitochondrial probes like rhodamine derivatives, such as tetra-methyl-rhodamine methyl ester (TMRM) or tetra-methylrhodamine ethyl ester (TMRE) [131,142,214] or the carbocyanine JC-1 [140]. Rhodamine probes accumulate into intact mitochondria, but once platelets experience loss of the mitochondrial membrane potential, they escape and fluorescence decreases [215]. The JC-1 probe naturally exhibits green fluorescence. Its accumulation into intact mitochondria induces formation of probe aggregates that induce a fluorescence emission shift from green to red. Therefore, the red/green fluorescence intensity ratio is an indicator of the mitochondrial potential allowing the detection of mitochondrial depolarization by a decrease in the red/green fluorescence ratio [215].

Table 1.
Activation endpoints of procoagulant platelets and common flow cytometry markers to detect and discriminate them.
Because procoagulant platelets lose their properties to aggregate, the PAC-1 binding assay is another interesting approach to discriminate procoagulant platelets from non-coagulant aggregating platelets [131,142,216,217].
Last but not least, procoagulant endpoint is the coating of α-granule proteins on the surface of procoagulant COAT platelets [127,146,218,219]. This approach relies on the analysis of the surface retention of α-granule proteins with specific monoclonal antibodies. This technique is not often employed by clinical diagnostic laboratories, but can be performed in research laboratories, as it requires a specialized method and technical expertise to detect it properly.
4.3.2. Assessment of the Overall Coagulation Potential and Procoagulant Activity of Platelets
An arsenal of different complementary methods, which we have briefly summarized in Table 2, are available to assess the procoagulant potential in biological samples. The procoagulant activity of PS expressed by platelets and PMPs can be directly measured in plasma by functional tests (clot or chromogenic based assays), which take advantage of the anionic phospholipid dependent acceleration exerted by PS on prothrombin activation by the FXa-FVa complex [220,221].

Table 2.
A non-exhaustive list of techniques to assess coagulation potential and procoagulant activity.
Thrombin generation assay (TGA) is a sophisticated technique capable of assessing the delicate balance of procoagulant and anticoagulant pathways involved in the haemostatic process, thus providing a global view of the coagulation potential of an individual. The standard reference method for measuring thrombin generation (TG) is the calibrated automated thrombogram (CAT) developed by Hemker [222]. TGA can be performed using various types of biological material: most commonly, the assay is performed in PRP or platelet poor plasma (PPP). PRP is useful to study the interaction of platelets with coagulation factors in the coagulation process. Working with PPP requires the addition of artificial phospholipids to the sample (as substitute for platelets in order to provide the negatively charged surface that sustains TG); PPP investigation focuses on the action of coagulation factors. A particular advantage of PPP is that the sample can be frozen (thus allowing storage) and thawed just before analysis. The measurement is performed in the presence of defined concentrations of tissue factor (low, normal or high), allowing the modulation of the sensitivity of the test (e.g., high concentration of tissue factor will make the test less sensitive to the intrinsic pathway). Thrombomodulin-modified TGA is a novel variant of the classical TGA, which allows the highlighting of the role of the protein C system in downregulating the coagulation process [223]. This might be of interest for investigating platelet-dependent TG because it has been demonstrated that platelet-derived activated coagulation factor Va (FVa) bound on the surface of procoagulant platelets is protected from inactivation catalysed by activated protein C [224]. Finally, interesting and innovative technologies based on a spatio-temporal model of haemostasis, have been used to measure the contribution of procoagulant platelets or PMPs to the growth of the fibrin clot [126].
A step closer to physiological coagulation is represented by ex vivo TG measurement in whole blood. However, this method is challenging due to the interference of erythrocytes on the stability of fluorescence signal and requires expert operators. An alternative method to overcome the problem of the turbidity or colour of the blood sample is based on monitoring TG by electrochemistry. Such a method was developed by Thuerlemann et al. [225] using a single-use electrochemical biosensor sensible to the electric variations produced by an amperogenic substrate cleaved by thrombin. The variation of electric signal is recorded and the raw data values used to build a TG curve.
To exclude the effect of plasmatic factors, platelets can be isolated by gel filtration [201] or washing steps [131]. The specific contribution of procoagulant platelets to TG can be assessed by modified TG assays [126,201]. Gel filtered/washed platelets, once activated with specific agonists to the procoagulant phenotype, also generate procoagulant PMPs. The latter can be directly identified and investigated by flow cytometry based on their size (FSC) and specific fluorescent dye binding to exposed PS [125]. Flow cytometry is a powerful and preferred technique for investigating PMPs [226], since it allows counting, identifying their origin, and determining PS exposure by Annexin V binding [227]. Drawbacks of PMP measurements with flow cytometry are the small and heterogeneous size (0.1 to 1 μm) of PMPs, which can be very close to the instrument background and the difficult of calibration. It is possible to overcome these limitations by using fluorochrome tagging PMPs (e.g., molecules incorporating the phospholipid bilayer) and size-calibrated fluorescent beads together with background noise reduction (through 0.1 μm liquid filtration). Nevertheless a good expertise and high resolution flow cytometers are required [227]. PMPs generated from procoagulant platelets can be further processed to obtain a pure PMP preparation by subsequent centrifugation steps and used to measure PMP-dependent TG [126].
4.3.3. In-Vivo Investigations of Procoagulant Platelets
Intravital microscopy permits the study of physiological haemostasis and the appreciation the heterogeneous structure of a growing thrombus [243,244]. More and more publications are present in the literature assessing the heterogeneous platelet activation status with a particular focus on the role of procoagulant platelets [134,245,246]. Very recently, Nechipurenko et al. demonstrated that, during the in vivo formation of the thrombus, the procoagulant platelets are located at the periphery of the clot, which is driven by their mechanical extrusion as a result of the clot contraction [247]. These increasing new data provided by intravital microscopy and future experimentation with genetically-engineered mouse models, such as TMEM16F-deficient mice [246], will increase our knowledge on the in vivo role of procoagulant platelets. Obviously, this can be extended to other thrombocytopathies, where we can also obtain a real time monitoring of thrombus formation in pathophysiological conditions [248,249,250].
Nevertheless, one should be aware that such experiments still lack standardization, and inter-laboratory replicability is laborious. We should also keep in mind that even though this technique allows a step closer in studying haemostasis and thrombosis, experiments have thus far been performed with non-physiological injuries and in murine models.
5. Thrombocytopathy Associated to COVID-19
The current ongoing outbreak of coronavirus disease 2019 (COVID-19) is caused by a viral infection from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [251]. Even though SARS-CoV-2 infection initially results in excessive inflammation and mild to acute respiratory distress syndrome, patients also experience a hypercoagulable state characterized by immuno-thrombosis [252,253]. Therefore, venous and arterial thrombotic complications are an important cause of morbidity and mortality in COVID-19 patients [254].
Although the research on mechanisms implicated on platelet dysfunction in COVID-19 is still ongoing, at the time of this review there is some emerging evidence of COVID-19-associated thrombocytopathy [255,256,257,258]. In addition to a mild thrombocytopenia, which is frequent among COVID-19 patients, studies have described altered platelet function and reactivity [257,259,260,261].
Platelets seem to circulate in an activated state as demonstrated by a higher expression of specific platelet activation markers, such as P-selectin (CD62P), LAMP-3, and GPIIb/IIIa in unstimulated platelets from COVID-19 patients compared to healthy controls [259,261,262]. Platelets from SARS-CoV-2 infected patients increased basal reactive oxygen species (ROS), but basal surface expression of PS was not altered [261,263].
In addition, platelets from COVID-19 patients are hyper-responsive. Platelets had increased aggregation response to subthreshold concentrations of agonists, as well as increased adhesion and spreading [259,260,261]. This could be linked to the observed increased expression of adhesive receptors, such as VWF- and fibrinogen-receptors, respectively GPIbα/GPIX and GPIIb/IIIa [259]. Of note, COVID-19 patients had a reduced procoagulant platelet response ex vivo [263]. This was observed with a reduced mitochondrial depolarization and externalization of PS, compared to controls.
Mechanisms leading to thrombocytopathy in COVID-19 still need to be understood. However, based on the literature, platelet hyper-responsiveness may be induced by increased circulating VWF (endothelial injury) [264], hypoxia [265,266,267], and/or a hyperinflammatory environment with high cytokine levels [268,269,270], and increased oxidative stress [271].
On current observations, it seems that procoagulant platelets should not contribute to the pathophysiology of COVID-19 patients, but the hyperreactive adhesion and aggregation may be implicated.
6. Conclusions
Thrombocytopathies are a diagnostic challenge. The introduction of flow cytometry, as an extension to routine diagnostic work-up by LTA and secretion assays, greatly improved management of patients with a bleeding diathesis in whom previous laboratory analysis could not identify a cause [87]. Moreover, in addition to the traditional platelet aggregation assays, flow cytometry has the advantage of rapidly acquiring intrinsic properties from thousands of single platelets, of requiring small blood volumes thus enabling the analysis of samples from thrombocytopenic patients, and the exploration of more than only one endpoint of the heterogeneous profiles, as performed with traditional aggregation assays. Flow cytometry is therefore able to point out surface membrane receptor deficiencies, such as BSS (adhesion endpoint) or GT (aggregation endpoint), as well as secretion endpoint defects (dense granule content and secretion by means of mepacrine, or alpha-granules, by investigating e.g., VWF content or surface expression of P-selectin). Finally, as highlighted in this review, flow cytometry is also able to cover the important procoagulant aspect of the pleomorphic platelet activation endpoints.
Wide systematic investigation of the procoagulant activity of platelets is increasingly described in the literature. This accumulating evidence indicates that the ability to generate procoagulant platelets at and beyond the extremes of the wide normal reference range [87] is associated with clinically relevant bleeding or thrombotic disease. Specifically, the generation of procoagulant platelets at levels <20% or >50% seems to worsen bleeding or thrombotic episodes, respectively. Moreover, the individual potential to generate procoagulant platelets at the time of the clinical event (e.g., stroke) seems to be strongly related to prognosis. It remains to be investigated whether an individual baseline potential to generate high or low level procoagulant platelets would also be a risk stratification for cardiovascular diseases before their clinical manifestation.
However, most of the publications were monocentric pilot studies and/or performed with relatively small cohort sizes and/or with short follow-up timeframes. The flow cytometric investigation of procoagulant platelets still needs standardization to allow proper meta-analysis and generalization of its use. In parallel, future research and experimentation on the procoagulant status of platelets and in vivo thrombus formation models will help to better appreciate the crucial role of procoagulant platelets in haemostatic diseases. These approaches will help to dissect the role of procoagulant platelets in thrombotic and haemorrhagic events.
Author Contributions
Conceptualization, A.A., D.B.C. and L.A.; Funding acquisition, A.A. and L.A.; Supervision, L.A.; Writing—original draft, A.A., D.B.C. and L.A.; Writing—review & editing, A.A., D.B.C., M.G.Z., M.M. and L.A. All authors have read and agreed to the published version of the manuscript.
Funding
Our research is supported by grants from Dr. Henri Dubois-Ferrière Dinu Lipatti Foundation, Novartis Foundation for Medical-Biological Research (Grant #18B074), Swiss Heart Foundation (Grant FF19117), and the Swiss National Science Foundation (SNSF grant 320030-197392).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Quach, M.E.; Chen, W.; Li, R. Mechanisms of platelet clearance and translation to improve platelet storage. Blood 2018, 131, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Jobe, S.M.; Di Paola, J. Congenital and acquired disorders of platelet function and number. In Consultative Hemostasis and Thrombosis; Elsevier: Amsterdam, The Netherlands, 2019; pp. 145–166. [Google Scholar]
- Shen, Y.M.; Frenkel, E.P. Acquired platelet dysfunction. Hematol. Oncol. Clin. North. Am. 2007, 21, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Cherry-Bukowiec, J.; Napolitano, L. What platelet disorders occur in the intensive care unit and how should they be treated? In Evidence-Based Practice of Critical Care; Elsevier: Amsterdam, The Netherlands, 2010; pp. 645–660. [Google Scholar]
- Bye, A.P.; Unsworth, A.J.; Gibbins, J.M. Platelet signaling: A complex interplay between inhibitory and activatory networks. J. Thromb. Haemost. 2016, 14, 918–930. [Google Scholar] [CrossRef]
- Stegner, D.; Nieswandt, B. Platelet receptor signaling in thrombus formation. J. Mol. Med. 2011, 89, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk, J.W.; Bevers, E.M.; Lindhout, T. Platelet activation and blood coagulation. Thromb. Haemost. 2002, 88, 186–193. [Google Scholar] [CrossRef] [PubMed]
- van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef]
- Andrews, R.K.; Lopez, J.A.; Berndt, M.C. Molecular mechanisms of platelet adhesion and activation. Int. J. Biochem. Cell Biol. 1997, 29, 91–105. [Google Scholar] [CrossRef]
- Lopez, J.A.; Dong, J.F. Structure and function of the glycoprotein ib-ix-v complex. Curr. Opin. Hematol. 1997, 4, 323–329. [Google Scholar] [CrossRef]
- Li, R.; Emsley, J. The organizing principle of the platelet glycoprotein ib-ix-v complex. J. Thromb. Haemost. 2013, 11, 605–614. [Google Scholar] [CrossRef]
- Andrews, R.K.; Berndt, M.C. The gpib-ix-v complex. In Platelets; Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 195–213. [Google Scholar]
- Romo, G.M.; Dong, J.F.; Schade, A.J.; Gardiner, E.E.; Kansas, G.S.; Li, C.Q.; McIntire, L.V.; Berndt, M.C.; Lopez, J.A. The glycoprotein ib-ix-v complex is a platelet counterreceptor for p-selectin. J. Exp. Med. 1999, 190, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.I.; Chen, Z.; Xu, H.; Li, C.Q.; Dong, J.; McIntire, L.V.; Ballantyne, C.M.; Zhang, L.; Furman, M.I.; Berndt, M.C.; et al. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin mac-1 (cd11b/cd18). J. Exp. Med. 2000, 192, 193–204. [Google Scholar] [CrossRef]
- Wang, Y.; Sakuma, M.; Chen, Z.; Ustinov, V.; Shi, C.; Croce, K.; Zago, A.C.; Lopez, J.; Andre, P.; Plow, E.; et al. Leukocyte engagement of platelet glycoprotein ibalpha via the integrin mac-1 is critical for the biological response to vascular injury. Circulation 2005, 112, 2993–3000. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Simon, D.I. Inflammation and thrombosis: The clot thickens. Circulation 2001, 103, 1718–1720. [Google Scholar] [CrossRef]
- Berndt, M.C.; Andrews, R.K. Bernard-soulier syndrome. Haematologica 2011, 96, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, F.; Pudas, R.; Heikkinen, O.; Permi, P.; Kilpelainen, I.; Munday, A.D.; Hartwig, J.H.; Stossel, T.P.; Ylanne, J. The structure of the gpib-filamin a complex. Blood 2006, 107, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Kanaji, T.; Russell, S.; Ware, J. Amelioration of the macrothrombocytopenia associated with the murine bernard-soulier syndrome. Blood 2002, 100, 2102–2107. [Google Scholar] [CrossRef]
- Cranmer, S.L.; Pikovski, I.; Mangin, P.; Thompson, P.E.; Domagala, T.; Frazzetto, M.; Salem, H.H.; Jackson, S.P. Identification of a unique filamin a binding region within the cytoplasmic domain of glycoprotein ibalpha. Biochem. J. 2005, 387, 849–858. [Google Scholar] [CrossRef]
- Nurden, A.T. Qualitative disorders of platelets and megakaryocytes. J. Thromb. Haemost. 2005, 3, 1773–1782. [Google Scholar] [CrossRef]
- Lopez, J.A.; Andrews, R.K.; Afshar-Kharghan, V.; Berndt, M.C. Bernard-soulier syndrome. Blood 1998, 91, 4397–4418. [Google Scholar] [CrossRef]
- Bernard, J.; Soulier, J.P. On a new variety of congenital thrombocytary hemo-ragiparous dystrophy. Sem. Hop. 1948, 24, 3217–3223. [Google Scholar] [PubMed]
- Lanza, F. Bernard-soulier syndrome (hemorrhagiparous thrombocytic dystrophy). Orphanet J. Rare Dis. 2006, 1, 46. [Google Scholar] [CrossRef]
- Savoia, A.; Kunishima, S.; De Rocco, D.; Zieger, B.; Rand, M.L.; Pujol-Moix, N.; Caliskan, U.; Tokgoz, H.; Pecci, A.; Noris, P.; et al. Spectrum of the mutations in bernard-soulier syndrome. Hum. Mutat. 2014, 35, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Lyle, V.A.; Cunningham, D. Mutation of leucine-57 to phenylalanine in a platelet glycoprotein ib alpha leucine tandem repeat occurring in patients with an autosomal dominant variant of bernard-soulier disease. Blood 1992, 79, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Kahn, M.L.; Diacovo, T.G.; Bainton, D.F.; Lanza, F.; Trejo, J.; Coughlin, S.R. Glycoprotein v-deficient platelets have undiminished thrombin responsiveness and do not exhibit a bernard-soulier phenotype. Blood 1999, 94, 4112–4121. [Google Scholar] [CrossRef]
- Weiss, H.J.; Tschopp, T.B.; Baumgartner, H.R.; Sussman, I.I.; Johnson, M.M.; Egan, J.J. Decreased adhesion of giant (bernard-soulier) platelets to subendothelium: Further implications on the role of the von willebrand factor in hemostasis. Am. J. Med. 1974, 57, 920–925. [Google Scholar] [CrossRef]
- Jamieson, G.A.; Okumura, T. Reduced thrombin binding and aggregation in bernard-soulier platelets. J. Clin. Investig. 1978, 61, 861–864. [Google Scholar] [CrossRef]
- Dormann, D.; Clemetson, K.J.; Kehrel, B.E. The gpib thrombin-binding site is essential for thrombin-induced platelet procoagulant activity. Blood 2000, 96, 2469–2478. [Google Scholar] [CrossRef]
- Bevers, E.M.; Comfurius, P.; Nieuwenhuis, H.K.; Levy-Toledano, S.; Enouf, J.; Belluci, S.; Caen, J.P.; Zwaal, R.F. Platelet prothrombin converting activity in hereditary disorders of platelet function. Br. J. Haematol. 1986, 63, 335–345. [Google Scholar] [CrossRef]
- Pham, A.; Wang, J. Bernard-soulier syndrome: An inherited platelet disorder. Arch. Pathol. Lab. Med. 2007, 131, 1834–1836. [Google Scholar] [CrossRef] [PubMed]
- Balduini, C.L.; Iolascon, A.; Savoia, A. Inherited thrombocytopenias: From genes to therapy. Haematologica 2002, 87, 860–880. [Google Scholar]
- Harrison, P.; Robinson, M.; Liesner, R.; Khair, K.; Cohen, H.; Mackie, I.; Machin, S. The pfa-100: A potential rapid screening tool for the assessment of platelet dysfunction. Clin. Lab. Haematol. 2002, 24, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Bolton-Maggs, P.H.; Chalmers, E.A.; Collins, P.W.; Harrison, P.; Kitchen, S.; Liesner, R.J.; Minford, A.; Mumford, A.D.; Parapia, L.A.; Perry, D.J.; et al. A review of inherited platelet disorders with guidelines for their management on behalf of the ukhcdo. Br. J. Haematol. 2006, 135, 603–633. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim-Kosta, M.; Alessi, M.C.; Hezard, N. Laboratory techniques used to diagnose constitutional platelet dysfunction. Hämostaseologie 2020, 40, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.K.; Berndt, M.C. Bernard-soulier syndrome: An update. Semin. Thromb. Hemost. 2013, 39, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Cohn, R.J.; Sherman, G.G.; Glencross, D.K. Flow cytometric analysis of platelet surface glycoproteins in the diagnosis of bernard-soulier syndrome. Pediatr. Hematol. Oncol. 1997, 14, 43–50. [Google Scholar] [CrossRef]
- Othman, M.; Emsley, J. Gene of the issue: Gp1ba gene mutations associated with bleeding. Platelets 2017, 28, 832–836. [Google Scholar] [CrossRef]
- Tait, A.S.; Cranmer, S.L.; Jackson, S.P.; Dawes, I.W.; Chong, B.H. Phenotype changes resulting in high-affinity binding of von willebrand factor to recombinant glycoprotein ib-ix: Analysis of the platelet-type von willebrand disease mutations. Blood 2001, 98, 1812–1818. [Google Scholar] [CrossRef] [PubMed]
- Othman, M. Platelet-type von willebrand disease: Three decades in the life of a rare bleeding disorder. Blood Rev. 2011, 25, 147–153. [Google Scholar] [CrossRef]
- Franchini, M.; Montagnana, M.; Lippi, G. Clinical, laboratory and therapeutic aspects of platelet-type von willebrand disease. Int. J. Lab. Hematol. 2008, 30, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Boselli, B.D.; Kupinski, J.M. In vivo interaction of von willebrand factor with platelets following cryoprecipitate transfusion in platelet-type von willebrand’s disease. Blood 1984, 63, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J. 2b or not 2b? What is the role of vwf in platelet-matrix interactions? And what is the role of the vwf:Cb in vwd diagnostics? These are the questions. J. Thromb. Haemost. 2006, 4, 892–894. [Google Scholar] [CrossRef]
- Othman, M.; Gresele, P. Guidance on the diagnosis and management of platelet-type von willebrand disease: A communication from the platelet physiology subcommittee of the isth. J. Thromb. Haemost. 2020, 18, 1855–1858. [Google Scholar] [CrossRef]
- Giannini, S.; Cecchetti, L.; Mezzasoma, A.M.; Gresele, P. Diagnosis of platelet-type von willebrand disease by flow cytometry. Haematologica 2010, 95, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, H.H.; Heemskerk, J.W.; Levi, M.; Reitsma, P.H. New fundamentals in hemostasis. Physiol Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef]
- Sharda, A.; Flaumenhaft, R. The life cycle of platelet granules. F1000Research 2018, 7, 236. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.S.; Heijnen, H.F.; Raposo, G. Lysosome-related organelles: Unusual compartments become mainstream. Curr. Opin. Cell Biol. 2013, 25, 495–505. [Google Scholar] [CrossRef]
- Heijnen, H.F.; Debili, N.; Vainchencker, W.; Breton-Gorius, J.; Geuze, H.J.; Sixma, J.J. Multivesicular bodies are an intermediate stage in the formation of platelet alpha-granules. Blood 1998, 91, 2313–2325. [Google Scholar] [CrossRef]
- Youssefian, T.; Cramer, E.M. Megakaryocyte dense granule components are sorted in multivesicular bodies. Blood 2000, 95, 4004–4007. [Google Scholar] [CrossRef] [PubMed]
- Maynard, D.M.; Heijnen, H.F.; Horne, M.K.; White, J.G.; Gahl, W.A. Proteomic analysis of platelet alpha-granules using mass spectrometry. J. Thromb. Haemost. 2007, 5, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Coppinger, J.A.; Cagney, G.; Toomey, S.; Kislinger, T.; Belton, O.; McRedmond, J.P.; Cahill, D.J.; Emili, A.; Fitzgerald, D.J.; Maguire, P.B. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood 2004, 103, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef]
- Flaumenhaft, R. Platelet secretion. In Platelets in Thrombotic and Non-Thrombotic Disorders; Gresele, P., Kleiman, N.S., Lopez, J.A., Page, C.P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 353–366. [Google Scholar]
- Youssefian, T.; Massé, J.-M.; Rendu, F.; Guichard, J.; Cramer, E.M. Platelet and megakaryocyte dense-granules contain glycoproteins ib and iib-iiia. Blood 1997, 89, 4047–4057. [Google Scholar] [CrossRef] [PubMed]
- Rendu, F.; Brohard-Bohn, B. The platelet release reaction: Granules’ constituents, secretion and functions. Platelets 2001, 12, 261–273. [Google Scholar] [CrossRef]
- Weiss, H.J.; Witte, L.D.; Kaplan, K.L.; Lages, B.A.; Chernoff, A.; Nossel, H.L.; Goodman, D.S.; Baumgartner, H.R. Heterogeneity in storage pool deficiency: Studies on granule-bound substances in 18 patients including variants deficient in alpha-granules, platelet factor 4, beta-thromboglobulin, and platelet-derived growth factor. Blood 1979, 54, 1296–1319. [Google Scholar] [CrossRef]
- Mumford, A.D.; Frelinger, A.L., 3rd; Gachet, C.; Gresele, P.; Noris, P.; Harrison, P.; Mezzano, D. A review of platelet secretion assays for the diagnosis of inherited platelet secretion disorders. Thromb. Haemost. 2015, 114, 14–25. [Google Scholar] [CrossRef]
- Nurden, A.; Nurden, P. Advances in our understanding of the molecular basis of disorders of platelet function. J. Thromb. Haemost. 2011, 9 (Suppl. S1), 76–91. [Google Scholar] [CrossRef] [PubMed]
- Sandrock, K.; Zieger, B. Current strategies in diagnosis of inherited storage pool defects. Transfus. Med. Hemother. 2010, 37, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, H.; van der Sluijs, P. Platelet secretory behaviour: As diverse as the granules... or not? J. Thromb. Haemost. 2015, 13, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Raccuglia, G. Gray platelet syndrome. A variety of qualitative platelet disorder. Am. J. Med. 1971, 51, 818–828. [Google Scholar] [CrossRef]
- Gerrard, J.M.; Phillips, D.R.; Rao, G.H.; Plow, E.F.; Walz, D.A.; Ross, R.; Harker, L.A.; White, J.G. Biochemical studies of two patients with the gray platelet syndrome. Selective deficiency of platelet alpha granules. J. Clin. Investig. 1980, 66, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Nurden, A.T.; Nurden, P. The gray platelet syndrome: Clinical spectrum of the disease. Blood Rev. 2007, 21, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Jantunen, E.; Hanninen, A.; Naukkarinen, A.; Vornanen, M.; Lahtinen, R. Gray platelet syndrome with splenomegaly and signs of extramedullary hematopoiesis: A case report with review of the literature. Am. J. Hematol. 1994, 46, 218–224. [Google Scholar] [CrossRef]
- Caen, J.P.; Deschamps, J.F.; Bodevin, E.; Bryckaert, M.C.; Dupuy, E.; Wasteson, A. Megakaryocytes and myelofibrosis in gray platelet syndrome. Nouv. Rev. Fr. Hematol. 1987, 29, 109–114. [Google Scholar] [PubMed]
- Sims, M.C.; Mayer, L.; Collins, J.H.; Bariana, T.K.; Megy, K.; Lavenu-Bombled, C.; Seyres, D.; Kollipara, L.; Burden, F.S.; Greene, D.; et al. Novel manifestations of immune dysregulation and granule defects in gray platelet syndrome. Blood 2020, 136, 1956–1967. [Google Scholar] [CrossRef] [PubMed]
- Breton-Gorius, J.; Vainchenker, W.; Nurden, A.; Levy-Toledano, S.; Caen, J. Defective alpha-granule production in megakaryocytes from gray platelet syndrome: Ultrastructural studies of bone marrow cells and megakaryocytes growing in culture from blood precursors. Am. J. Pathol. 1981, 102, 10–19. [Google Scholar] [PubMed]
- Simon, D.; Kunicki, T.; Nugent, D. Platelet function defects. Haemophilia 2008, 14, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Mayer, L.; Jasztal, M.; Pardo, M.; Aguera de Haro, S.; Collins, J.; Bariana, T.K.; Smethurst, P.A.; Grassi, L.; Petersen, R.; Nurden, P.; et al. Nbeal2 interacts with dock7, sec16a, and vac14. Blood 2018, 131, 1000–1011. [Google Scholar] [CrossRef]
- Lo, R.W.; Li, L.; Leung, R.; Pluthero, F.G.; Kahr, W.H.A. Nbeal2 (neurobeachin-like 2) is required for retention of cargo proteins by -granules during their production by megakaryocytes. Arter. Thromb. Vasc. Biol. 2018, 38, 2435–2447. [Google Scholar] [CrossRef]
- Nurden, A.T.; Nurden, P. Should any genetic defect affecting alpha-granules in platelets be classified as gray platelet syndrome? Am. J. Hematol. 2016, 91, 714–718. [Google Scholar] [CrossRef]
- Villeneuve, J.; Block, A.; Le Bousse-Kerdiles, M.C.; Lepreux, S.; Nurden, P.; Ripoche, J.; Nurden, A.T. Tissue inhibitors of matrix metalloproteinases in platelets and megakaryocytes: A novel organization for these secreted proteins. Exp. Hematol. 2009, 37, 849–856. [Google Scholar] [CrossRef]
- Rosa, J.P.; George, J.N.; Bainton, D.F.; Nurden, A.T.; Caen, J.P.; McEver, R.P. Gray platelet syndrome. Demonstration of alpha granule membranes that can fuse with the cell surface. J. Clin. Investig. 1987, 80, 1138–1146. [Google Scholar] [CrossRef]
- Drouin, A.; Favier, R.; Masse, J.M.; Debili, N.; Schmitt, A.; Elbim, C.; Guichard, J.; Adam, M.; Gougerot-Pocidalo, M.A.; Cramer, E.M. Newly recognized cellular abnormalities in the gray platelet syndrome. Blood 2001, 98, 1382–1391. [Google Scholar] [CrossRef]
- Lages, B.; Sussman, I.I.; Levine, S.P.; Coletti, D.; Weiss, H.J. Platelet alpha granule deficiency associated with decreased p-selectin and selective impairment of thrombin-induced activation in a new patient with gray platelet syndrome (alpha-storage pool deficiency). J. Lab. Clin. Med. 1997, 129, 364–375. [Google Scholar] [CrossRef]
- Shahraki, H.; Dorgalaleh, A.; Bain, B.J. Gray platelet syndrome (gps). In Congenital Bleeding Disorders: Diagnosis and Management; Dorgalaleh, A., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 379–396. [Google Scholar]
- Podda, G.; Femia, E.A.; Pugliano, M.; Cattaneo, M. Congenital defects of platelet function. Platelets 2012, 23, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Huizing, M.; Helip-Wooley, A.; Westbroek, W.; Gunay-Aygun, M.; Gahl, W.A. Disorders of lysosome-related organelle biogenesis: Clinical and molecular genetics. Annu Rev. Genom. Hum. Genet. 2008, 9, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, A.; Bordet, J.C.; Eckly, A.; Gachet, C. Platelet delta-storage pool disease: An update. J. Clin. Med. 2020, 9, 2508. [Google Scholar] [CrossRef] [PubMed]
- Woldie, I.; Guo, R.; Ososki, R.; Dyson, G.; Mohamad, S.; Raval, K.K.; Gabali, A.M. Clinical Characteristics of Patients Diagnosed with Delta Granule Platelet Storage Pool Deficiency (Δ-PSPD); The Detroit Medical Center (DMC): Detroit, MI, USA, 2017. [Google Scholar]
- Masliah-Planchon, J.; Darnige, L.; Bellucci, S. Molecular determinants of platelet delta storage pool deficiencies: An update. Br. J. Haematol. 2013, 160, 5–11. [Google Scholar] [CrossRef]
- Nieuwenhuis, H.K.; Akkerman, J.W.; Sixma, J.J. Patients with a prolonged bleeding time and normal aggregation tests may have storage pool deficiency: Studies on one hundred six patients. Blood 1987, 70, 620–623. [Google Scholar] [CrossRef]
- White, J.G. Use of the electron microscope for diagnosis of platelet disorders. Semin. Thromb. Hemost. 1998, 24, 163–168. [Google Scholar] [CrossRef]
- Wall, J.E.; Buijswilts, M.; Arnold, J.T.; Wang, W.; White, M.M.; Jennings, L.K.; Jackson, C.W. A flow cytometric assay using mepacrine for study of uptake and release of platelet dense granule contents. Br. J. Haematol. 1995, 89, 380–385. [Google Scholar] [CrossRef]
- Daskalakis, M.; Colucci, G.; Keller, P.; Rochat, S.; Silzle, T.; Biasiutti, F.D.; Barizzi, G.; Alberio, L. Decreased generation of procoagulant platelets detected by flow cytometric analysis in patients with bleeding diathesis. Cytometry B Clin. Cytom 2014, 86, 397–409. [Google Scholar] [CrossRef]
- Gordon, N.; Thom, J.; Cole, C.; Baker, R. Rapid detection of hereditary and acquired platelet storage pool deficiency by flow cytometry. Br. J. Haematol. 1995, 89, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Mullier, F.; Frotscher, B.; Briquel, M.E.; Toussaint, M.; Massin, F.; Lecompte, T.; Latger-Cannard, V. Usefulness of flow cytometric mepacrine uptake/release combined with cd63 assay in diagnosis of patients with suspected platelet dense granule disorder. Semin. Thromb. Hemost. 2016, 42, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Holmsen, H.; Weiss, H.J. Secretable storage pools in platelets. Annu. Rev. Med. 1979, 30, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Shattil, S.J.; Kashiwagi, H.; Pampori, N. Integrin signaling: The platelet paradigm. Blood 1998, 91, 2645–2657. [Google Scholar] [CrossRef]
- Poon, M.C.; Di Minno, G.; d’Oiron, R.; Zotz, R. New insights into the treatment of glanzmann thrombasthenia. Transfus. Med. Rev. 2016, 30, 92–99. [Google Scholar] [CrossRef]
- George, J.N.; Caen, J.P.; Nurden, A.T. Glanzmann’s thrombasthenia: The spectrum of clinical disease. Blood 1990, 75, 1383–1395. [Google Scholar] [CrossRef]
- D’Andrea, G.; Margaglione, M.; Glansmann’s Thrombasthemia Italian T. Glanzmann’s thrombasthenia: Modulation of clinical phenotype by alpha2c807t gene polymorphism. Haematologica 2003, 88, 1378–1382. [Google Scholar] [CrossRef]
- Di Minno, G.; Zotz, R.B.; d’Oiron, R.; Bindslev, N.; Di Minno, M.N.; Poon, M.C.; Glanzmann Thrombasthenia Registry Investigators. The international, prospective glanzmann thrombasthenia registry: Treatment modalities and outcomes of non-surgical bleeding episodes in patients with glanzmann thrombasthenia. Haematologica 2015, 100, 1031–1037. [Google Scholar] [CrossRef]
- Nurden, A.T. Glanzmann thrombasthenia. Orphanet J. Rare Dis. 2006, 1, 10. [Google Scholar] [CrossRef]
- Bellucci, S.; Caen, J. Molecular basis of glanzmann’s thrombasthenia and current strategies in treatment. Blood Rev. 2002, 16, 193–202. [Google Scholar] [CrossRef]
- Botero, J.P.; Lee, K.; Branchford, B.R.; Bray, P.F.; Freson, K.; Lambert, M.P.; Luo, M.; Mohan, S.; Ross, J.E.; Bergmeier, W.; et al. Glanzmann thrombasthenia: Genetic basis and clinical correlates. Haematologica 2020, 105, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Caen, J.P. Glanzmann’s thrombasthenia. Baillieres Clin. Haematol. 1989, 2, 609–625. [Google Scholar] [CrossRef]
- Linden, M.D.; Frelinger, A.L., 3rd; Barnard, M.R.; Przyklenk, K.; Furman, M.I.; Michelson, A.D. Application of flow cytometry to platelet disorders. Semin. Thromb. Hemost. 2004, 30, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Weiss, H.J.; Turitto, V.T.; Baumgartner, H.R. Further evidence that glycoprotein iib-iiia mediates platelet spreading on subendothelium. Thromb. Haemost. 1991, 65, 202–205. [Google Scholar] [CrossRef]
- Weiss, H.J.; Turitto, V.T.; Baumgartner, H.R. Platelet adhesion and thrombus formation on subendothelium in platelets deficient in glycoproteins iib-iiia, ib, and storage granules. Blood 1986, 67, 322–330. [Google Scholar] [CrossRef]
- Jurk, K.; Kehrel, B.E. Inherited and acquired disorders of platelet function. Transfus. Med. Hemotherapy 2007, 34, 6–19. [Google Scholar] [CrossRef]
- Gobbi, G.; Sponzilli, I.; Mirandola, P.; Tazzari, P.L.; Caimi, L.; Cacchioli, A.; Matteucci, A.; Giuliani Piccari, G.; Cocco, L.; Vitale, M. Efficient platelet delta-granule release induced by [Ca2+]i elevation is modulated by gpiibiiia. Int. J. Mol. Med. 2006, 18, 309–313. [Google Scholar]
- Dorgalaleh, A.; Poon, M.; Shiravand, Y. Glanzmann thrombasthenia. In Congenital Bleeding Disorders; Dorgalaleh, A., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Gresele, P.; Subcommittee on Platelet Physiology of the International Society on T. Hemostasis. Diagnosis of inherited platelet function disorders: Guidance from the ssc of the isth. J. Thromb. Haemost. 2015, 13, 314–322. [Google Scholar] [CrossRef]
- Alessi, M.C.; Sie, P.; Payrastre, B. Strengths and weaknesses of light transmission aggregometry in diagnosing hereditary platelet function disorders. J. Clin. Med. 2020, 9, 763. [Google Scholar] [CrossRef]
- Nurden, A.T.; Pillois, X.; Wilcox, D.A. Glanzmann thrombasthenia: State of the art and future directions. Semin Thromb. Hemost. 2013, 39, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Lanza, F.; Stierle, A.; Fournier, D.; Morales, M.; Andre, G.; Nurden, A.T.; Cazenave, J.P. A new variant of glanzmann’s thrombasthenia (strasbourg i). Platelets with functionally defective glycoprotein iib-iiia complexes and a glycoprotein iiia 214arg—214trp mutation. J. Clin. Investig. 1992, 89, 1995–2004. [Google Scholar] [CrossRef]
- Nagata, S.; Sakuragi, T.; Segawa, K. Flippase and scramblase for phosphatidylserine exposure. Curr. Opin. Immunol. 2020, 62, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Schoenwaelder, S.M. Procoagulant platelets: Are they necrotic? Blood 2010, 116, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- van Kruchten, R.; Mattheij, N.J.; Saunders, C.; Feijge, M.A.; Swieringa, F.; Wolfs, J.L.; Collins, P.W.; Heemskerk, J.W.; Bevers, E.M. Both tmem16f-dependent and tmem16f-independent pathways contribute to phosphatidylserine exposure in platelet apoptosis and platelet activation. Blood 2013, 121, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Umeda, M.; Sims, P.J.; Nagata, S. Calcium-dependent phospholipid scrambling by tmem16f. Nature 2010, 468, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, A.; David, T.; Palmer, D.; Jin, T.; Tien, J.; Huang, F.; Cheng, T.; Coughlin, S.R.; Jan, Y.N.; et al. Tmem16f forms a ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation. Cell 2012, 151, 111–122. [Google Scholar] [CrossRef]
- Podoplelova, N.A.; Sveshnikova, A.N.; Kotova, Y.N.; Eckly, A.; Receveur, N.; Nechipurenko, D.Y.; Obydennyi, S.I.; Kireev, I.I.; Gachet, C.; Ataullakhanov, F.I.; et al. Coagulation factors bound to procoagulant platelets concentrate in cap structures to promote clotting. Blood 2016, 128, 1745–1755. [Google Scholar] [CrossRef]
- Swieringa, F.; Spronk, H.M.H.; Heemskerk, J.W.M.; van der Meijden, P.E.J. Integrating platelet and coagulation activation in fibrin clot formation. Res. Pract. Thromb. Haemost. 2018, 2, 450–460. [Google Scholar] [CrossRef]
- Zwaal, R.F.; Comfurius, P.; Bevers, E.M. Lipid-protein interactions in blood coagulation. Biochim. Biophys. Acta 1998, 1376, 433–453. [Google Scholar] [CrossRef]
- Stenflo, J.; Fernlund, P.; Egan, W.; Roepstorff, P. Vitamin k dependent modifications of glutamic acid residues in prothrombin. Proc. Natl. Acad. Sci. USA 1974, 71, 2730–2733. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, C. Gamma-carboxyglutamate-containing proteins and the vitamin k-dependent carboxylase. Biochem. J. 1990, 266, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, Y.Z.; Tajkhorshid, E. Distinct structural and adhesive roles of ca2+ in membrane binding of blood coagulation factors. Structure 2008, 16, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Rigby, A.C.; Morelli, X.; Grant, M.A.; Huang, G.; Furie, B.; Seaton, B.; Furie, B.C. Structural basis of membrane binding by gla domains of vitamin k-dependent proteins. Nat. Struct. Biol. 2003, 10, 751–756. [Google Scholar] [CrossRef]
- Morel, O.; Toti, F.; Hugel, B.; Bakouboula, B.; Camoin-Jau, L.; Dignat-George, F.; Freyssinet, J.M. Procoagulant microparticles: Disrupting the vascular homeostasis equation? Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2594–2604. [Google Scholar] [CrossRef] [PubMed]
- Owens, A.P., 3rd; Mackman, N. Microparticles in hemostasis and thrombosis. Circ. Res. 2011, 108, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Morel, O.; Jesel, L.; Freyssinet, J.M.; Toti, F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 15–26. [Google Scholar] [CrossRef]
- Dale, G.L.; Remenyi, G.; Friese, P. Quantitation of microparticles released from coated-platelets. J. Thromb. Haemost. 2005, 3, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Sinauridze, E.I.; Kireev, D.A.; Popenko, N.Y.; Pichugin, A.V.; Panteleev, M.A.; Krymskaya, O.V.; Ataullakhanov, F.I. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb. Haemost. 2007, 97, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Alberio, L.; Safa, O.; Clemetson, K.J.; Esmon, C.T.; Dale, G.L. Surface expression and functional characterization of alpha-granule factor v in human platelets: Effects of ionophore a23187, thrombin, collagen, and convulxin. Blood 2000, 95, 1694–1702. [Google Scholar] [CrossRef]
- Shaw, A.W.; Pureza, V.S.; Sligar, S.G.; Morrissey, J.H. The local phospholipid environment modulates the activation of blood clotting. J. Biol. Chem. 2007, 282, 6556–6563. [Google Scholar] [CrossRef] [PubMed]
- Reddy, E.C.; Rand, M.L. Procoagulant phosphatidylserine-exposing platelets in vitro and in vivo. Front. Cardiovasc. Med. 2020, 7, 15. [Google Scholar] [CrossRef]
- Agbani, E.O.; Poole, A.W. Procoagulant platelets: Generation, function, and therapeutic targeting in thrombosis. Blood 2017, 130, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Alberio, L.; Ravanat, C.; Hechler, B.; Mangin, P.H.; Lanza, F.; Gachet, C. Delayed-onset of procoagulant signalling revealed by kinetic analysis of coat platelet formation. Thromb. Haemost. 2017, 117, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Dale, G.L. Coated-platelets: An emerging component of the procoagulant response. J. Thromb. Haemost. 2005, 3, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, A.C.; Stoner, J.A.; Dale, G.L.; Rabadi, M.; Prodan, C.I. Higher coated-platelet levels in acute stroke are associated with lower cognitive scores at three months post infarction. J. Stroke Cerebrovasc. Dis. 2019, 28, 2398–2406. [Google Scholar] [CrossRef]
- Agbani, E.O.; van den Bosch, M.T.; Brown, E.; Williams, C.M.; Mattheij, N.J.; Cosemans, J.M.; Collins, P.W.; Heemskerk, J.W.; Hers, I.; Poole, A.W. Coordinated membrane ballooning and procoagulant spreading in human platelets. Circulation 2015, 132, 1414–1424. [Google Scholar] [CrossRef]
- Kulkarni, S.; Jackson, S.P. Platelet factor xiii and calpain negatively regulate integrin alphaiibbeta3 adhesive function and thrombus growth. J. Biol. Chem. 2004, 279, 30697–30706. [Google Scholar] [CrossRef] [PubMed]
- Mazepa, M.; Hoffman, M.; Monroe, D. Superactivated platelets: Thrombus regulators, thrombin generators, and potential clinical targets. Arterioscler Thromb. Vasc. Biol. 2013, 33, 1747–1752. [Google Scholar] [CrossRef]
- Pecci, A.; Balduini, C.L. Desmopressin and super platelets. Blood 2014, 123, 1779–1780. [Google Scholar] [CrossRef][Green Version]
- Storrie, B. A tip of the cap to procoagulant platelets. Blood 2016, 128, 1668–1669. [Google Scholar] [CrossRef][Green Version]
- Heemskerk, J.W. Procoagulant ’Zombie’ Platelets. Available online: https://academy.isth.org/isth/2017/berlin/186727/johan.heemskerk.procoagulant.zombie.platelets.html (accessed on 25 January 2021).
- Hua, V.M.; Abeynaike, L.; Glaros, E.; Campbell, H.; Pasalic, L.; Hogg, P.J.; Chen, V.M. Necrotic platelets provide a procoagulant surface during thrombosis. Blood 2015, 126, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Obydennyy, S.I.; Sveshnikova, A.N.; Ataullakhanov, F.I.; Panteleev, M.A. Dynamics of calcium spiking, mitochondrial collapse and phosphatidylserine exposure in platelet subpopulations during activation. J. Thromb. Haemost. 2016, 14, 1867–1881. [Google Scholar] [CrossRef]
- Aliotta, A.; Bertaggia Calderara, D.; Zermatten, M.G.; Alberio, L. Sodium-calcium exchanger reverse mode sustains dichotomous ion fluxes required for procoagulant coat platelet formation. Thromb. Haemost. 2020, 121, 309–321. [Google Scholar] [CrossRef]
- Kholmukhamedov, A.; Janecke, R.; Choo, H.J.; Jobe, S.M. The mitochondrial calcium uniporter regulates procoagulant platelet formation. J. Thromb. Haemost 2018, 16, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Mattheij, N.J.; Gilio, K.; van Kruchten, R.; Jobe, S.M.; Wieschhaus, A.J.; Chishti, A.H.; Collins, P.; Heemskerk, J.W.; Cosemans, J.M. Dual mechanism of integrin alphaiibbeta3 closure in procoagulant platelets. J. Biol. Chem. 2013, 288, 13325–13336. [Google Scholar] [CrossRef] [PubMed]
- London, F.S.; Marcinkiewicz, M.; Walsh, P.N. Par-1-stimulated factor ixa binding to a small platelet subpopulation requires a pronounced and sustained increase of cytoplasmic calcium. Biochemistry 2006, 45, 7289–7298. [Google Scholar] [CrossRef][Green Version]
- Dale, G.L.; Friese, P.; Batar, P.; Hamilton, S.F.; Reed, G.L.; Jackson, K.W.; Clemetson, K.J.; Alberio, L. Stimulated platelets use serotonin to enhance their retention of procoagulant proteins on the cell surface. Nature 2002, 415, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Prodan, C.I.; Joseph, P.M.; Vincent, A.S.; Dale, G.L. Coated-platelet levels are influenced by smoking, aspirin, and selective serotonin reuptake inhibitors. J. Thromb. Haemost. 2007, 5, 2149–2151. [Google Scholar] [CrossRef] [PubMed]
- Dale, G.L. Procoagulant platelets: Further details but many more questions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1596–1597. [Google Scholar] [CrossRef]
- Aliotta, A.; Krusi, M.; Bertaggia Calderara, D.; Zermatten, M.G.; Gomez, F.J.; Batista Mesquita Sauvage, A.P.; Alberio, L. Characterization of procoagulant coat platelets in patients with glanzmann thrombasthenia. Int. J. Mol. Sci. 2020, 21, 9515. [Google Scholar] [CrossRef] [PubMed]
- Weiss, H.J.; Vicic, W.J.; Lages, B.A.; Rogers, J. Isolated deficiency of platelet procoagulant activity. Am. J. Med. 1979, 67, 206–213. [Google Scholar] [CrossRef]
- Zwaal, R.F.; Comfurius, P.; Bevers, E.M. Scott syndrome, a bleeding disorder caused by defective scrambling of membrane phospholipids. Biochim. Biophys. Acta 2004, 1636, 119–128. [Google Scholar] [CrossRef] [PubMed]
- van Geffen, J.P.; Swieringa, F.; Heemskerk, J.W. Platelets and coagulation in thrombus formation: Aberrations in the scott syndrome. Thromb. Res. 2016, 141 (Suppl. S2), S12–S16. [Google Scholar] [CrossRef]
- Adler, M.; Kaufmann, J.; Alberio, L.; Nagler, M. Diagnostic utility of the isth bleeding assessment tool in patients with suspected platelet function disorders. J. Thromb. Haemost. 2019, 17, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Prodan, C.I.; Vincent, A.S.; Padmanabhan, R.; Dale, G.L. Coated-platelet levels are low in patients with spontaneous intracerebral hemorrhage. Stroke 2009, 40, 2578–2580. [Google Scholar] [CrossRef]
- Prodan, C.I.; Vincent, A.S.; Dale, G.L. Coated platelet levels correlate with bleed volume in patients with spontaneous intracerebral hemorrhage. Stroke 2010, 41, 1301–1303. [Google Scholar] [CrossRef]
- Prodan, C.I.; Stoner, J.A.; Dale, G.L. Lower coated-platelet levels are associated with increased mortality after spontaneous intracerebral hemorrhage. Stroke 2015, 46, 1819–1825. [Google Scholar] [CrossRef]
- Prodan, C.I.; Vincent, A.S.; Kirkpatrick, A.C.; Hoover, S.L.; Dale, G.L. Higher levels of coated-platelets are observed in patients with subarachnoid hemorrhage but lower levels are associated with increased mortality at 30 days. J. Neurol. Sci. 2013, 334, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Prodan, C.I.; Stoner, J.A.; Gordon, D.L.; Dale, G.L. Cerebral microbleeds in nonlacunar brain infarction are associated with lower coated-platelet levels. J. Stroke Cerebrovasc. Dis. 2014, 23, e325–e330. [Google Scholar] [CrossRef] [PubMed]
- Prodan, C.I.; Stoner, J.A.; Cowan, L.D.; Dale, G.L. Lower coated-platelet levels are associated with early hemorrhagic transformation in patients with non-lacunar brain infarction. J. Thromb. Haemost. 2010, 8, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Saxena, K.; Pethe, K.; Dale, G.L. Coated-platelet levels may explain some variability in clinical phenotypes observed with severe hemophilia. J. Thromb. Haemost. 2010, 8, 1140–1142. [Google Scholar] [CrossRef]
- Lastrapes, K.K.; Mohammed, B.M.; Mazepa, M.A.; Martin, E.J.; Barrett, J.C.; Massey, G.V.; Kuhn, J.G.; Nolte, M.E.; Hoffman, M.; Monroe, D.M.; et al. Coated platelets and severe haemophilia a bleeding phenotype: Is there a connection? Haemophilia 2016, 22, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Remenyi, G.; Szasz, R.; Debreceni, I.B.; Szarvas, M.; Batar, P.; Nagy, B., Jr.; Kappelmayer, J.; Udvardy, M. Comparison of coated-platelet levels in patients with essential thrombocythemia with and without hydroxyurea treatment. Platelets 2013, 24, 486–492. [Google Scholar] [CrossRef]
- Prodan, C.I.; Joseph, P.M.; Vincent, A.S.; Dale, G.L. Coated-platelets in ischemic stroke: Differences between lacunar and cortical stroke. J. Thromb. Haemost. 2008, 6, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Prodan, C.I.; Vincent, A.S.; Dale, G.L. Coated-platelet levels are elevated in patients with transient ischemic attack. Transl. Res. 2011, 158, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Prodan, C.I.; Stoner, J.A.; Cowan, L.D.; Dale, G.L. Higher coated-platelet levels are associated with stroke recurrence following nonlacunar brain infarction. J. Cereb. Blood Flow Metab. 2013, 33, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, A.C.; Vincent, A.S.; Dale, G.L.; Prodan, C.I. Coated-platelets predict stroke at 30 days following tia. Neurology 2017, 89, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, A.C.; Stoner, J.A.; Dale, G.L.; Prodan, C.I. Elevated coated-platelets in symptomatic large-artery stenosis patients are associated with early stroke recurrence. Platelets 2014, 25, 93–96. [Google Scholar] [CrossRef]
- Kirkpatrick, A.C.; Tafur, A.J.; Vincent, A.S.; Dale, G.L.; Prodan, C.I. Coated-platelets improve prediction of stroke and transient ischemic attack in asymptomatic internal carotid artery stenosis. Stroke 2014, 45, 2995–3001. [Google Scholar] [CrossRef][Green Version]
- Kirkpatrick, A.C.; Vincent, A.S.; Dale, G.L.; Prodan, C.I. Increased platelet procoagulant potential predicts recurrent stroke and tia after lacunar infarction. J. Thromb. Haemost. 2020, 18, 660–668. [Google Scholar] [CrossRef]
- Wang, L.; Bi, Y.; Yu, M.; Li, T.; Tong, D.; Yang, X.; Zhang, C.; Guo, L.; Wang, C.; Kou, Y.; et al. Phosphatidylserine-exposing blood cells and microparticles induce procoagulant activity in non-valvular atrial fibrillation. Int. J. Cardiol. 2018, 258, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Pasalic, L.; Wing-Lun, E.; Lau, J.K.; Campbell, H.; Pennings, G.J.; Lau, E.; Connor, D.; Liang, H.P.; Muller, D.; Kritharides, L.; et al. Novel assay demonstrates that coronary artery disease patients have heightened procoagulant platelet response. J. Thromb. Haemost. 2018, 16, 1198–1210. [Google Scholar] [CrossRef]
- Kou, Y.; Zou, L.; Liu, R.; Zhao, X.; Wang, Y.; Zhang, C.; Dong, Z.; Kou, J.; Bi, Y.; Fu, L.; et al. Intravascular cells and circulating microparticles induce procoagulant activity via phosphatidylserine exposure in heart failure. J. Thromb. Thrombolysis 2019, 48, 187–194. [Google Scholar] [CrossRef]
- Ray, B.; Pandav, V.M.; Mathews, E.A.; Thompson, D.M.; Ford, L.; Yearout, L.K.; Bohnstedt, B.N.; Chaudhary, S.; Dale, G.L.; Prodan, C.I. Coated-platelet trends predict short-term clinical outcomeafter subarachnoid hemorrhage. Transl. Stroke Res. 2017, 9, 459–470. [Google Scholar] [CrossRef]
- Ray, B.; Ross, S.R.; Danala, G.; Aghaei, F.; Nouh, C.D.; Ford, L.; Hollabaugh, K.M.; Karfonta, B.N.; Santucci, J.A.; Cornwell, B.O.; et al. Systemic response of coated-platelet and peripheral blood inflammatory cell indices after aneurysmal subarachnoid hemorrhage and long-term clinical outcome. J. Crit. Care 2019, 52, 1–9. [Google Scholar] [CrossRef]
- Jenkins, A.J.; Gosmanova, A.K.; Lyons, T.J.; May, K.D.; Dashti, A.; Baker, M.Z.; Olansky, L.; Aston, C.E.; Dale, G.L. Coated-platelet levels in patients with type 1 and with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2008, 81, e8–e10. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, A.C.; Vincent, A.S.; Dale, G.L.; Prodan, C.I. Clopidogrel use and smoking cessation result in lower coated-platelet levels after stroke. Platelets 2019, 31, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Vulliamy, P.; Gillespie, S.; Armstrong, P.C.; Allan, H.E.; Warner, T.D.; Brohi, K. Histone h4 induces platelet ballooning and microparticle release during trauma hemorrhage. Proc. Natl. Acad. Sci. USA 2019, 116, 17444–17449. [Google Scholar] [CrossRef]
- Prodan, C.I.; Szasz, R.; Vincent, A.S.; Ross, E.D.; Dale, G.L. Coated-platelets retain amyloid precursor protein on their surface. Platelets 2006, 17, 56–60. [Google Scholar] [CrossRef]
- Prodan, C.I.; Ross, E.D.; Vincent, A.S.; Dale, G.L. Coated-platelets correlate with disease progression in alzheimer disease. J. Neurol. 2007, 254, 548–549. [Google Scholar] [CrossRef] [PubMed]
- Prodan, C.I.; Ross, E.D.; Vincent, A.S.; Dale, G.L. Rate of progression in alzheimer’s disease correlates with coated-platelet levels--a longitudinal study. Transl. Res. 2008, 152, 99–102. [Google Scholar] [CrossRef]
- Prodan, C.I.; Ross, E.D.; Vincent, A.S.; Dale, G.L. Coated-platelets are higher in amnestic versus nonamnestic patients with mild cognitive impairment. Alzheimer Dis. Assoc. Disord. 2007, 21, 259–261. [Google Scholar] [CrossRef]
- Prodan, C.I.; Ross, E.D.; Stoner, J.A.; Cowan, L.D.; Vincent, A.S.; Dale, G.L. Coated-platelet levels and progression from mild cognitive impairment to alzheimer disease. Neurology 2011, 76, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Valaydon, Z.S.; Lee, P.; Dale, G.L.; Januszewski, A.S.; Rowley, K.G.; Nandurkar, H.; Karschimkus, C.; Best, J.D.; Lyons, T.J.; Jenkins, A.J. Increased coated-platelet levels in chronic haemodialysis patients. Nephrology 2009, 14, 148–154. [Google Scholar] [CrossRef]
- Foley, J.H.; Conway, E.M. Cross talk pathways between coagulation and inflammation. Circ. Res. 2016, 118, 1392–1408. [Google Scholar] [CrossRef]
- Kulkarni, S.; Woollard, K.J.; Thomas, S.; Oxley, D.; Jackson, S.P. Conversion of platelets from a proaggregatory to a proinflammatory adhesive phenotype: Role of paf in spatially regulating neutrophil adhesion and spreading. Blood 2007, 110, 1879–1886. [Google Scholar] [CrossRef]
- Charania, R.; Smith, J.; Vesely, S.K.; Dale, G.L.; Holter, J. Quantitation of coated platelet potential during collection, storage, and transfusion of apheresis platelets. Transfusion 2011, 51, 2690–2694. [Google Scholar] [CrossRef] [PubMed]
- Bertaggia Calderara, D.; Crettaz, D.; Aliotta, A.; Barelli, S.; Tissot, J.D.; Prudent, M.; Alberio, L. Generation of procoagulant collagen- and thrombin-activated platelets in platelet concentrates derived from buffy coat: The role of processing, pathogen inactivation, and storage. Transfusion 2018, 58, 2395–2406. [Google Scholar] [CrossRef]
- Gerber, B.; Alberio, L.; Rochat, S.; Stenner, F.; Manz, M.G.; Buser, A.; Schanz, U.; Stussi, G. Safety and efficacy of cryopreserved autologous platelet concentrates in hla-alloimmunized patients with hematologic malignancies. Transfusion 2016, 56, 2426–2437. [Google Scholar] [CrossRef]
- Kotova, Y.N.; Ataullakhanov, F.I.; Panteleev, M.A. Formation of coated platelets is regulated by the dense granule secretion of adenosine 5’diphosphate acting via the p2y12 receptor. J. Thromb. Haemost. 2008, 6, 1603–1605. [Google Scholar] [CrossRef]
- Norgard, N.B.; Saya, S.; Hann, C.L.; Hennebry, T.A.; Schechter, E.; Dale, G.L. Clopidogrel attenuates coated-platelet production in patients undergoing elective coronary catheterization. J. Cardiovasc. Pharmacol. 2008, 52, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Norgard, N.B.; Hann, C.L.; Dale, G.L. Cangrelor attenuates coated-platelet formation. Clin. Appl. Thromb. Hemost. 2009, 15, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Judge, H.M.; Buckland, R.J.; Sugidachi, A.; Jakubowski, J.A.; Storey, R.F. The active metabolite of prasugrel effectively blocks the platelet p2y12 receptor and inhibits procoagulant and pro-inflammatory platelet responses. Platelets 2008, 19, 125–133. [Google Scholar] [CrossRef]
- Hamilton, S.F.; Miller, M.W.; Thompson, C.A.; Dale, G.L. Glycoprotein iib/iiia inhibitors increase coat-platelet production in vitro. J. Lab. Clin. Med. 2004, 143, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Vermylen, J.; Hoylaerts, M.; Arnout, J. Increased mortality with long-term platelet glycoprotein iib/iiia antagonists: An explanation? Circulation 2001, 104, E109. [Google Scholar] [CrossRef] [PubMed]
- Topalov, N.N.; Kotova, Y.N.; Vasil’ev, S.A.; Panteleev, M.A. Identification of signal transduction pathways involved in the formation of platelet subpopulations upon activation. Br. J. Haematol. 2012, 157, 105–115. [Google Scholar] [CrossRef]
- van der Meijden, P.E.; Feijge, M.A.; Swieringa, F.; Gilio, K.; Nergiz-Unal, R.; Hamulyak, K.; Heemskerk, J.W. Key role of integrin alpha(iib)beta (3) signaling to syk kinase in tissue factor-induced thrombin generation. Cell. Mol. Life Sci. 2012, 69, 3481–3492. [Google Scholar] [CrossRef]
- Razmara, M.; Hu, H.; Masquelier, M.; Li, N. Glycoprotein iib/iiia blockade inhibits platelet aminophospholipid exposure by potentiating translocase and attenuating scramblase activity. Cell. Mol. Life Sci. 2007, 64, 999–1008. [Google Scholar] [CrossRef]
- Mannucci, P.M.; Ruggeri, Z.M.; Pareti, F.I.; Capitanio, A. 1-deamino-8-d-arginine vasopressin: A new pharmacological approach to the management of haemophilia and von willebrands’ diseases. Lancet 1977, 1, 869–872. [Google Scholar] [CrossRef]
- Tomasiak, M.M.; Stelmach, H.; Bodzenta-Lukaszyk, A.; Tomasiak, M. Involvement of na+/h+ exchanger in desmopressin-induced platelet procoagulant response. Acta Biochim. Pol. 2004, 51, 773–788. [Google Scholar] [CrossRef]
- Tomasiak, M.; Stelmach, H.; Rusak, T.; Ciborowski, M.; Radziwon, P. Vasopressin acts on platelets to generate procoagulant activity. Blood Coagul. Fibrinolysis 2008, 19, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Colucci, G.; Stutz, M.; Rochat, S.; Conte, T.; Pavicic, M.; Reusser, M.; Giabbani, E.; Huynh, A.; Thurlemann, C.; Keller, P.; et al. The effect of desmopressin on platelet function: A selective enhancement of procoagulant coat platelets in patients with primary platelet function defects. Blood 2014, 123, 1905–1916. [Google Scholar] [CrossRef] [PubMed]
- Swieringa, F.; Lance, M.D.; Fuchs, B.; Feijge, M.A.; Solecka, B.A.; Verheijen, L.P.; Hughes, K.R.; van Oerle, R.; Deckmyn, H.; Kannicht, C.; et al. Desmopressin treatment improves platelet function under flow in patients with postoperative bleeding. J. Thromb. Haemost. 2015, 13, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.T. Auranofin, a thioredoxin reductase inhibitor, causes platelet death through calcium overload. Platelets 2019, 30, 98–104. [Google Scholar] [CrossRef]
- Tseng, Y.L.; Braun, A.; Chang, J.P.; Chiang, M.L.; Tseng, C.Y.; Chen, W. Micromolar concentrations of citalopram or escitalopram inhibit glycoprotein vi-mediated and integrin alphaiibbeta3-mediated signaling in human platelets. Toxicol. Appl. Pharmacol. 2019, 364, 106–113. [Google Scholar] [CrossRef]
- Galan, A.M.; Lopez-Vilchez, I.; Diaz-Ricart, M.; Navalon, F.; Gomez, E.; Gasto, C.; Escolar, G. Serotonergic mechanisms enhance platelet-mediated thrombogenicity. Thromb. Haemost. 2009, 102, 511–519. [Google Scholar] [CrossRef]
- Laporte, S.; Chapelle, C.; Caillet, P.; Beyens, M.N.; Bellet, F.; Delavenne, X.; Mismetti, P.; Bertoletti, L. Bleeding risk under selective serotonin reuptake inhibitor (ssri) antidepressants: A meta-analysis of observational studies. Pharmacol. Res. 2017, 118, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Mezei, G.; Debreceni, I.B.; Kerenyi, A.; Remenyi, G.; Szasz, R.; Illes, A.; Kappelmayer, J.; Batar, P. Dasatinib inhibits coated-platelet generation in patients with chronic myeloid leukemia. Platelets 2019, 30, 836–843. [Google Scholar] [CrossRef]
- Deb, S.; Boknas, N.; Sjostrom, C.; Tharmakulanathan, A.; Lotfi, K.; Ramstrom, S. Varying effects of tyrosine kinase inhibitors on platelet function-a need for individualized cml treatment to minimize the risk for hemostatic and thrombotic complications? Cancer Med. 2020, 9, 313–323. [Google Scholar] [CrossRef]
- Tullemans, B.M.E.; Nagy, M.; Sabrkhany, S.; Griffioen, A.W.; Oude Egbrink, M.G.A.; Aarts, M.; Heemskerk, J.W.M.; Kuijpers, M.J.E. Tyrosine kinase inhibitor pazopanib inhibits platelet procoagulant activity in renal cell carcinoma patients. Front. Cardiovasc. Med. 2018, 5, 142. [Google Scholar] [CrossRef]
- Cao, H.; Umbach, A.T.; Bissinger, R.; Gawaz, M.; Lang, F. Inhibition of collagen related peptide induced platelet activation and apoptosis by ceritinib. Cell Physiol. Biochem. 2018, 45, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Keuren, J.F.; Wielders, S.J.; Ulrichts, H.; Hackeng, T.; Heemskerk, J.W.; Deckmyn, H.; Bevers, E.M.; Lindhout, T. Synergistic effect of thrombin on collagen-induced platelet procoagulant activity is mediated through protease-activated receptor-1. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1499–1505. [Google Scholar] [CrossRef]
- Agbani, E.O.; Williams, C.M.; Hers, I.; Poole, A.W. Membrane ballooning in aggregated platelets is synchronised and mediates a surge in microvesiculation. Sci. Rep. 2017, 7, 2770. [Google Scholar] [CrossRef]
- Denorme, F.; Manne, B.K.; Portier, I.; Eustes, A.S.; Kosaka, Y.; Kile, B.T.; Rondina, M.T.; Campbell, R.A. Platelet necrosis mediates ischemic stroke outcome in mice. Blood 2020, 135, 429–440. [Google Scholar] [CrossRef]
- Choo, H.J.; Saafir, T.B.; Mkumba, L.; Wagner, M.B.; Jobe, S.M. Mitochondrial calcium and reactive oxygen species regulate agonist-initiated platelet phosphatidylserine exposure. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2946–2955. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Sodergren, A.L.; Ramstrom, S. Platelet subpopulations remain despite strong dual agonist stimulation and can be characterised using a novel six-colour flow cytometry protocol. Sci. Rep. 2018, 8, 1441. [Google Scholar] [CrossRef]
- Topalov, N.N.; Yakimenko, A.O.; Canault, M.; Artemenko, E.O.; Zakharova, N.V.; Abaeva, A.A.; Loosveld, M.; Ataullakhanov, F.I.; Nurden, A.T.; Alessi, M.C.; et al. Two types of procoagulant platelets are formed upon physiological activation and are controlled by integrin alpha(iib)beta(3). Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2475–2483. [Google Scholar] [CrossRef]
- Szasz, R.; Dale, G.L. Coat platelets. Curr. Opin. Hematol. 2003, 10, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Abaeva, A.A.; Canault, M.; Kotova, Y.N.; Obydennyy, S.I.; Yakimenko, A.O.; Podoplelova, N.A.; Kolyadko, V.N.; Chambost, H.; Mazurov, A.V.; Ataullakhanov, F.I.; et al. Procoagulant platelets form an alpha-granule protein-covered “cap” on their surface that promotes their attachment to aggregates. J. Biol. Chem. 2013, 288, 29621–29632. [Google Scholar] [CrossRef]
- Aupeix, K.; Hugel, B.; Martin, T.; Bischoff, P.; Lill, H.; Pasquali, J.L.; Freyssinet, J.M. The significance of shed membrane particles during programmed cell death in vitro, and in vivo, in hiv-1 infection. J. Clin. Investig. 1997, 99, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Bohling, S.D.; Pagano, M.B.; Stitzel, M.R.; Ferrell, C.; Yeung, W.; Chandler, W.L. Comparison of clot-based vs chromogenic factor xa procoagulant phospholipid activity assays. Am. J. Clin. Pathol. 2012, 137, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Hemker, H.C.; Wielders, S.; Kessels, H.; Beguin, S. Continuous registration of thrombin generation in plasma, its use for the determination of the thrombin potential. Thromb. Haemost. 1993, 70, 617–624. [Google Scholar] [CrossRef]
- Zermatten, M.G.; Fraga, M.; Calderara, D.B.; Aliotta, A.; Moradpour, D.; Alberio, L. Biomarkers of liver dysfunction correlate with a prothrombotic and not with a prohaemorrhagic profile in patients with cirrhosis. JHEP Rep. Innov. Hepatol. 2020, 2, 100120. [Google Scholar] [CrossRef] [PubMed]
- Camire, R.M.; Kalafatis, M.; Simioni, P.; Girolami, A.; Tracy, P.B. Platelet-derived factor va/va leiden cofactor activities are sustained on the surface of activated platelets despite the presence of activated protein c. Blood 1998, 91, 2818–2829. [Google Scholar] [CrossRef]
- Thuerlemann, C.; Haeberli, A.; Alberio, L. Monitoring thrombin generation by electrochemistry: Development of an amperometric biosensor screening test for plasma and whole blood. Clin. Chem. 2009, 55, 505–512. [Google Scholar] [CrossRef]
- Jy, W.; Horstman, L.L.; Jimenez, J.J.; Ahn, Y.S.; Biro, E.; Nieuwland, R.; Sturk, A.; Dignat-George, F.; Sabatier, F.; Camoin-Jau, L.; et al. Measuring circulating cell-derived microparticles. J. Thromb. Haemost. 2004, 2, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, R.; Robert, S.; Poncelet, P.; Dignat-George, F. Overcoming limitations of microparticle measurement by flow cytometry. Semin. Thromb. Hemost. 2010, 36, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Kessels, H.; Beguin, S.; Andree, H.; Hemker, H.C. Measurement of thrombin generation in whole blood--the effect of heparin and aspirin. Thromb. Haemost. 1994, 72, 78–83. [Google Scholar] [CrossRef]
- Ninivaggi, M.; Apitz-Castro, R.; Dargaud, Y.; de Laat, B.; Hemker, H.C.; Lindhout, T. Whole-blood thrombin generation monitored with a calibrated automated thrombogram-based assay. Clin. Chem. 2012, 58, 1252–1259. [Google Scholar] [CrossRef]
- Prior, S.M.; Mann, K.G.; Freeman, K.; Butenas, S. Continuous thrombin generation in whole blood: New applications for assessing activators and inhibitors of coagulation. Anal. Biochem. 2018, 551, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Konings, J.; Yan, Q.; Kelchtermans, H.; Kremers, R.; de Laat, B.; Roest, M. A novel assay for studying the involvement of blood cells in whole blood thrombin generation. J. Thromb. Haemost. 2020, 18, 1291–1301. [Google Scholar] [CrossRef]
- Hemker, H.C.; Giesen, P.L.; Ramjee, M.; Wagenvoord, R.; Beguin, S. The thrombogram: Monitoring thrombin generation in platelet-rich plasma. Thromb. Haemost. 2000, 83, 589–591. [Google Scholar] [CrossRef]
- Douxfils, J.; Morimont, L.; Bouvy, C.; de Saint-Hubert, M.; Devalet, B.; Devroye, C.; Dincq, A.S.; Dogne, J.M.; Guldenpfennig, M.; Baudar, J.; et al. Assessment of the analytical performances and sample stability on st genesia system using the stg-drugscreen application. J. Thromb. Haemost. 2019, 17, 1273–1287. [Google Scholar] [CrossRef]
- Talon, L.; Sinegre, T.; Lecompte, T.; Pereira, B.; Massoulie, S.; Abergel, A.; Lebreton, A. Hypercoagulability (thrombin generation) in patients with cirrhosis is detected with st-genesia. J. Thromb. Haemost. 2020, 18, 2177–2190. [Google Scholar] [CrossRef] [PubMed]
- Bertaggia Calderara, D.; Zermatten, M.G.; Aliotta, A.; Batista Mesquita Sauvage, A.P.; Carle, V.; Heinis, C.; Alberio, L. Tissue factor-independent coagulation correlates with clinical phenotype in factor xi deficiency and replacement therapy. Thromb. Haemost. 2021, 121, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Koltsova, E.M.; Kuprash, A.D.; Dashkevich, N.M.; Vardanyan, D.M.; Chernyakov, A.V.; Kumskova, M.A.; Nair, S.C.; Srivastava, A.; Ataullakhanov, F.I.; Panteleev, M.A.; et al. Determination of fibrin clot growth and spatial thrombin propagation in the presence of different types of phospholipid surfaces. Platelets 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Aswad, M.H.; Kissova, J.; Rihova, L.; Zavrelova, J.; Ovesna, P.; Penka, M. High level of circulating microparticles in patients with bcr/abl negative myeloproliferative neoplasm—A pilot study. Klein. Onkol. 2019, 32, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Mooberry, M.J.; Bradford, R.; Hobl, E.L.; Lin, F.C.; Jilma, B.; Key, N.S. Procoagulant microparticles promote coagulation in a factor xi-dependent manner in human endotoxemia. J. Thromb. Haemost. 2016, 14, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Exner, T.; Joseph, J.E.; Connor, D.; Low, J.; Ma, D.D. Increased procoagulant phospholipid activity in blood from patients with suspected acute coronary syndromes: A pilot study. Blood Coagul. Fibrinolysis 2005, 16, 375–379. [Google Scholar] [CrossRef]
- Marchetti, M.; Tartari, C.J.; Russo, L.; Panova-Noeva, M.; Leuzzi, A.; Rambaldi, A.; Finazzi, G.; Woodhams, B.; Falanga, A. Phospholipid-dependent procoagulant activity is highly expressed by circulating microparticles in patients with essential thrombocythemia. Am. J. Hematol. 2014, 89, 68–73. [Google Scholar] [CrossRef]
- van Dreden, P.; Rousseau, A.; Fontaine, S.; Woodhams, B.J.; Exner, T. Clinical evaluation of a new functional test for detection of plasma procoagulant phospholipids. Blood Coagul. Fibrinolysis 2009, 20, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.K.; Abdel-Monem, H.; Niravath, P.; Le, A.; Bellera, R.V.; Langlois, K.; Nagata, S.; Rumbaut, R.E.; Thiagarajan, P. Lactadherin and clearance of platelet-derived microvesicles. Blood 2009, 113, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Polak, D.; Talar, M.; Watala, C.; Przygodzki, T. Intravital assessment of blood platelet function. A review of the methodological approaches with examples of studies of selected aspects of blood platelet function. Int. J. Mol. Sci. 2020, 21, 8334. [Google Scholar] [CrossRef] [PubMed]
- Montague, S.J.; Lim, Y.J.; Lee, W.M.; Gardiner, E.E. Imaging platelet processes and function-current and emerging approaches for imaging in vitro and in vivo. Front. Immunol. 2020, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Marcinczyk, N.; Golaszewska, A.; Misztal, T.; Gromotowicz-Poplawska, A.; Rusak, T.; Chabielska, E. New approaches for the assessment of platelet activation status in thrombus under flow condition using confocal microscopy. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Sakata, A.; Nishimura, S.; Eto, K.; Nagata, S. Tmem16f is required for phosphatidylserine exposure and microparticle release in activated mouse platelets. Proc. Natl. Acad. Sci. USA 2015, 112, 12800–12805. [Google Scholar] [CrossRef]
- Nechipurenko, D.Y.; Receveur, N.; Yakimenko, A.O.; Shepelyuk, T.O.; Yakusheva, A.A.; Kerimov, R.R.; Obydennyy, S.I.; Eckly, A.; Leon, C.; Gachet, C.; et al. Clot contraction drives the translocation of procoagulant platelets to thrombus surface. Arter. Thromb. Vasc. Biol. 2019, 39, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Kennedy, D.R.; Lin, L.; Huang, M.; Merrill-Skoloff, G.; Furie, B.C.; Furie, B. Protein disulfide isomerase capture during thrombus formation in vivo depends on the presence of beta3 integrins. Blood 2012, 120, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Denis, C.; Methia, N.; Frenette, P.S.; Rayburn, H.; Ullman-Cullere, M.; Hynes, R.O.; Wagner, D.D. A mouse model of severe von willebrand disease: Defects in hemostasis and thrombosis. Proc. Natl. Acad. Sci. USA 1998, 95, 9524–9529. [Google Scholar] [CrossRef] [PubMed]
- Deppermann, C.; Cherpokova, D.; Nurden, P.; Schulz, J.N.; Thielmann, I.; Kraft, P.; Vogtle, T.; Kleinschnitz, C.; Dutting, S.; Krohne, G.; et al. Gray platelet syndrome and defective thrombo-inflammation in nbeal2-deficient mice. J. Clin. Investig. 2013, 123, 3331–3342. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe sars-cov-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef]
- Gasecka, A.; Borovac, J.A.; Guerreiro, R.A.; Giustozzi, M.; Parker, W.; Caldeira, D.; Chiva-Blanch, G. Thrombotic complications in patients with covid-19: Pathophysiological mechanisms, diagnosis, and treatment. Cardiovasc. Drugs Ther. 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.X.; Tyagi, T.; Jain, K.; Gu, V.W.; Lee, S.H.; Hwa, J.M.; Kwan, J.M.; Krause, D.S.; Lee, A.I.; Halene, S.; et al. Thrombocytopathy and endotheliopathy: Crucial contributors to covid-19 thromboinflammation. Nat. Rev. Cardiol. 2020, 1–16. [Google Scholar] [CrossRef]
- Larsen, J.B.; Pasalic, L.; Hvas, A.M. Platelets in coronavirus disease 2019. Semin. Thromb. Hemost. 2020, 46, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M.; Freedman, J.E. Platelets and covid-19: Inflammation, hyperactivation and additional questions. Circ. Res. 2020, 127, 1419–1421. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M. Potential role of platelets in covid-19: Implications for thrombosis. Res. Pract. Thromb. Haemost. 2020, 4, 737–740. [Google Scholar] [CrossRef]
- Bongiovanni, D.; Klug, M.; Lazareva, O.; Weidlich, S.; Biasi, M.; Ursu, S.; Warth, S.; Buske, C.; Lukas, M.; Spinner, C.D.; et al. Sars-cov-2 infection is associated with a pro-thrombotic platelet phenotype. Cell Death. Dis. 2021, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Zaid, Y.; Puhm, F.; Allaeys, I.; Naya, A.; Oudghiri, M.; Khalki, L.; Limami, Y.; Zaid, N.; Sadki, K.; Ben El Haj, R.; et al. Platelets can associate with sars-cov-2 rna and are hyperactivated in covid-19. Circ. Res. 2020, 127, 1404–1418. [Google Scholar] [CrossRef]
- Manne, B.K.; Denorme, F.; Middleton, E.A.; Portier, I.; Rowley, J.W.; Stubben, C.; Petrey, A.C.; Tolley, N.D.; Guo, L.; Cody, M.; et al. Platelet gene expression and function in patients with covid-19. Blood 2020, 136, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Hottz, E.D.; Azevedo-Quintanilha, I.G.; Palhinha, L.; Teixeira, L.; Barreto, E.A.; Pao, C.R.R.; Righy, C.; Franco, S.; Souza, T.M.L.; Kurtz, P.; et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe covid-19. Blood 2020, 136, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Denorme, F.; Manne, B.K.; Portier, I.; Petrey, A.C.; Middleton, E.A.; Kile, B.T.; Rondina, M.T.; Campbell, R.A. Covid-19 patients exhibit reduced procoagulant platelet responses. J. Thromb. Haemost. 2020, 18, 3067–3073. [Google Scholar] [CrossRef] [PubMed]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in covid-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef]
- Maclay, J.D.; McAllister, D.A.; Johnston, S.; Raftis, J.; McGuinnes, C.; Deans, A.; Newby, D.E.; Mills, N.L.; MacNee, W. Increased platelet activation in patients with stable and acute exacerbation of copd. Thorax 2011, 66, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, T.; Mairbaurl, H.; Pleisch, B.; Maggiorini, M.; Bartsch, P.; Reinhart, W.H. Platelet count and function at high altitude and in high-altitude pulmonary edema. J. Appl. Physiol. 2006, 100, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, T.; Ahmad, S.; Gupta, N.; Sahu, A.; Ahmad, Y.; Nair, V.; Chatterjee, T.; Bajaj, N.; Sengupta, S.; Ganju, L.; et al. Altered expression of platelet proteins and calpain activity mediate hypoxia-induced prothrombotic phenotype. Blood 2014, 123, 1250–1260. [Google Scholar] [CrossRef]
- Yan, S.L.; Russell, J.; Granger, D.N. Platelet activation and platelet-leukocyte aggregation elicited in experimental colitis are mediated by interleukin-6. Inflamm. Bowel Dis. 2014, 20, 353–362. [Google Scholar] [CrossRef]
- Senchenkova, E.Y.; Komoto, S.; Russell, J.; Almeida-Paula, L.D.; Yan, L.S.; Zhang, S.; Granger, D.N. Interleukin-6 mediates the platelet abnormalities and thrombogenesis associated with experimental colitis. Am. J. Pathol. 2013, 183, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S.A.; Peng, J.; Friese, P.; Wolf, R.F.; Harrison, P.; Downs, T.; Hamilton, K.; Comp, P.; Dale, G.L. Cytokine-induced alteration of platelet and hemostatic function. Stem Cells 1996, 14 (Suppl. S1), 154–162. [Google Scholar] [CrossRef] [PubMed]
- Masselli, E.; Pozzi, G.; Vaccarezza, M.; Mirandola, P.; Galli, D.; Vitale, M.; Carubbi, C.; Gobbi, G. Ros in platelet biology: Functional aspects and methodological insights. Int. J. Mol. Sci. 2020, 21, 4866. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).