When Should We Think of Myelodysplasia or Bone Marrow Failure in a Thrombocytopenic Patient? A Practical Approach to Diagnosis
Abstract
1. Introduction
2. Clinical Presentations and Symptoms of MDS and BMF
2.1. Myelodysplastic Syndromes
2.1.1. Definition and Pathogenesis of MDS
2.1.2. Epidemiology of MDS and Risk Factors
2.1.3. Presentation and Symptoms of Patients with MDS
2.2. Bone Marrow Failure Syndromes
2.2.1. Definition and Pathogenesis of BMF
2.2.2. Epidemiology of BMF and Risk Factors
2.2.3. General Presentation and Symptoms of Patients with BMF
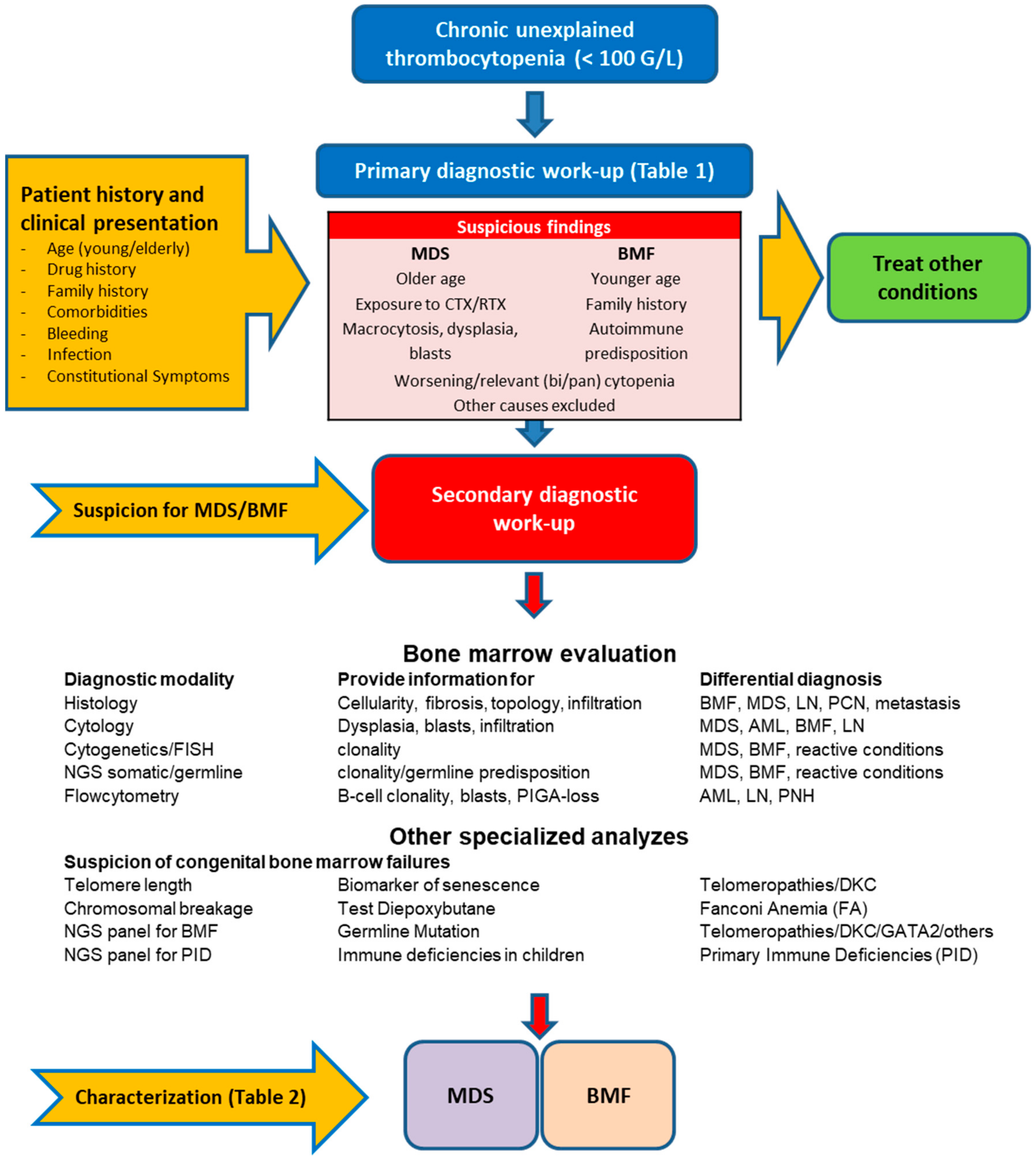
3. Diagnostic Approach for MDS and BMF
3.1. Primary Diagnostic Work-Up
3.2. Secondary Diagnostic Work-Up
3.3. Peripheral Blood Smear
3.4. Bone Marrow Cytomorphology
3.5. Bone Marrow Histopathology
3.6. Multiparameter Flow Cytometry
3.7. Cytogenetics
3.8. Next-Generation Sequencing
3.9. Role of Next-Generation Sequencing in BMF
3.10. Discrimination of Germline from Somatic Mutations
4. Characterization of MDS and BMF
4.1. Challenges in Finding the Diagnosis of MDS and BMF
4.2. Characterization of MDS
4.3. Relevance of Thrombocytopenia in the Context of MDS Patients
4.4. Characterization of BMF
4.5. Isolated Thrombocytopenia as First Presentation of a BMF
4.6. Aplastic Anemia and PNH
4.7. Inherited Bone Marrow Failures (iBMF)
4.8. Future Challenges: Unexplained Thrombocytopenia with Clonal Hematopoiesis
5. Conclusions
- Unexplained chronic thrombocytopenia has to be considered as an early and unusual presentation in MDS or BMF.
- Patient’s history remains crucial to identify suspicious cases and for the correct interpretation of primary laboratory values.
- Various diagnostic modalities are required to confirm or exclude MDS or BMF and an interdisciplinary workup is frequently required, especially in difficult cases.
- Meticulous assessment of the PB smear, BM cyto- and histomorphology, as well as cytogenetics are the mainstay of diagnostic evaluation, and is nowadays complemented by NGS and other specialized analyses (telomere length, DNA breakage).
- Repeated bone marrow investigation may be necessary, especially in cases with hypocellular BM for the distinction of sampling errors, reactive-toxic conditions, BMF, and hypoplastic MDS.
- In some occasions, conclusive diagnosis is only possible after follow-up. However, NGS has substantially contributed in identifying early conditions of clonal hematopoiesis, but additional challenges arise for classification and prognostication.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neunert, C.; Terrell, D.R.; Arnold, D.M.; Buchanan, G.; Cines, D.B.; Cooper, N.; Cuker, A.; Despotovic, J.M.; George, J.N.; Grace, R.F.; et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019, 3, 3829–3866. [Google Scholar] [CrossRef]
- Roman, E.; Smith, A.; Appleton, S.; Crouch, S.; Kelly, R.; Kinsey, S.; Cargo, C.; Patmore, R. Myeloid malignancies in the real-world: Occurrence, progression and survival in the UK’s population-based Haematological Malignancy Research Network 2004–15. Cancer Epidemiol. 2016, 42, 186–198. [Google Scholar] [CrossRef]
- Hellström-Lindberg, E.; Tobiasson, M.; Greenberg, P. Myelodysplastic syndromes: Moving towards personalized management. Haematologica 2020, 105, 1765–1779. [Google Scholar] [CrossRef]
- Corey, S.J.; Minden, M.D.; Barber, D.L.; Kantarjian, H.; Wang, J.C.Y.; Schimmer, A.D. Myelodysplastic syndromes: The complexity of stem-cell diseases. Nat. Rev. Cancer 2007, 7, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Steensma, D.P. Clinical Implications of Clonal Hematopoiesis. Mayo Clin. Proc. 2018, 93, 1122–1130. [Google Scholar] [CrossRef]
- Steensma, D.P. Predicting therapy-related myeloid neoplasms-and preventing them? Lancet Oncol. 2017, 18, 11–13. [Google Scholar] [CrossRef]
- Neukirchen, J.; Schoonen, W.M.; Strupp, C.; Gattermann, N.; Aul, C.; Haas, R.; Germing, U. Incidence and prevalence of myelodysplastic syndromes: Data from the Düsseldorf MDS-registry. Leuk. Res. 2011, 35, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Foran, J.M.; Shammo, J.M. Clinical Presentation, Diagnosis, and Prognosis of Myelodysplastic Syndromes. Am. J. Med. 2012, 125 (Suppl. S7), S6–S13. [Google Scholar] [CrossRef]
- Strom, S.S.; Gu, Y.; Gruschkus, S.K.; Pierce, S.; Estey, E.H. Risk factors of myelodysplastic syndromes: A case–control study. Leukemia 2005, 19, 1912–1918. [Google Scholar] [CrossRef]
- Bonadies, N.; Feller, A.; Rovo, A.; Ruefer, A.; Blum, S.; Gerber, B.; Stuessi, G.; Benz, R.; Cantoni, N.; Holbro, A.; et al. Trends of classification, incidence, mortality, and survival of MDS patients in Switzerland between 2001 and 2012. Cancer Epidemiol. 2017, 46, 85–92. [Google Scholar] [CrossRef]
- Rollison, D.E.; Howlader, N.; Smith, M.T.; Strom, S.S.; Merritt, W.D.; Ries, L.A.; Edwards, B.K.; List, A.F. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood 2008, 112, 45–52. [Google Scholar] [CrossRef]
- Babushok, D.V.; Bessler, M. Genetic predisposition syndromes: When should they be considered in the work-up of MDS? Best Pract. Res. Clin. Haematol. 2015, 28, 55–68. [Google Scholar] [CrossRef]
- Steensma, D.P.; Bennett, J.M. The Myelodysplastic Syndromes: Diagnosis and Treatment. Mayo Clin. Proc. 2006, 81, 104–130. [Google Scholar] [CrossRef]
- Thota, S.; Gerds, A.T. Myelodysplastic and myeloproliferative neoplasms: Updates on the overlap syndromes. Leuk. Lymphoma 2017, 59, 803–812. [Google Scholar] [CrossRef]
- Kipfer, B.; Daikeler, T.; Kuchen, S.; Hallal, M.; Andina, N.; Allam, R.; Bonadies, N. Increased cardiovascular comorbidities in patients with myelodysplastic syndromes and chronic myelomonocytic leukemia presenting with systemic inflammatory and autoimmune manifestations. Semin. Hematol. 2018, 55, 242–247. [Google Scholar] [CrossRef]
- Mekinian, A.; Grignano, E.; Braun, T.; Decaux, O.; Liozon, E.; Costedoat-Chalumeau, N.; Kahn, J.-E.; Hamidou, M.; Park, S.; Puéchal, X.; et al. Systemic inflammatory and autoimmune manifestations associated with myelodysplastic syndromes and chronic myelomonocytic leukaemia: A French multicentre retrospective study. Rheumatology 2016, 55, 291–300. [Google Scholar] [CrossRef]
- Risitano, A.M.; Maciejewski, J.P.; Green, S.; Plasilova, M.; Zeng, W.; Young, N.S. In-vivo dominant immune responses in aplastic anaemia: Molecular tracking of putatively pathogenetic T-cell clones by TCR beta-CDR3 sequencing. Lancet 2004, 364, 355–364. [Google Scholar] [CrossRef]
- Young, N.S. Current concepts in the pathophysiology and treatment of aplastic anemia. Hematol. Am. Soc. Hematol. Educ. Program 2013, 2013, 76–81. [Google Scholar] [CrossRef]
- Min, K.-W.; Jung, H.Y.; Han, H.S.; Hwang, T.S.; Kim, S.-Y.; Kim, W.S.; Lim, S.D.; Kim, W.Y. Ileal mass-like lesion induced by Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in a patient with aplastic anemia. APMIS 2014, 123, 81–86. [Google Scholar] [CrossRef]
- Brown, K.E.; Idrees, M.; Shah, S.A.; Butt, S.; Butt, A.M.; Ali, L.; Hussain, A.; Rehman, I.U.; Ali, M. Hepatitis-associated aplastic anemia. N. Engl. J. Med. 1997, 336, 1059–1064. [Google Scholar] [CrossRef]
- Young, N.S. Flaviviruses and bone marrow failure. JAMA 1990, 263, 3065–3068. [Google Scholar] [CrossRef] [PubMed]
- Locasciulli, A.; Bacigalupo, A.; Bruno, B.; Montante, B.; Marsh, J.; Tichelli, A.; Socié, G.; Passweg, J. Hepatitis-associated aplastic anaemia: Epidemiology and treatment results obtained in Europe. A report of The EBMT aplastic anaemia working party. Br. J. Haematol. 2010, 149, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Calado, R.T.; Cooper, J.N.; Padilla-Nash, H.M.; Sloand, E.M.; Wu, C.O.; Scheinberg, P.; Ried, T.; Young, N.S. Short telomeres result in chromosomal instability in hematopoietic cells and precede malignant evolution in human aplastic anemia. Leukemmia 2011, 26, 700–707. [Google Scholar] [CrossRef]
- Dumitriu, B.; Feng, X.; Townsley, D.M.; Ueda, Y.; Yoshizato, T.; Calado, R.T.; Yang, Y.; Wakabayashi, Y.; Kajigaya, S.; Ogawa, S.; et al. Telomere attrition and candidate gene mutations preceding monosomy 7 in aplastic anemia. Blood 2015, 125, 706–709. [Google Scholar] [CrossRef]
- Townsley, D.M.; Dumitriu, B.; Young, N.S. Bone marrow failure and the telomeropathies. Blood 2014, 124, 2775–2783. [Google Scholar] [CrossRef]
- Bono, E.; McLornan, D.; Travaglino, E.; Gandhi, S.; Gallì, A.; Khan, A.A.; Kulasekararaj, A.G.; Boveri, E.; Raj, K.; Elena, C.; et al. Clinical, histopathological and molecular characterization of hypoplastic myelodysplastic syndrome. Leukemia 2019, 33, 2495–2505. [Google Scholar] [CrossRef]
- Stanley, N.; Olson, T.S.; Babushok, D.V. Recent advances in understanding clonal haematopoiesis in aplastic anaemia. Br. J. Haematol. 2017, 177, 509–525. [Google Scholar] [CrossRef]
- Kulasekararaj, A.G.; Jiang, J.; Smith, A.E.; Mohamedali, A.M.; Mian, S.; Gandhi, S.; Gaken, J.; Czepulkowski, B.; Marsh, J.C.; Mufti, G.J. Somatic mutations identify a subgroup of aplastic anemia patients who progress to myelodysplastic syndrome. Blood 2014, 124, 2698–2704. [Google Scholar] [CrossRef]
- Triemstra, J.; Pham, A.; Rhodes, L.; Waggoner, D.J.; Onel, K. A Review of Fanconi Anemia for the Practicing Pediatrician. Pediatr. Ann. 2015, 44, 444–452. [Google Scholar] [CrossRef]
- Farooqui, S.M.; Ward, R.; Aziz, M. Shwachman-Diamond Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Geddis, A.E. Inherited Thrombocytopenia: Congenital Amegakaryocytic Thrombocytopenia and Thrombocytopenia with Absent Radii. Semin. Hematol. 2006, 43, 196–203. [Google Scholar] [CrossRef]
- Kojima, S. Why is the incidence of aplastic anemia higher in Asia? Expert Rev. Hematol. 2017, 10, 277–279. [Google Scholar] [CrossRef]
- Young, N.S.; Kaufman, D.W. The epidemiology of acquired aplastic anemia. Haematologica 2008, 93, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Song, E.Y.; Kang, H.J.; Shin, H.Y.; Ahn, H.S.; Kim, I.; Yoon, S.-S.; Park, S.; Kim, B.K.; Park, M.H. Association of human leukocyte antigen class II alleles with response to immunosuppressive therapy in Korean aplastic anemia patients. Hum. Immunol. 2010, 71, 88–92. [Google Scholar] [CrossRef]
- Sugimori, C.; Yamazaki, H.; Feng, X.; Mochizuki, K.; Kondo, Y.; Takami, A.; Chuhjo, T.; Kimura, A.; Teramura, M.; Mizoguchi, H.; et al. Roles of DRB1 *1501 and DRB1 *1502 in the pathogenesis of aplastic anemia. Exp. Hematol. 2007, 35, 13–20. [Google Scholar] [CrossRef][Green Version]
- Yoshida, N.; Yagasaki, H.; Takahashi, Y.; Yamamoto, T.; Liang, J.; Wang, Y.; Tanaka, M.; Hama, A.; Nishio, N.; Kobayashi, R.; et al. Clinical impact of HLA-DR15, a minor population of paroxysmal nocturnal haemoglobinuria-type cells, and an aplastic anaemia-associated autoantibody in children with acquired aplastic anaemia. Br. J. Haematol. 2008, 142, 427–435. [Google Scholar] [CrossRef]
- Wang, M.; Nie, N.; Feng, S.; Shi, J.; Ge, M.; Li, X.; Shao, Y.; Huang, J.; Zheng, Y. The polymorphisms of human leukocyte antigen loci may contribute to the susceptibility and severity of severe aplastic anemia in Chinese patients. Hum. Immunol. 2014, 75, 867–872. [Google Scholar] [CrossRef]
- Jeong, T.-D.; Mun, Y.-C.; Chung, H.-S.; Seo, D.; Im, J.; Huh, J. Novel deletion mutation of HLA-B*40:02 gene in acquired aplastic anemia. HLA 2016, 89, 47–51. [Google Scholar] [CrossRef]
- Maluf, E.; Hamerschlak, N.; Cavalcanti, A.B.; Júnior, Á.A.; Eluf-Neto, J.; Falcão, R.P.; Lorand-Metze, I.G.; Goldenberg, D.; Santana, C.L.; Rodrigues, D.D.O.W.; et al. Incidence and risk factors of aplastic anemia in Latin American countries: The LATIN case-control study. Haematology 2009, 94, 1220–1226. [Google Scholar] [CrossRef]
- Yoshizato, T.; Dumitriu, B.; Hosokawa, K.; Makishima, H.; Yoshida, K.; Townsley, D.; Sato-Otsubo, A.; Sato, Y.; Liu, D.; Suzuki, H.; et al. Somatic Mutations and Clonal Hematopoiesis in Aplastic Anemia. N. Engl. J. Med. 2015, 373, 35–47. [Google Scholar] [CrossRef]
- Kallen, M.E.; Dulau-Florea, A.; Wang, W.; Calvo, K.R. Acquired and germline predisposition to bone marrow failure: Diagnostic features and clinical implications. Semin. Hematol. 2019, 56, 69–82. [Google Scholar] [CrossRef]
- Killick, S.B.; Bown, N.; Cavenagh, J.; Dokal, I.; Foukaneli, T.; Hill, A.; Hillmen, P.; Ireland, R.; Kulasekararaj, A.G.; Mufti, G.J.; et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br. J. Haematol. 2016, 172, 187–207. [Google Scholar] [CrossRef]
- Shimamura, A.; Alter, B.P. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev. 2010, 24, 101–122. [Google Scholar] [CrossRef]
- Invernizzi, R.; Quaglia, F.; Della Porta, M.G. Importance of classical morphology in the diagnosis of myelodysplastic syndrome. Mediterr. J. Hematol. Infect. Dis. 2015, 7, e2015035. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.; Cox, C.; Lebeau, M.M.; Fenaux, P.; Morel, P.; Sanz, G.; Sanz, M.; Vallespi, T.; Hamblin, T.; Oscier, D.; et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997, 89, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.F.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised International Prognostic Scoring System for Myelodysplastic Syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef]
- Bonadies, N.; Bacher, V.U. What role can next-generation sequencing play in myelodysplastic syndrome care? Expert Rev. Hematol. 2019, 12, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Silzle, T.; Blum, S.; Schuler, E.; Kaivers, J.; Rudelius, M.; Hildebrandt, B.; Gattermann, N.; Haas, R.; Germing, U. Lymphopenia at diagnosis is highly prevalent in myelodysplastic syndromes and has an independent negative prog-nostic value in IPSS-R-low-risk patients. Blood Cancer J. 2019, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Go, R.S.; Lust, J.A.; Phyliky, R.L. Aplastic anemia and pure red cell aplasia associated with large granular lymphocyte leukemia. Semin. Hematol. 2003, 40, 196–200. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Y.; Tang, J.; Zhan, Q.; Liao, Y. CD4(−)/CD8(−)/CD56(+)/TCRgammadelta(+) T-cell large granular lymphocyte leukemia presenting as aplastic ane-mia: A case report and literature review. Zhonghua Xue Ye Xue Za Zhi 2019, 40, 525–527. [Google Scholar]
- Tichelli, A.; Gratwohl, A.; Nissen, C.; Signer, E.; Gysi, C.S.; Speck, B. Morphology in patients with severe aplastic anemia treated with antilymphocyte globulin. Blood 1992, 80, 337–345. [Google Scholar] [CrossRef]
- Rovó, A.; Ebmt, O.B.O.T.S.-W.; Tichelli, A.; Dufour, C. Diagnosis of acquired aplastic anemia. Bone Marrow Transplant. 2012, 48, 162–167. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Bain, B.J.; Clark, D.M.; Wilkins, B.S. Bone Marrow Pathology, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Bain, B.J. Bone marrow trephine biopsy. J. Clin. Pathol. 2001, 54, 737–742. [Google Scholar] [CrossRef]
- Rovo, A.; Kulasekararaj, A.; Medinger, M.; Chevallier, P.; Ribera, J.M.; Peffault de Latour, R.; Knol, C.; Iacobelli, S.; Kanfer, E.; Bruno, B.; et al. Association of aplastic anaemia and lymphoma: A report from the severe aplastic anaemia working party of the Euro-pean Society of Blood and Bone Marrow Transplantation. Br. J. Haematol. 2019, 184, 294–298. [Google Scholar] [CrossRef]
- Medinger, M.; Buser, A.; Stern, M.; Heim, D.; Halter, J.; Rovó, A.; Tzankov, A.; Tichelli, A.; Passweg, J. Aplastic anemia in association with a lymphoproliferative neoplasm: Coincidence or causality? Leuk. Res. 2012, 36, 250–251. [Google Scholar] [CrossRef]
- Zonder, J.A.; Keating, M.; Schiffer, C.A. Chronic lymphocytic leukemia presenting in association with aplastic anemia. Am. J. Hematol. 2002, 71, 323–327. [Google Scholar] [CrossRef]
- Westers, T.M.; Ireland, R.; Kern, W.; Alhan, C.C.; Balleisen, J.S.; Bettelheim, P.; Burbury, K.; Cullen, M.; Cutler, J.; Della Porta, M.G.; et al. Standardization of flow cytometry in myelodysplastic syndromes: A report from an international consortium and the European LeukemiaNet Working Group. Leukemia 2012, 26, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Stojkov, K.; Silzle, T.; Stussi, G.; Schwappach, D.; Bernhard, J.; Bowen, D.; Čermák, J.; Dinmohamed, A.G.; Eeltink, C.; Eggmann, S.; et al. Guideline-based indicators for adult patients with myelodysplastic syndromes. Blood Adv. 2020, 4, 4029–4044. [Google Scholar] [CrossRef]
- Brodsky, R.A. Paroxysmal nocturnal hemoglobinuria. Blood 2014, 124, 2804–2811. [Google Scholar] [CrossRef]
- Schanz, J.; Tüchler, H.; Solé, F.; Mallo, M.; Luño, E.; Cervera, J.; Granada, I.; Hildebrandt, B.; Slovak, M.L.; Ohyashiki, K.; et al. New Comprehensive Cytogenetic Scoring System for Primary Myelodysplastic Syndromes (MDS) and Oligoblastic Acute Myeloid Leukemia After MDS Derived from an International Database Merge. J. Clin. Oncol. 2012, 30, 820–829. [Google Scholar] [CrossRef]
- Gupta, V.; Brooker, C.; Tooze, J.A.; Yi, Q.-L.; Sage, D.; Turner, D.; Kangasabapathy, P.; Marsh, J.C.W. Clinical relevance of cytogenetic abnormalities at diagnosis of acquired aplastic anaemia in adults. Br. J. Haematol. 2006, 134, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, K.; Katagiri, T.; Sugimori, N.; Ishiyama, K.; Sasaki, Y.; Seiki, Y.; Sato-Otsubo, A.; Sanada, M.; Ogawa, S.; Nakao, S. Favorable outcome of patients who have 13q deletion: A suggestion for revision of the WHO ’MDS-U’ designation. Haematologica 2012, 97, 1845–1849. [Google Scholar] [CrossRef] [PubMed]
- Holbro, A.; Jotterand, M.; Passweg, J.R.; Buser, A.; Tichelli, A.; Rovó, A. Comment to “Favorable outcome of patients who have 13q deletion: A suggestion for revision of the WHO ’MDS-U’ designation”. Haematologica 2013, 98, e46–e47. [Google Scholar] [CrossRef][Green Version]
- Heinrichs, S.; Li, C.; Look, A.T. SNP array analysis in hematologic malignancies: Avoiding false discoveries. Blood 2010, 115, 4157–4161. [Google Scholar] [CrossRef][Green Version]
- Ouahchi, I.; Zhang, L.; Brito, R.B.; Benz, R.; Müller, R.; Bonadies, N.; Tchinda, J. Microarray-based comparative genomic hybridisation reveals additional recurrent aberrations in adult patients evaluated for myelodysplastic syndrome with normal karyotype. Br. J. Haematol. 2019, 184, 282–287. [Google Scholar] [CrossRef]
- Kamps, R.; Brandão, R.D.; Bosch, B.J.V.D.; Paulussen, A.D.C.; Xanthoulea, S.; Blok, M.J.; Romano, A. Next-Generation Sequencing in Oncology: Genetic Diagnosis, Risk Prediction and Cancer Classification. Int. J. Mol. Sci. 2017, 18, 308. [Google Scholar] [CrossRef] [PubMed]
- Braggio, E.; Egan, J.B.; Fonseca, R.; Stewart, A.K. Lessons from next-generation sequencing analysis in hematological malignancies. Blood Cancer J. 2013, 3, e127. [Google Scholar] [CrossRef]
- Merker, J.D.; Valouev, A.; Gotlib, J. Next-generation sequencing in hematologic malignancies: What will be the dividends? Ther. Adv. Hematol. 2012, 3, 333–339. [Google Scholar] [CrossRef]
- Chirnomas, S.D.; Kupfer, G.M. The Inherited Bone Marrow Failure Syndromes. Pediatr. Clin. N. Am. 2013, 60, 1291–1310. [Google Scholar] [CrossRef]
- Mangaonkar, A.A.; Patnaik, M.M. Hereditary Predisposition to Hematopoietic Neoplasms: When Bloodline Matters for Blood Cancers. Mayo Clin. Proc. 2020, 95, 1482–1498. [Google Scholar] [CrossRef]
- Wegman-Ostrosky, T.; Savage, S.A. The genomics of inherited bone marrow failure: From mechanism to the clinic. Br. J. Haematol. 2017, 177, 526–542. [Google Scholar] [CrossRef]
- Alter, B.P. Diagnosis, Genetics, and Management of Inherited Bone Marrow Failure Syndromes. Hematol. Am. Soc. Hematol. Educ. Program. 2007, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Walne, A.J.; Collopy, L.; Cardoso, S.; Ellison, A.; Plagnol, V.; Albayrak, C.; Albayrak, D.; Kilic, S.S.; Patıroglu, T.; Akar, H.; et al. Marked overlap of four genetic syndromes with dyskeratosis congenita confounds clinical diagnosis. Haematologica 2016, 101, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Bluteau, O.; Sebert, M.; Leblanc, T.; De Latour, R.P.; Quentin, S.; Lainey, E.; Hernandez, L.; Dalle, J.-H.; De Fontbrune, F.S.; Lengline, E.; et al. A landscape of germ line mutations in a cohort of inherited bone marrow failure patients. Blood 2018, 131, 717–732. [Google Scholar] [CrossRef]
- Ogawa, S. Genetics of MDS. Blood 2019, 133, 1049–1059. [Google Scholar] [CrossRef]
- Li, M.M.; Chao, E.; Esplin, E.D.; Miller, D.T.; Nathanson, K.L.; Plon, S.E.; Scheuner, M.T.; Stewart, D.R.; ACMG Professional Practice and Guidelines Committee. Points to consider for reporting of germline variation in patients undergoing tumor testing: A statement of the Ameri-can College of Medical Genetics and Genomics (ACMG). Genet. Med. 2020, 22, 1142–1148. [Google Scholar] [CrossRef]
- Malcovati, L.; Gallì, A.; Travaglino, E.; Ambaglio, I.; Rizzo, E.; Molteni, E.; Elena, C.; Ferretti, V.V.; Catricalà, S.; Bono, E.; et al. Clinical significance of somatic mutation in unexplained blood cytopenia. Blood 2017, 129, 3371–3378. [Google Scholar] [CrossRef]
- Valent, P.; Orazi, A.; Steensma, D.P.; Ebert, B.L.; Haase, D.; Malcovati, L.; Van De Loosdrecht, A.A.; Haferlach, T.; Westers, T.M.; Wells, D.A.; et al. Proposed minimal diagnostic criteria for myelodysplastic syndromes (MDS) and potential pre-MDS conditions. Oncotarget 2017, 8, 73483–73500. [Google Scholar] [CrossRef]
- Malcovati, L.; Germing, U.; Kuendgen, A.; Della Porta, M.G.; Pascutto, C.; Invernizzi, R.; Giagounidis, A.; Hildebrandt, B.; Bernasconi, P.; Knipp, S.; et al. Time-Dependent Prognostic Scoring System for Predicting Survival and Leukemic Evolution in Myelodysplastic Syndromes. J. Clin. Oncol. 2007, 25, 3503–3510. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before alloge-neic HCT. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef]
- Sorror, M.L.; Storb, R.F.; Sandmaier, B.M.; Maziarz, R.T.; Pulsipher, M.A.; Maris, M.B.; Bhatia, S.; Ostronoff, F.; Deeg, H.J.; Syrjala, K.L.; et al. Comorbidity-Age Index: A Clinical Measure of Biologic Age Before Allogeneic Hematopoietic Cell Transplantation. J. Clin. Oncol. 2014, 32, 3249–3256. [Google Scholar] [CrossRef]
- Della Porta, M.G.; Malcovati, L.; Strupp, C.; Ambaglio, I.; Kuendgen, A.; Zipperer, E.; Travaglino, E.; Invernizzi, R.; Pascutto, C.; Lazzarino, M.; et al. Risk stratification based on both disease status and extra-hematologic comorbidities in patients with myelo-dysplastic syndrome. Haematologica 2011, 96, 441–449. [Google Scholar] [CrossRef]
- Swinkels, M.; Rijkers, M.; Voorberg, J.; Vidarsson, G.; Leebeek, F.W.G.; Jansen, A.J.G. Emerging Concepts in Immune Thrombocytopenia. Front. Immunol. 2018, 9, 880. [Google Scholar] [CrossRef]
- Waisbren, J.; Dinner, S.; Altman, J.; Frankfurt, O.; Helenowski, I.; Gao, J.; McMahon, B.J.; Stein, B.L. Disease characteristics and prognosis of myelodysplastic syndrome presenting with isolated thrombocytopenia. Int. J. Hematol. 2016, 105, 44–51. [Google Scholar] [CrossRef]
- Kuroda, J.; Kimura, S.; Kobayashi, Y.; Wada, K.; Uoshima, N.; Yoshikawa, T. Unusual myelodysplastic syndrome with the initial presentation mimicking idiopathic thrombocytopenic purpura. Acta Haematol. 2002, 108, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Bryan, J.; Jabbour, E.; Prescott, H.; Kantarjian, H. Thrombocytopenia in patients with myelodysplastic syndromes. Semin. Hematol. 2010, 47, 274–280. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mittelman, M. Good news for patients with myelodysplastic syndromes and thrombocytopenia. Lancet Haematol. 2018, 5, e100–e101. [Google Scholar] [CrossRef]
- Manoharan, A.; Brighton, T.; Gemmell, R.; Lopez, K.; Moran, S.; Kyle, P. Platelet Dysfunction in Myelodysplastic Syndromes: A Clinicopathological Study. Int. J. Hematol. 2002, 76, 272–278. [Google Scholar] [CrossRef]
- Galera, P.; Dulau-Florea, A.; Calvo, K.R. Inherited thrombocytopenia and platelet disorders with germline predisposition to myeloid neoplasia. Int. J. Lab. Hematol. 2019, 41 (Suppl. S1), 131–141. [Google Scholar] [CrossRef]
- Sekeres, M.A.; Schoonen, W.M.; Kantarjian, H.; List, A.; Fryzek, J.; Paquette, R.; Maciejewski, J.P. Characteristics of US Patients with Myelodysplastic Syndromes: Results of Six Cross-sectional Physician Surveys. J. Natl. Cancer Inst. 2008, 100, 1542–1551. [Google Scholar] [CrossRef]
- Klymenko, S.V.; Belyi, D.A.; Ross, J.R.; Owzar, K.; Jiang, C.; Li, Z.; Minchenko, J.N.; Kovalenko, A.N.; Bebeshko, V.G.; Chao, N.J. Hematopoietic cell infusion for the treatment of nuclear disaster victims: New data from the Chernobyl accident. Int. J. Radiat. Biol. 2011, 87, 846–850. [Google Scholar] [CrossRef]
- Camitta, B.M.; Rappeport, J.M.; Parkman, R.; Nathan, D.G. Selection of patients for bone marrow transplantation in severe aplastic anemia. Blood 1975, 45, 355–363. [Google Scholar] [CrossRef]
- Bacigalupo, A.; Hows, J.; Gluckman, E.; Nissen, C.; Marsh, J.; Van Lint, M.T.; Congiu, M.; De Planque, M.M.; Ernst, P.; McCann, S.; et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): A report of the EBMT SAA Working Party. Br. J. Haematol. 1988, 70, 177–182. [Google Scholar] [CrossRef]
- Gross, S.; Kiwanuka, J. Chronic ITP terminating in aplastic anemia. Am. J. Pediatr. Hematol. Oncol. 1981, 3, 446–448. [Google Scholar]
- Yun, G.-W.; Yang, Y.-J.; Song, I.-C.; Baek, S.-W.; Lee, K.-S.; Lee, H.-J.; Yun, H.-J.; Kwon, K.-C.; Kim, S.; Jo, D.-Y. Long-term outcome of isolated thrombocytopenia accompanied by hypocellular marrow. Korean J. Hematol. 2011, 46, 128–134. [Google Scholar] [CrossRef][Green Version]
- Levy, I.; Laor, R.; Jiries, N.; Bejar, J.; Polliack, A.; Tadmor, T. Amegakaryocytic Thrombocytopenia and Subsequent Aplastic Anemia Associated with Apparent Epstein-Barr Virus Infection. Acta Haematol. 2018, 139, 7–11. [Google Scholar] [CrossRef]
- King, J.A.C.; ElKhalifa, M.Y.; Latour, L.F. Rapid Progression of Acquired Amegakaryocytic Thrombocytopenia to Aplastic Anemia. South. Med. J. 1997, 90, 91–94. [Google Scholar] [CrossRef]
- Young, N.S.; Maciejewski, J.P.; Sloand, E.; Chen, G.; Zeng, W.; Risitano, A.; Miyazato, A. The relationship of aplastic anemia and PNH. Int. J. Hematol. 2002, 76, 168–172. [Google Scholar] [CrossRef]
- Pu, J.J.; Mukhina, G.; Wang, H.; Savage, W.J.; Brodsky, R.A. Natural history of paroxysmal nocturnal hemoglobinuria clones in patients presenting as aplastic anemia. Eur. J. Haematol. 2011, 87, 37–45. [Google Scholar] [CrossRef]
- Sutherland, D.R.; Illingworth, A.; Marinov, I.; Ortiz, F.; Andrea, I.; Payne, D.; Wallace, P.K.; Keeney, M. ICCS/ESCCA Consensus Guidelines to detect GPI-deficient cells in Paroxysmal Nocturnal Hemoglobinuria (PNH) and related Disorders Part 2—Reagent Selection and Assay Optimization for High-Sensitivity Testing. Cytom. Part B Clin. Cytom. 2018, 94, 23–48. [Google Scholar] [CrossRef]
- Auerbach, A.D. Fanconi anemia and its diagnosis. Mutat. Res. Mol. Mech. Mutagen. 2009, 668, 4–10. [Google Scholar] [CrossRef]
- Malcovati, L.; Hellström-Lindberg, E.; Bowen, D.; Adès, L.; Cermak, J.; Del Cañizo, C.; Della Porta, M.G.; Fenaux, P.; Gattermann, N.; Germing, U.; et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: Recommendations from the European LeukemiaNet. Blood 2013, 122, 2943–2964. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, O.; Figueroa, M.E. Interpreting new molecular genetics in myelodysplastic syndromes. Haematology 2012, 2012, 56–64. [Google Scholar] [CrossRef]
- Heuser, M.; Thol, F.; Ganser, A. Clonal Hematopoiesis of Indeterminate Potential. Dtsch. Aerzteblatt Int. 2016, 113, 317–322. [Google Scholar] [CrossRef]
- Jaiswal, S.; Fontanillas, P.; Flannick, J.; Manning, A.; Grauman, P.V.; Mar, B.G.; Lindsley, R.C.; Mermel, C.H.; Burtt, N.; Chavez, A.; et al. Age-Related Clonal Hematopoiesis Associated with Adverse Outcomes. N. Engl. J. Med. 2014, 371, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Genovese, G.; Kähler, A.K.; Handsaker, R.E.; Lindberg, J.; Rose, S.A.; Bakhoum, S.F.; Chambert, K.; Mick, E.; Neale, B.M.; Fromer, M.; et al. Clonal Hematopoiesis and Blood-Cancer Risk Inferred from Blood DNA Sequence. N. Engl. J. Med. 2014, 371, 2477–2487. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Lu, C.; Wang, J.; McLellan, M.D.; Johnson, K.J.; Wendl, M.C.; McMichael, J.F.; Schmidt, H.K.; Yellapantula, V.; Miller, C.A.; et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat. Med. 2014, 20, 1472–1478. [Google Scholar] [CrossRef]
- Steensma, D.P.; Bejar, R.; Jaiswal, S.; Lindsley, R.C.; Sekeres, M.A.; Hasserjian, R.P.; Ebert, B.L. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015, 126, 9–16. [Google Scholar] [CrossRef] [PubMed]
| Laboratory Test | Provide Information on |
|---|---|
| Automated blood count (MPV, IPF), reticulocytes | CBC. Quantitative values. Platelet size and production capacity of the bone marrow. |
| Blood smear | Pseudothrombocytopenia, schistocytes, dysplasia, blasts, general cell line; changes and maturation |
| Substrates (folate, Vitamin B12/holoTC, iron tests) | Substrate deficiency |
| PT, INR, aPTT, Fibrinogen | Coagulopathy (DIC, TTP) |
| Liver and kidney tests function | Liver or kidney disease |
| Infections (HIV, HCV, HBV, CMV, EBV, Parvo B19) | Viral infection |
| Protein electrophoresis with immunofixation | Lymphoid neoplasms, plasma cell neoplasms |
| Free light chains | plasma cell neoplasms |
| LDH, bilirubin, haptoglobin, DAT | Hemolysis |
| TSH (ANA, ANCA, RF) | Autoimmunity |
| Abdominal ultrasound | Liver disease, splenomegaly, lymph nodes enlargement |
| Parameters | Hypoplastic MDS | Aplastic Anemia |
|---|---|---|
| Cytopenia | Present | Present |
| BM cellularity | Hypocellular | Aplastic (<10% cellularity or significantly hypocellular) |
| BM hematopoiesis | ||
| Erythropoiesis | Present | Present in nests, “hot spots” |
| Granulopoiesis | Present | Typically decreased |
| Megakaryopoiesis | Present | Decreased or absent |
| BM fat replacement | Possible | Typical |
| Dysplasia | ||
| Erythroid dysplasia | Frequent | Possible |
| Granulocytic dysplasia | Frequent | Normal morphology |
| Megakaryocytic dysplasia | Frequent | Normal morphology |
| Ring sideroblasts | Possible | Absent |
| Blasts | Variable | Absent |
| CD34+ or CD117+ immunohistochemistry | Normal or increased | No increase |
| Marrow fibrosis | Possible | Absent |
| PNH clone | Unusual | Frequent |
| Splenomegaly at diagnosis | Possible | Absent |
| Karyotype | Abnormal ~50% | Clonal abnormality possible (~12%) |
| Recurrent cytogenetic abnormalities | -Y, del(11q), −5/del(5q), del(12p), del(20q), −7/del(7q), +8, +19, i(17q), inv(3)/t(3q)/del(3q) | At Diagnosis: del(13q), +8 Evolution: −7, −5/del (5q), del(20q) |
| Complex cytogenetics (≥3 abnormalities) | Possible | Absent |
| Acquired CN-LOH | Possible | Possible (<20%) |
| Somatic mutated genes | SF3B1, SRSF2, U2AF1, ZRSR2, TET2, DNMT3A, IDH1, IDH2, ASXL1, EZH2, RUNX1, NRAS, BCOR, TP53, STAG2 | Particularly PIGA, ASXL1, BCOR, BCORL1; 5–52% of patients will present MDS-associated mutations |
| Germline mutations | Should be investigated in patients with suspicion of underlying germline predisposition. | Should be investigated in patients with suspicion of underlying congenital BMF. |
| Cytological/Histological Variables | |
|---|---|
| Requisite criteria | Scoring points |
| Bone marrow blasts AND/OR CD34 + cells ≥5% | 2 |
| Bone marrow blasts AND/OR CD34 + cells 2–4% | 1 |
| Fibrosis grade 2–3 | 1 |
| Dysmegakaryopoiesis | 1 |
| Co-criteria | |
| Ring sideroblasts ≥15% | 2 |
| Ring sideroblasts 5–14% * | 1 |
| Severe dysgranulopoiesis | 1 |
| Karyotype (co-criterion) | |
| Presumptive cytogenetic abnormality * | 2 |
| Somatic mutation (co-criterion) | |
| Specific high-risk mutation pattern ** | 1 |
| Criteria | Major Diagnostic Tests |
|---|---|
| Prerequisite criteria (both must be fulfilled) | |
| Constant cytopenia | Blood counts (over 6 months) |
| Exclusion of all other diseases as primary cause of cytopenia⁄dysplasia | BM smear and BM histology, cytogenetics, flow cytometry, molecular markers, other relevant investigations * |
| MDS-related criteria (one of these must be fulfilled) | |
| Morphological dysplasia in one of the three major lineages | BM and PB smear, in certain situations BM histology |
| Blast count ≥5% | BM smear and histology |
| Ring sideroblasts ≥15% or ≥5% and SF3B1 mutation | Iron staining |
| Typical karyotype anomaly | Conventional karyotyping and/or FISH |
| Co-criteria | |
| Monoclonality of myeloid cells | Molecular markers and mutations |
| BM stem cell function | Circulating CFC, reticulocytes |
| Abnormal immunophenotype of BM cells | Multicolor flow cytometry, immunohistochemistry |
| Abnormal gene expression profile | mRNA profiling assays |
| Subtype 1 | No. of Dysplastic Lineages | No. of Cytopenic Lineages 2 | % RS of all Erythroid Cells in BM | % Blasts in PB or BM AR: Auer Rods | Conventional Cytogenetics | |||
|---|---|---|---|---|---|---|---|---|
| wtSF3B1 | mSF3B1 | BM | PB | AR | ||||
| MDS-SLD | 1 | 1 or 2 | <15 | <5 | <5 | <1 | - | |
| MDS-MLD | 2 or 3 | 1–3 | <15 | <5 | <5 | <1 | - | |
| MDS RS-SLD | 1 | 1 or 2 | ≥15 | ≥5 | <5 | <1 | - | |
| MDS RS-MLD | 2 or 3 | 1–3 | ≥15 | ≥5 | <5 | <1 | - | |
| MDS del(5q) | 1–3 | 1 or 2 | n.a. | n.a. | <5 | <1 | - | Isolated del(5q) +/− 1 add. aberration without del(7q)/−7 |
| MDS EB-1 | 0–3 | 1–3 | n.a. | n.a. | 5–9 | 2–4 | - | |
| MDS EB-2 | 0–3 | 1–3 | n.a. | n.a. | 10–19 | 5–19 | + | |
| MDS-U | <15 | <5 | <5 | <1 | - | |||
| (a) 1% blasts in PB | 1–3 | 1–3 | n.a. | n.a. | <5 | 1 3 | - | |
| (b) SLD with pancytopenia | 1 | 3 | n.a. | n.a. | <5 | <1 | - | |
| (c) defining cytogenetic aberration | 0 | 1–3 | <15 4 | n.a. | <5 | <1 | - | MDS defining cytogenetic aberration |
| RCC | 1–3 | 1–3 | <15 | ≤5 | <5 | <1 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonadies, N.; Rovó, A.; Porret, N.; Bacher, U. When Should We Think of Myelodysplasia or Bone Marrow Failure in a Thrombocytopenic Patient? A Practical Approach to Diagnosis. J. Clin. Med. 2021, 10, 1026. https://doi.org/10.3390/jcm10051026
Bonadies N, Rovó A, Porret N, Bacher U. When Should We Think of Myelodysplasia or Bone Marrow Failure in a Thrombocytopenic Patient? A Practical Approach to Diagnosis. Journal of Clinical Medicine. 2021; 10(5):1026. https://doi.org/10.3390/jcm10051026
Chicago/Turabian StyleBonadies, Nicolas, Alicia Rovó, Naomi Porret, and Ulrike Bacher. 2021. "When Should We Think of Myelodysplasia or Bone Marrow Failure in a Thrombocytopenic Patient? A Practical Approach to Diagnosis" Journal of Clinical Medicine 10, no. 5: 1026. https://doi.org/10.3390/jcm10051026
APA StyleBonadies, N., Rovó, A., Porret, N., & Bacher, U. (2021). When Should We Think of Myelodysplasia or Bone Marrow Failure in a Thrombocytopenic Patient? A Practical Approach to Diagnosis. Journal of Clinical Medicine, 10(5), 1026. https://doi.org/10.3390/jcm10051026








