Diagnosis of Pancreatic Solid Lesions, Subepithelial Lesions, and Lymph Nodes Using Endoscopic Ultrasound
Abstract
1. Introduction
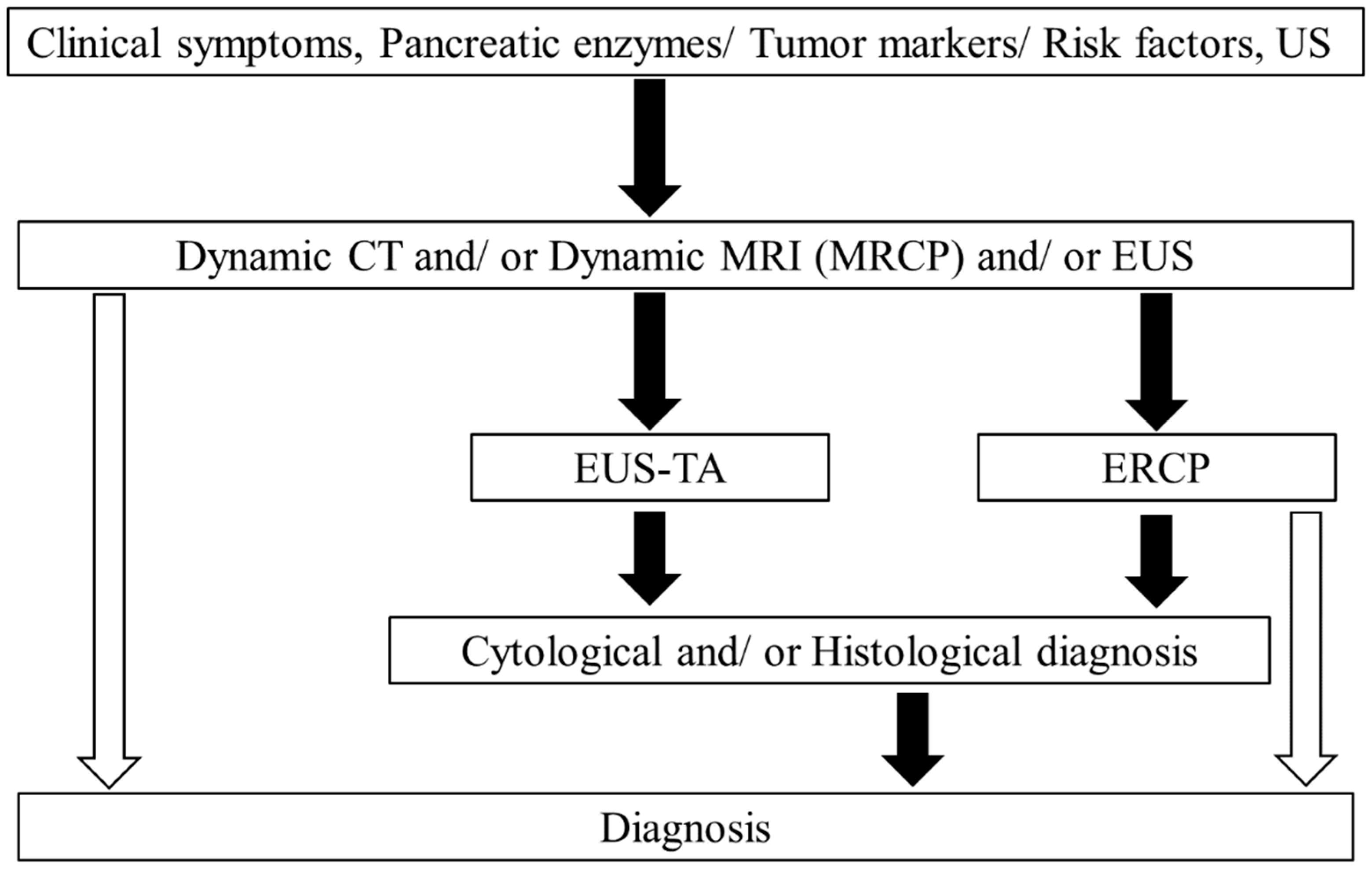
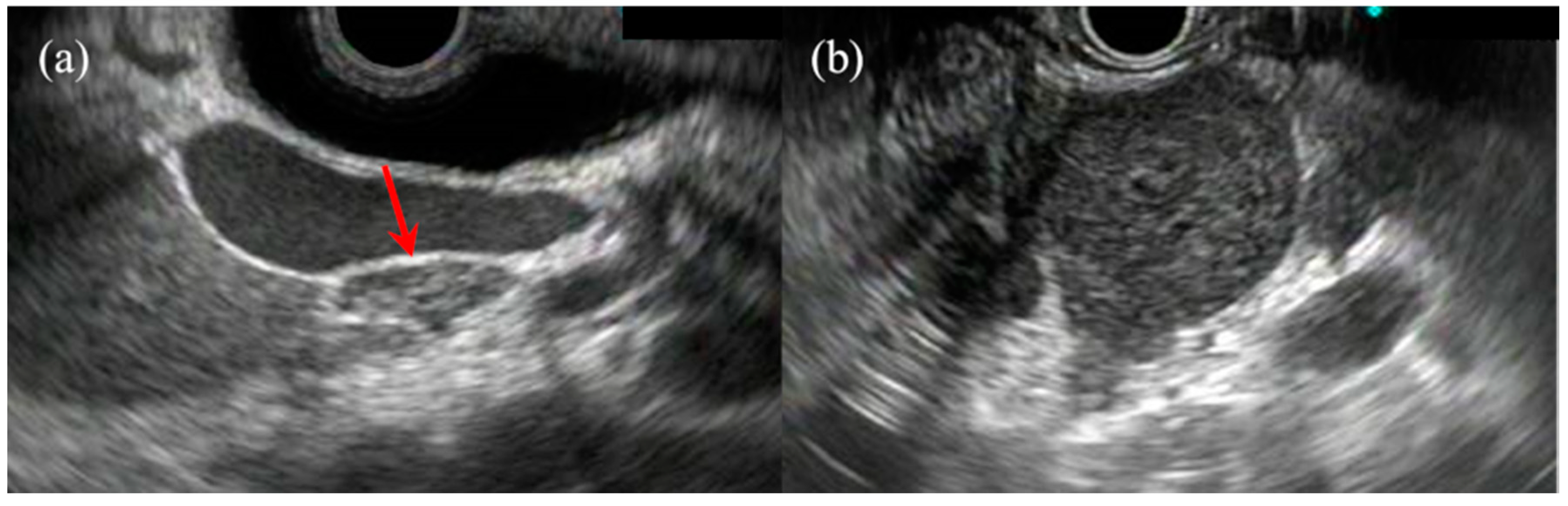
2. Pancreatic Solid Lesions
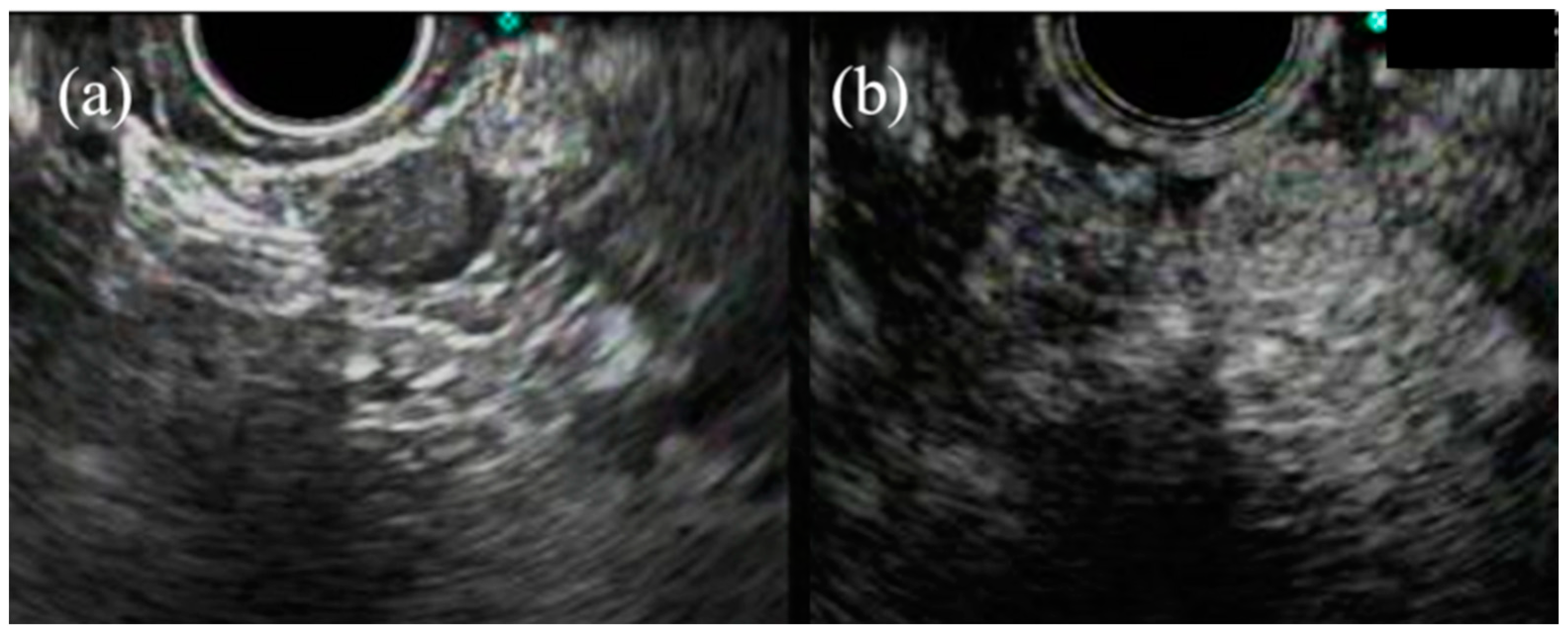
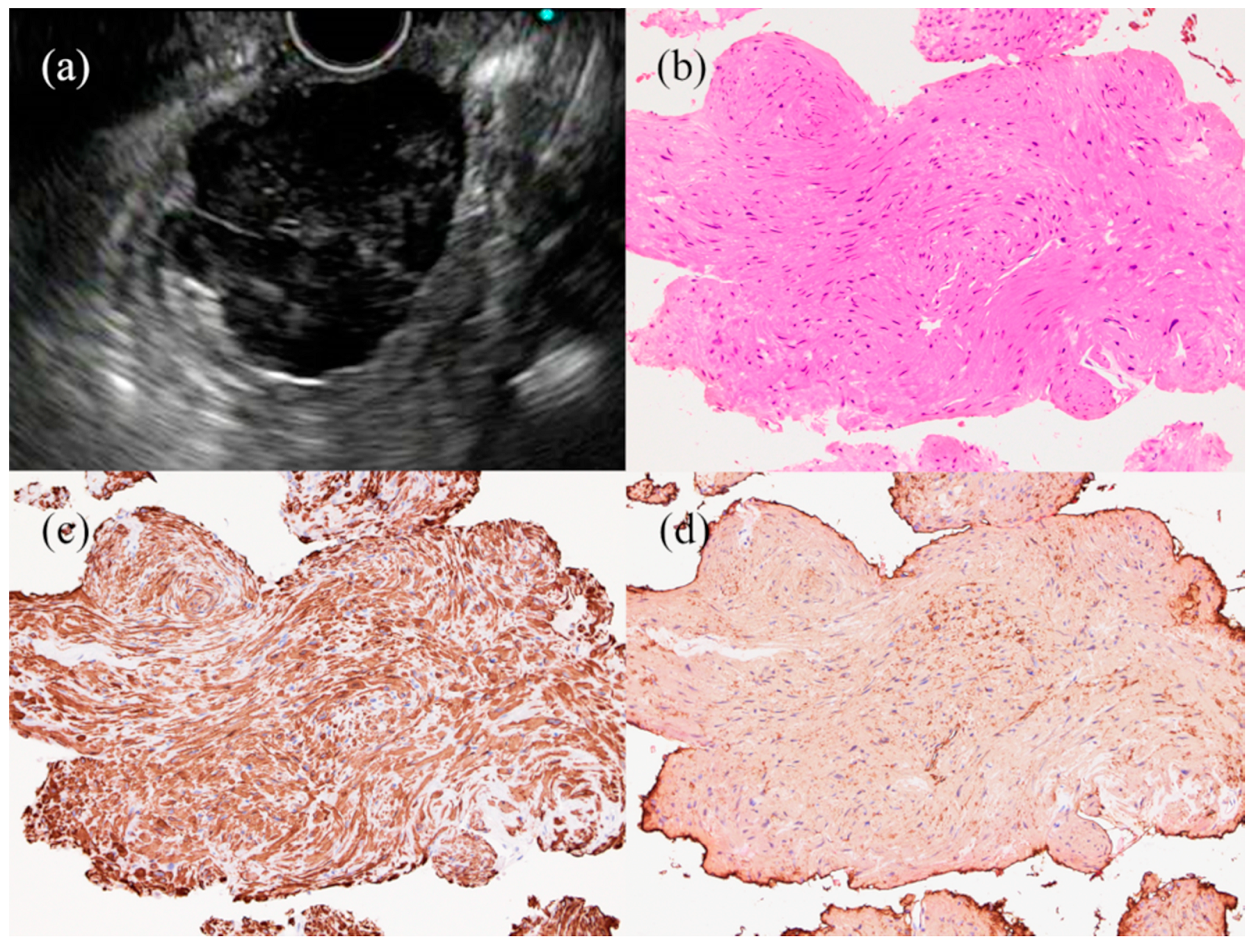
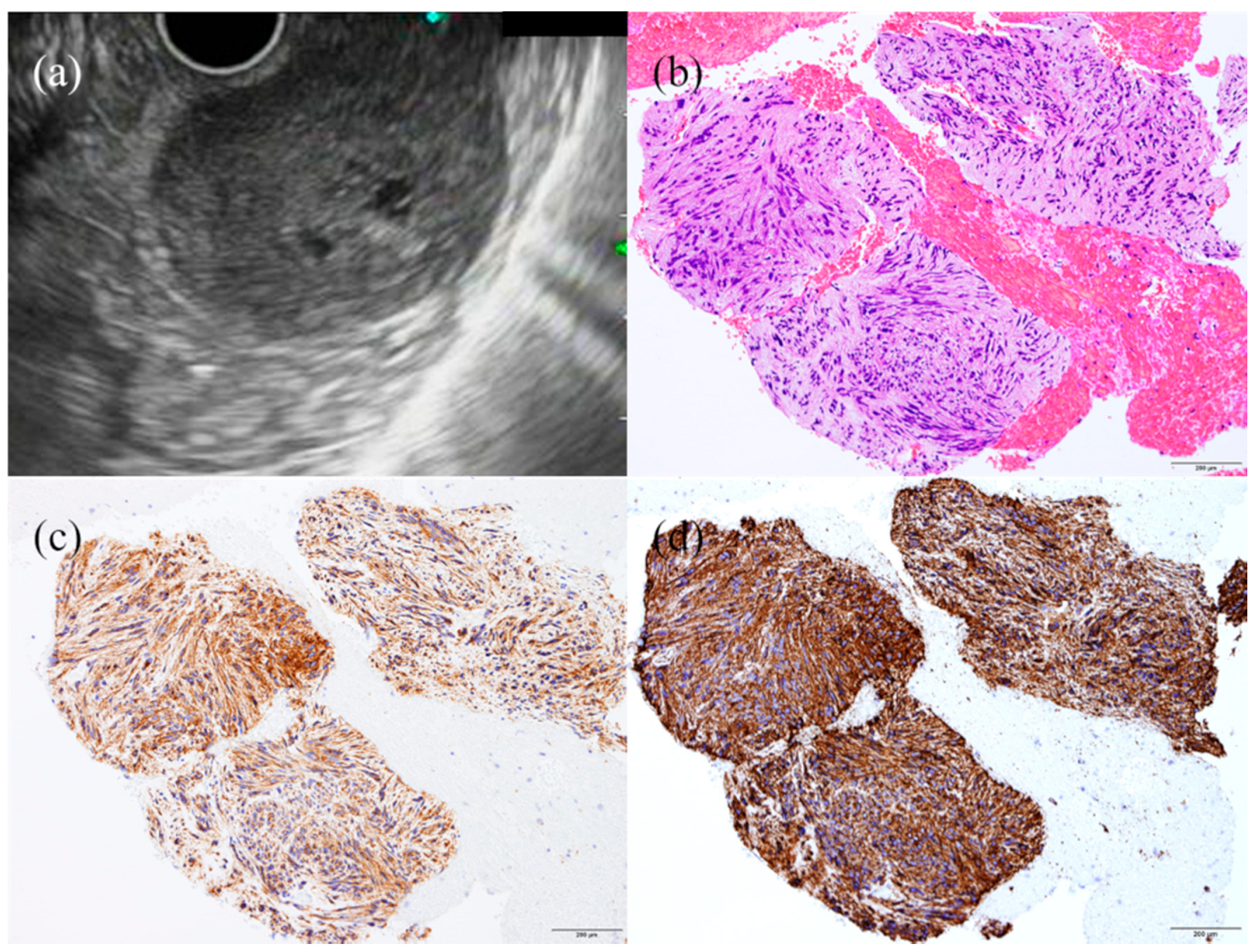
3. Subepithelial Lesions
4. Lymph Nodes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Irisawa, A.; Yamao, K. Curved linear array EUS technique in the pancreas and biliary tree: Focusing on the stations. Gastrointest. Endosc. 2009, 69, S84–S89. [Google Scholar] [CrossRef]
- Matsumoto, K.; Takeda, Y.; Onoyama, T.; Kawata, S.; Kurumi, H.; Koda, H.; Yamashita, T.; Isomoto, H. Endoscopic ultrasound-guided fine-needle aspiration biopsy—Recent topics and technical tips. World J. Clin. Cases 2019, 7, 1775–1783. [Google Scholar] [CrossRef]
- Cazacu, I.M.; Luzuriaga Chavez, A.A.; Saftoiu, A.; Vilmann, P.; Bhutani, M.S. A quarter century of EUS-FNA: Progress, milestones, and future directions. Endosc. Ultrasound 2018, 7, 141–160. [Google Scholar] [CrossRef]
- Vilmann, P.; Jacobsen, G.K.; Henriksen, F.W.; Hancke, S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest. Endosc. 1992, 38, 172–173. [Google Scholar] [CrossRef]
- Ryozawa, S.; Kitoh, H.; Gondo, T.; Urayama, N.; Yamashita, H.; Ozawa, H.; Yanai, H.; Okita, K. Usefulness of endoscopic ultrasound-guided fine-needle aspiration biopsy for the diagnosis of pancreatic cancer. J. Gastroenterol. 2005, 40, 907–911. [Google Scholar] [CrossRef]
- Yasuda, I.; Tsurumi, H.; Omar, S.; Iwashita, T.; Kojima, Y.; Yamada, T.; Sawada, M.; Takami, T.; Moriwaki, H.; Soehendra, N. Endoscopic ultrasound-guided fine-needle aspiration biopsy for lymphadenopathy of unknown origin. Endoscopy 2006, 38, 919–924. [Google Scholar] [CrossRef]
- Mekky, M.A.; Yamao, K.; Sawaki, A.; Mizuno, N.; Hara, K.; Nafeh, M.A.; Osman, A.M.; Koshikawa, T.; Yatabe, Y.; Bhatia, V. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest. Endosc. 2010, 71, 913–919. [Google Scholar] [CrossRef]
- Horwhat, J.D.; Paulson, E.K.; McGrath, K.; Branch, M.S.; Baillie, J.; Tyler, D.; Pappas, T.; Enns, R.; Robuck, G.; Stiffler, H.; et al. A randomized comparison of EUS-guided FNA versus CT or US-guided FNA for the evaluation of pancreatic mass lesions. Gastrointest. Endosc. 2006, 63, 966–975. [Google Scholar] [CrossRef]
- Wang, K.X.; Ben, Q.W.; Jin, Z.D.; Du, Y.Q.; Zou, D.W.; Liao, Z.; Li, Z.S. Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest. Endosc. 2011, 73, 283–290. [Google Scholar] [CrossRef]
- Mizuide, M.; Ryozawa, S.; Fujita, A.; Ogawa, T.; Katsuda, H.; Suzuki, M.; Noguchi, T.; Tanisaka, Y. Complications of Endoscopic Ultrasound-Guided Fine Needle Aspiration: A Narrative Review. Diagn. (BaselSwitz.) 2020, 10, 964. [Google Scholar] [CrossRef]
- Khalid, A.; Nodit, L.; Zahid, M.; Bauer, K.; Brody, D.; Finkelstein, S.D.; McGrath, K.M. Endoscopic ultrasound fine needle aspirate DNA analysis to differentiate malignant and benign pancreatic masses. Am. J. Gastroenterol. 2006, 101, 2493–2500. [Google Scholar] [CrossRef]
- Eloubeidi, M.A.; Chen, V.K.; Eltoum, I.A.; Jhala, D.; Chhieng, D.C.; Jhala, N.; Vickers, S.M.; Wilcox, C.M. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: Diagnostic accuracy and acute and 30-day complications. Am. J. Gastroenterol. 2003, 98, 2663–2668. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.F.; Rosenblatt, M.L.; Chak, A.; Sivak, M.V., Jr.; Scheiman, J.; Gress, F. Endoscopic ultrasound-guided fine needle aspiration in the diagnosis of mediastinal masses of unknown origin. Am. J. Gastroenterol. 2002, 97, 2559–2565. [Google Scholar] [CrossRef][Green Version]
- Tournoy, K.G.; Praet, M.M.; Van Maele, G.; Van Meerbeeck, J.P. Esophageal endoscopic ultrasound with fine-needle aspiration with an on-site cytopathologist: High accuracy for the diagnosis of mediastinal lymphadenopathy. Chest 2005, 128, 3004–3009. [Google Scholar] [CrossRef]
- Yasuda, I.; Goto, N.; Tsurumi, H.; Nakashima, M.; Doi, S.; Iwashita, T.; Kanemura, N.; Kasahara, S.; Adachi, S.; Hara, T.; et al. Endoscopic ultrasound-guided fine needle aspiration biopsy for diagnosis of lymphoproliferative disorders: Feasibility of immunohistological, flow cytometric, and cytogenetic assessments. Am. J. Gastroenterol. 2012, 107, 397–404. [Google Scholar] [CrossRef]
- Philipper, M.; Hollerbach, S.; Gabbert, H.E.; Heikaus, S.; Bocking, A.; Pomjanski, N.; Neuhaus, H.; Frieling, T.; Schumacher, B. Prospective comparison of endoscopic ultrasound-guided fine-needle aspiration and surgical histology in upper gastrointestinal submucosal tumors. Endoscopy 2010, 42, 300–305. [Google Scholar] [CrossRef]
- Singh, P.; Mukhopadhyay, P.; Bhatt, B.; Patel, T.; Kiss, A.; Gupta, R.; Bhat, S.; Erickson, R.A. Endoscopic ultrasound versus CT scan for detection of the metastases to the liver: Results of a prospective comparative study. J. Clin. Gastroenterol. 2009, 43, 367–373. [Google Scholar] [CrossRef]
- DeWitt, J.; Alsatie, M.; LeBlanc, J.; McHenry, L.; Sherman, S. Endoscopic ultrasound-guided fine-needle aspiration of left adrenal gland masses. Endoscopy 2007, 39, 65–71. [Google Scholar] [CrossRef]
- Lee, J.H.; Salem, R.; Aslanian, H.; Chacho, M.; Topazian, M. Endoscopic ultrasound and fine-needle aspiration of unexplained bile duct strictures. Am. J. Gastroenterol. 2004, 99, 1069–1073. [Google Scholar] [CrossRef]
- Eloubeidi, M.A.; Chen, V.K.; Jhala, N.C.; Eltoum, I.E.; Jhala, D.; Chhieng, D.C.; Syed, S.A.; Vickers, S.M.; Mel Wilcox, C. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin. Gastroenterol. Hepatol. 2004, 2, 209–213. [Google Scholar] [CrossRef]
- Tanaka, M.; Fernandez-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef]
- Hirooka, Y.; Goto, H.; Itoh, A.; Hashimoto, S.; Niwa, K.; Ishikawa, H.; Okada, N.; Itoh, T.; Kawashima, H. Case of intraductal papillary mucinous tumor in which endosonography-guided fine-needle aspiration biopsy caused dissemination. J. Gastroenterol. Hepatol. 2003, 18, 1323–1324. [Google Scholar] [CrossRef]
- Fujita, A.; Ryozawa, S.; Tanisaka, Y.; Ogawa, T.; Suzuki, M.; Noguchi, T.; Katsuda, H.; Mizuide, M. Current Status of Needles in the Optimization of Endoscopic Ultrasound-Guided Procedures. Diagn. (BaselSwitz.) 2020, 10, 463. [Google Scholar] [CrossRef]
- Hanada, K.; Okazaki, A.; Hirano, N.; Izumi, Y.; Teraoka, Y.; Ikemoto, J.; Kanemitsu, K.; Hino, F.; Fukuda, T.; Yonehara, S. Diagnostic strategies for early pancreatic cancer. J. Gastroenterol. 2015, 50, 147–154. [Google Scholar] [CrossRef]
- Kanno, A.; Masamune, A.; Hanada, K.; Maguchi, H.; Shimizu, Y.; Ueki, T.; Hasebe, O.; Ohtsuka, T.; Nakamura, M.; Takenaka, M.; et al. Multicenter study of early pancreatic cancer in Japan. Pancreatology 2018, 18, 61–67. [Google Scholar] [CrossRef]
- Okusaka, T.; Nakamura, M.; Yoshida, M.; Kitano, M.; Uesaka, K.; Ito, Y.; Furuse, J.; Hanada, K.; Okazaki, K. Clinical Practice Guidelines for Pancreatic Cancer 2019 From the Japan Pancreas Society: A Synopsis. Pancreas 2020, 49, 326–335. [Google Scholar] [CrossRef]
- Yamashita, Y.; Shimokawa, T.; Napoléon, B.; Fusaroli, P.; Gincul, R.; Kudo, M.; Kitano, M. Value of contrast-enhanced harmonic endoscopic ultrasonography with enhancement pattern for diagnosis of pancreatic cancer: A meta-analysis. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2019, 31, 125–133. [Google Scholar] [CrossRef]
- Kitano, M.; Kudo, M.; Yamao, K.; Takagi, T.; Sakamoto, H.; Komaki, T.; Kamata, K.; Imai, H.; Chiba, Y.; Okada, M.; et al. Characterization of small solid tumors in the pancreas: The value of contrast-enhanced harmonic endoscopic ultrasonography. Am. J. Gastroenterol. 2012, 107, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.K.; Ioncică, A.M.; Săftoiu, A.; Vilmann, P.; Bhutani, M.S. Contrast-enhanced endoscopic ultrasonography. World J. Gastroenterol. 2011, 17, 42–48. [Google Scholar] [CrossRef]
- Banafea, O.; Mghanga, F.P.; Zhao, J.; Zhao, R.; Zhu, L. Endoscopic ultrasonography with fine-needle aspiration for histological diagnosis of solid pancreatic masses: A meta-analysis of diagnostic accuracy studies. BMC Gastroenterol. 2016, 16, 108. [Google Scholar] [CrossRef]
- Chen, J.; Yang, R.; Lu, Y.; Xia, Y.; Zhou, H. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesion: A systematic review. J. Cancer Res. Clin. Oncol. 2012, 138, 1433–1441. [Google Scholar] [CrossRef]
- Hewitt, M.J.; McPhail, M.J.; Possamai, L.; Dhar, A.; Vlavianos, P.; Monahan, K.J. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest. Endosc. 2012, 75, 319–331. [Google Scholar] [CrossRef]
- Puli, S.R.; Bechtold, M.L.; Buxbaum, J.L.; Eloubeidi, M.A. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: A meta-analysis and systematic review. Pancreas 2013, 42, 20–26. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Qu, C.; Liang, S.; Zeng, B.; Luo, Z. Endoscopic ultrasound-guided fine needle core biopsy for the diagnosis of pancreatic malignant lesions: A systematic review and Meta-Analysis. Sci. Rep. 2016, 6, 22978. [Google Scholar] [CrossRef]
- Hébert-Magee, S.; Bae, S.; Varadarajulu, S.; Ramesh, J.; Frost, A.R.; Eloubeidi, M.A.; Eltoum, I.A. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: A meta-analysis. Cytopathology 2013, 24, 159–171. [Google Scholar] [CrossRef]
- Seicean, A.; Samarghitan, A.; Bolboacă, S.D.; Pojoga, C.; Rusu, I.; Rusu, D.; Sparchez, Z.; Gheorghiu, M.; Al Hajjar, N.; Seicean, R. Contrast-enhanced harmonic versus standard endoscopic ultrasound-guided fine-needle aspiration in solid pancreatic lesions: A single-center prospective randomized trial. Endoscopy 2020, 52, 1084–1090. [Google Scholar] [CrossRef]
- Oppong, K.W.; Bekkali, N.L.H.; Leeds, J.S.; Johnson, S.J.; Nayar, M.K.; Darné, A.; Egan, M.; Bassett, P.; Haugk, B. Fork-tip needle biopsy versus fine-needle aspiration in endoscopic ultrasound-guided sampling of solid pancreatic masses: A randomized crossover study. Endoscopy 2020, 52, 454–461. [Google Scholar] [CrossRef]
- Adler, D.G.; Witt, B.; Chadwick, B.; Wells, J.; Taylor, L.J.; Dimaio, C.; Schmidt, R. Pathologic evaluation of a new endoscopic ultrasound needle designed to obtain core tissue samples: A pilot study. Endosc. Ultrasound 2016, 5, 178–183. [Google Scholar] [CrossRef]
- Bang, J.Y.; Hebert-Magee, S.; Hasan, M.K.; Navaneethan, U.; Hawes, R.; Varadarajulu, S. Endoscopic ultrasonography-guided biopsy using a Franseen needle design: Initial assessment. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2017, 29, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Crinò, S.F.; Le Grazie, M.; Manfrin, E.; Conti Bellocchi, M.C.; Bernardoni, L.; Granato, A.; Locatelli, F.; Parisi, A.; Di Stefano, S.; Frulloni, L.; et al. Randomized trial comparing fork-tip and side-fenestrated needles for EUS-guided fine-needle biopsy of solid pancreatic lesions. Gastrointest. Endosc. 2020, 92, 648–658.e642. [Google Scholar] [CrossRef]
- Chen, Y.I.; Chatterjee, A.; Berger, R.; Kanber, Y.; Wyse, J.M.; Lam, E.; Gan, S.I.; Auger, M.; Kenshil, S.; Telford, J.; et al. EUS-guided fine needle biopsy alone vs. EUS-guided fine needle aspiration with rapid on-site evaluation of cytopathology in pancreatic lesions: A multicenter randomized trial. Endoscopy 2021. [Google Scholar] [CrossRef]
- Madhoun, M.F.; Wani, S.B.; Rastogi, A.; Early, D.; Gaddam, S.; Tierney, W.M.; Maple, J.T. The diagnostic accuracy of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of solid pancreatic lesions: A meta-analysis. Endoscopy 2013, 45, 86–92. [Google Scholar] [CrossRef]
- Alatawi, A.; Beuvon, F.; Grabar, S.; Leblanc, S.; Chaussade, S.; Terris, B.; Barret, M.; Prat, F. Comparison of 22G reverse-beveled versus standard needle for endoscopic ultrasound-guided sampling of solid pancreatic lesions. United Eur. Gastroenterol. J. 2015, 3, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Stasi, E.; Di Maso, M.; Serviddio, G.; Ali Hussein, M.S.; Muscatiello, N. Endoscopic ultrasound-guided fine needle aspiration of pancreatic lesions with 22 versus 25 Gauge needles: A meta-analysis. United Eur. Gastroenterol. J. 2017, 5, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.M.; Jia, H.Y.; Yan, L.L.; Li, S.S.; Zheng, Y. Comparison of two different size needles in endoscopic ultrasound-guided fine-needle aspiration for diagnosing solid pancreatic lesions: A meta-analysis of prospective controlled trials. Med. (Baltim.) 2017, 96, e5802. [Google Scholar] [CrossRef]
- Guedes, H.G.; Moura, D.T.H.; Duarte, R.B.; Cordero, M.A.C.; Santos, M.; Cheng, S.; Matuguma, S.E.; Chaves, D.M.; Bernardo, W.M.; Moura, E.G.H. A comparison of the efficiency of 22G versus 25G needles in EUS-FNA for solid pancreatic mass assessment: A systematic review and meta-analysis. Clin. (Sao PauloBraz.) 2018, 73, e261. [Google Scholar] [CrossRef]
- Ishii, Y.; Serikawa, M.; Tsuboi, T.; Kawamura, R.; Tsushima, K.; Nakamura, S.; Hirano, T.; Fukiage, A.; Mori, T.; Ikemoto, J.; et al. Role of Endoscopic Ultrasonography and Endoscopic Retrograde Cholangiopancreatography in the Diagnosis of Pancreatic Cancer. Diagn. (BaselSwitz.) 2021, 11, 238. [Google Scholar] [CrossRef]
- Minaga, K.; Kitano, M.; Enoki, E.; Kashida, H.; Kudo, M. Needle-Tract Seeding on the Proximal Gastric Wall After EUS-Guided Fine-Needle Aspiration of a Pancreatic Mass. Am. J. Gastroenterol. 2016, 111, 1515. [Google Scholar] [CrossRef]
- Shubert, C.R.; Bergquist, J.R.; Groeschl, R.T.; Habermann, E.B.; Wilson, P.M.; Truty, M.J.; Smoot, R.L.; Kendrick, M.L.; Nagorney, D.M.; Farnell, M.B. Overall survival is increased among stage III pancreatic adenocarcinoma patients receiving neoadjuvant chemotherapy compared to surgery first and adjuvant chemotherapy: An intention to treat analysis of the National Cancer Database. Surgery 2016, 160, 1080–1096. [Google Scholar] [CrossRef]
- de Geus, S.W.; Eskander, M.F.; Bliss, L.A.; Kasumova, G.G.; Ng, S.C.; Callery, M.P.; Tseng, J.F. Neoadjuvant therapy versus upfront surgery for resected pancreatic adenocarcinoma: A nationwide propensity score matched analysis. Surgery 2017, 161, 592–601. [Google Scholar] [CrossRef]
- Motoi, F.; Satoi, S.; Honda, G.; Wada, K.; Shinchi, H.; Matsumoto, I.; Sho, M.; Tsuchida, A.; Unno, M. A single-arm, phase II trial of neoadjuvant gemcitabine and S1 in patients with resectable and borderline resectable pancreatic adenocarcinoma: PREP-01 study. J. Gastroenterol. 2019, 54, 194–203. [Google Scholar] [CrossRef]
- Motoi, F.; Kosuge, T.; Ueno, H.; Yamaue, H.; Satoi, S.; Sho, M.; Honda, G.; Matsumoto, I.; Wada, K.; Furuse, J.; et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn. J. Clin. Oncol. 2019, 49, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Morisaka, H.; Sano, K.; Nagata, K.; Ryozawa, S.; Okamoto, K.; Ichikawa, T. Seeding of a Tumor in the Gastric Wall after Endoscopic Ultrasound-guided Fine-needle Aspiration of Solid Pseudopapillary Neoplasm of the Pancreas. Intern. Med. 2020, 59, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Hedenbro, J.L.; Ekelund, M.; Wetterberg, P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg. Endosc. 1991, 5, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Motoo, Y.; Okai, T.; Ohta, H.; Satomura, Y.; Watanabe, H.; Yamakawa, O.; Yamaguchi, Y.; Mouri, I.; Sawabu, N. Endoscopic ultrasonography in the diagnosis of extraluminal compressions mimicking gastric submucosal tumors. Endoscopy 1994, 26, 239–242. [Google Scholar] [CrossRef]
- Hwang, J.H.; Saunders, M.D.; Rulyak, S.J.; Shaw, S.; Nietsch, H.; Kimmey, M.B. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest. Endosc. 2005, 62, 202–208. [Google Scholar] [CrossRef]
- Polkowski, M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy 2005, 37, 635–645. [Google Scholar] [CrossRef]
- Cantor, M.J.; Davila, R.E.; Faigel, D.O. Yield of tissue sampling for subepithelial lesions evaluated by EUS: A comparison between forceps biopsies and endoscopic submucosal resection. Gastrointest. Endosc. 2006, 64, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Buscaglia, J.M.; Nagula, S.; Jayaraman, V.; Robbins, D.H.; Vadada, D.; Gross, S.A.; DiMaio, C.J.; Pais, S.; Patel, K.; Sejpal, D.V.; et al. Diagnostic yield and safety of jumbo biopsy forceps in patients with subepithelial lesions of the upper and lower GI tract. Gastrointest. Endosc. 2012, 75, 1147–1152. [Google Scholar] [CrossRef]
- Kida, M.; Kawaguchi, Y.; Miyata, E.; Hasegawa, R.; Kaneko, T.; Yamauchi, H.; Koizumi, S.; Okuwaki, K.; Miyazawa, S.; Iwai, T.; et al. Endoscopic ultrasonography diagnosis of subepithelial lesions. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2017, 29, 431–443. [Google Scholar] [CrossRef]
- Eckardt, A.J.; Jenssen, C. Current endoscopic ultrasound-guided approach to incidental subepithelial lesions: Optimal or optional? Ann. Gastroenterol. 2015, 28, 160–172. [Google Scholar] [PubMed]
- Sato, M.; Irisawa, A.; Bhutani, M.S.; Schnadig, V.; Takagi, T.; Shibukawa, G.; Wakatsuki, T.; Imamura, H.; Takahashi, Y.; Sato, A.; et al. Gastric bronchogenic cyst diagnosed by endosonographically guided fine needle aspiration biopsy. J. Clin. Ultrasound 2008, 36, 237–239. [Google Scholar] [CrossRef]
- Polkowski, M.; Gerke, W.; Jarosz, D.; Nasierowska-Guttmejer, A.; Rutkowski, P.; Nowecki, Z.I.; Ruka, W.; Regula, J.; Butruk, E. Diagnostic yield and safety of endoscopic ultrasound-guided trucut [corrected] biopsy in patients with gastric submucosal tumors: A prospective study. Endoscopy 2009, 41, 329–334. [Google Scholar] [CrossRef]
- Eckardt, A.J.; Adler, A.; Gomes, E.M.; Jenssen, C.; Siebert, C.; Gottschalk, U.; Koch, M.; Röcken, C.; Rösch, T. Endosonographic large-bore biopsy of gastric subepithelial tumors: A prospective multicenter study. Eur. J. Gastroenterol. Hepatol. 2012, 24, 1135–1144. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, K.B.; Lee, S.H.; Jeon, H.K.; Park, D.Y.; Jeon, T.Y.; Kim, D.H.; Song, G.A. Digital image analysis of endoscopic ultrasonography is helpful in diagnosing gastric mesenchymal tumors. Bmc Gastroenterol. 2014, 14, 7. [Google Scholar] [CrossRef]
- Kim, G.H.; Park, D.Y.; Kim, S.; Kim, D.H.; Kim, D.H.; Choi, C.W.; Heo, J.; Song, G.A. Is it possible to differentiate gastric GISTs from gastric leiomyomas by EUS? World J. Gastroenterol. 2009, 15, 3376–3381. [Google Scholar] [CrossRef] [PubMed]
- Ando, N.; Goto, H.; Niwa, Y.; Hirooka, Y.; Ohmiya, N.; Nagasaka, T.; Hayakawa, T. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest. Endosc. 2002, 55, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors—Definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001, 438, 1–12. [Google Scholar] [CrossRef]
- Joensuu, H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum. Pathol. 2008, 39, 1411–1419. [Google Scholar] [CrossRef]
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors: Review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch. Pathol. Lab. Med. 2006, 130, 1466–1478. [Google Scholar] [CrossRef]
- Miettinen, M.; Sobin, L.H.; Lasota, J. Gastrointestinal stromal tumors of the stomach: A clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am. J. Surg. Pathol. 2005, 29, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Akahoshi, K.; Oya, M.; Koga, T.; Koga, H.; Motomura, Y.; Kubokawa, M.; Gibo, J.; Nakamura, K. Clinical usefulness of endoscopic ultrasound-guided fine needle aspiration for gastric subepithelial lesions smaller than 2 cm. J. Gastrointestin. Liver Dis. 2014, 23, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Aso, A.; Ihara, E.; Kubo, H.; Osoegawa, T.; Oono, T.; Nakamura, K.; Ito, T.; Kakeji, Y.; Mikako, O.; Yamamoto, H.; et al. Gastric gastrointestinal stromal tumor smaller than 20 mm with liver metastasis. Clin. J. Gastroenterol. 2013, 6, 29–32. [Google Scholar] [CrossRef] [PubMed]
- West, R.B.; Corless, C.L.; Chen, X.; Rubin, B.P.; Subramanian, S.; Montgomery, K.; Zhu, S.; Ball, C.A.; Nielsen, T.O.; Patel, R.; et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am. J. Pathol. 2004, 165, 107–113. [Google Scholar] [CrossRef]
- Okasha, H.H.; Naguib, M.; El Nady, M.; Ezzat, R.; Al-Gemeie, E.; Al-Nabawy, W.; Aref, W.; Abdel-Moaty, A.; Essam, K.; Hamdy, A. Role of endoscopic ultrasound and endoscopic-ultrasound-guided fine-needle aspiration in endoscopic biopsy negative gastrointestinal lesions. Endosc. Ultrasound 2017, 6, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Akahoshi, K.; Sumida, Y.; Matsui, N.; Oya, M.; Akinaga, R.; Kubokawa, M.; Motomura, Y.; Honda, K.; Watanabe, M.; Nagaie, T. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J. Gastroenterol. 2007, 13, 2077–2082. [Google Scholar] [CrossRef] [PubMed]
- El Chafic, A.H.; Loren, D.; Siddiqui, A.; Mounzer, R.; Cosgrove, N.; Kowalski, T. Comparison of FNA and fine-needle biopsy for EUS-guided sampling of suspected GI stromal tumors. Gastrointest. Endosc. 2017, 86, 510–515. [Google Scholar] [CrossRef]
- Hoda, K.M.; Rodriguez, S.A.; Faigel, D.O. EUS-guided sampling of suspected GI stromal tumors. Gastrointest. Endosc. 2009, 69, 1218–1223. [Google Scholar] [CrossRef]
- Kim, G.H.; Cho, Y.K.; Kim, E.Y.; Kim, H.K.; Cho, J.W.; Lee, T.H.; Moon, J.S. Comparison of 22-gauge aspiration needle with 22-gauge biopsy needle in endoscopic ultrasonography-guided subepithelial tumor sampling. Scand. J. Gastroenterol. 2014, 49, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.R.; Binmoeller, K.F.; Hamerski, C.M.; Shergill, A.K.; Shaw, R.E.; Jaffee, I.M.; Stewart, L.; Shah, J.N. Yield and performance characteristics of endoscopic ultrasound-guided fine needle aspiration for diagnosing upper GI tract stromal tumors. Dig. Dis. Sci. 2011, 56, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, A.; Irisawa, A.; Bhutani, M.S.; Shibukawa, G.; Abe, Y.; Saito, A.; Imbe, K.; Hoshi, K.; Igarashi, R. Usefulness of endoscopic ultrasound-guided fine-needle aspiration with a forward-viewing and curved linear-array echoendoscope for small gastrointestinal subepithelial lesions. Endosc. Int. Open 2015, 3, E161–E164. [Google Scholar] [CrossRef] [PubMed]
- Ihara, E.; Matsuzaka, H.; Honda, K.; Hata, Y.; Sumida, Y.; Akiho, H.; Misawa, T.; Toyoshima, S.; Chijiiwa, Y.; Nakamura, K.; et al. Mucosal-incision assisted biopsy for suspected gastric gastrointestinal stromal tumors. World J. Gastrointest. Endosc. 2013, 5, 191–196. [Google Scholar] [CrossRef]
- Lee, C.K.; Chung, I.K.; Lee, S.H.; Lee, S.H.; Lee, T.H.; Park, S.H.; Kim, H.S.; Kim, S.J.; Cho, H.D. Endoscopic partial resection with the unroofing technique for reliable tissue diagnosis of upper GI subepithelial tumors originating from the muscularis propria on EUS (with video). Gastrointest. Endosc. 2010, 71, 188–194. [Google Scholar] [CrossRef]
- Akahoshi, K.; Oya, M.; Koga, T.; Shiratsuchi, Y. Current clinical management of gastrointestinal stromal tumor. World J. Gastroenterol. 2018, 24, 2806–2817. [Google Scholar] [CrossRef]
- Fujita, A.; Ryozawa, S.; Kobayashi, M.; Araki, R.; Nagata, K.; Minami, K.; Tanisaka, Y.; Kobatake, T.; Mizuide, M. Diagnostic ability of a 22G Franseen needle in endoscopic ultrasound-guided fine needle aspiration of subepithelial lesions. Mol. Clin. Oncol. 2018, 9, 527–531. [Google Scholar] [CrossRef]
- De Potter, T.; Flamen, P.; Van Cutsem, E.; Penninckx, F.; Filez, L.; Bormans, G.; Maes, A.; Mortelmans, L. Whole-body PET with FDG for the diagnosis of recurrent gastric cancer. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 525–529. [Google Scholar] [CrossRef]
- Habermann, C.R.; Weiss, F.; Riecken, R.; Honarpisheh, H.; Bohnacker, S.; Staedtler, C.; Dieckmann, C.; Schoder, V.; Adam, G. Preoperative staging of gastric adenocarcinoma: Comparison of helical CT and endoscopic US. Radiology 2004, 230, 465–471. [Google Scholar] [CrossRef]
- Annema, J.T.; Versteegh, M.I.; Veseliç, M.; Voigt, P.; Rabe, K.F. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of lung cancer and its impact on surgical staging. J. Clin. Oncol. 2005, 23, 8357–8361. [Google Scholar] [CrossRef]
- Bhutani, M.S.; Hawes, R.H.; Hoffman, B.J. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest. Endosc. 1997, 45, 474–479. [Google Scholar] [CrossRef]
- Kanamori, A.; Hirooka, Y.; Itoh, A.; Hashimoto, S.; Kawashima, H.; Hara, K.; Uchida, H.; Goto, J.; Ohmiya, N.; Niwa, Y.; et al. Usefulness of contrast-enhanced endoscopic ultrasonography in the differentiation between malignant and benign lymphadenopathy. Am. J. Gastroenterol. 2006, 101, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.K.; Eloubeidi, M.A. Endoscopic ultrasound-guided fine needle aspiration is superior to lymph node echofeatures: A prospective evaluation of mediastinal and peri-intestinal lymphadenopathy. Am. J. Gastroenterol. 2004, 99, 628–633. [Google Scholar] [CrossRef]
- Eloubeidi, M.A.; Wallace, M.B.; Reed, C.E.; Hadzijahic, N.; Lewin, D.N.; Van Velse, A.; Leveen, M.B.; Etemad, B.; Matsuda, K.; Patel, R.S.; et al. The utility of EUS and EUS-guided fine needle aspiration in detecting celiac lymph node metastasis in patients with esophageal cancer: A single-center experience. Gastrointest. Endosc. 2001, 54, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Kim, J.O.; Eun, S.H.; Cho, Y.D.; Jung, I.S.; Cheon, Y.K.; Moon, J.H.; Lee, J.S.; Lee, M.S.; Shim, C.S.; et al. Endoscopic Ultrasonograpic Findings of Benign Mediastinal and Abdominal Lymphadenopathy Confirmed by EUS-guided Fine Needle Aspiration. Gut Liver 2007, 1, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Tanisaka, Y.; Ryozawa, S.; Kobayashi, M.; Harada, M.; Kobatake, T.; Omiya, K.; Iwano, H.; Arai, S.; Nonaka, K.; Mashimo, Y. Usefulness of endoscopic ultrasound-guided fine needle aspiration for lymphadenopathy. Oncol. Lett. 2018, 15, 4759–4766. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Y.; Gao, X.; Lin, S.; He, L.; Luo, G.; Li, J.; Huang, C.; Wang, G.; Yang, Q.; et al. High Diagnostic Accuracy and Safety of Endoscopic Ultrasound-Guided Fine-Needle Aspiration in Malignant Lymph Nodes: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2020. [Google Scholar] [CrossRef]
- Nakahara, O.; Yamao, K.; Bhatia, V.; Sawaki, A.; Mizuno, N.; Takagi, T.; Shimizu, Y.; Koshikawa, T.; Yatabe, Y.; Baba, H. Usefulness of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for undiagnosed intra-abdominal lymphadenopathy. J. Gastroenterol. 2009, 44, 562–567. [Google Scholar] [CrossRef]
- Ribeiro, A.; Vazquez-Sequeiros, E.; Wiersema, L.M.; Wang, K.K.; Clain, J.E.; Wiersema, M.J. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest. Endosc. 2001, 53, 485–491. [Google Scholar] [CrossRef]
- Fujii, Y.; Kanno, Y.; Koshita, S.; Ogawa, T.; Kusunose, H.; Masu, K.; Sakai, T.; Yonamine, K.; Kawakami, Y.; Murabayashi, T.; et al. Predictive Factors for Inaccurate Diagnosis of Swollen Lymph Nodes in Endoscopic Ultrasound-Guided Fine Needle Aspiration. Clin. Endosc. 2019, 52, 152–158. [Google Scholar] [CrossRef]
- Gleeson, F.C.; Clain, J.E.; Karnes, R.J.; Rajan, E.; Topazian, M.D.; Wang, K.K.; Levy, M.J. Endoscopic-ultrasound-guided tissue sampling facilitates the detection of local recurrence and extra pelvic metastasis in pelvic urologic malignancy. Diagn. Ther. Endosc. 2012, 2012, 219521. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Reference | Year | Cases (n) | Sensitivity | Specificity |
|---|---|---|---|---|
| Hewitt [32] | 2012 | FNA: 4984 | 85 | 98 |
| Chen [31] | 2012 | FNA: 1860 | 92 | 96 |
| Puli [33] | 2013 | FNA: 4766 | 86.8 | 95.8 |
| Banafea [30] | 2016 | FNA: 2761 | 90.8 | 96.5 |
| Yang [34] | 2016 | FNB: 828 | 84 | 98 |
| SELs | EUS Layer | EUS Imaging Feature | Histology | Malignant Potential |
|---|---|---|---|---|
| Leiomyoma | 2nd or 4th | Hypoechoic (iso- or hypoechoic compared to muscle layer), homogeneous, sometimes calcifications | Desmin (+), α-SMA (+) | None (primary leiomyosarcoma: extremely rare) |
| Schwannoma | 3rd or 4th | Hypoechoic, round or oval, homogeneous, well-demarcated | S-100 (+) | Extremely rare |
| Ectopic pancreas | 3rd (and 4th) | Hypoechoic, or mixed echogenicity heterogeneous echotexture, umbilication, ductal structures, indistinct margins | Pancreatic tissue | Extremely rare |
| Lipoma | 3rd | Hyperechoic, smooth margins, homogeneous, may be polypoid | Mature lipocytes | None |
| Brunnerioma | 3rd | Hyperechoic, smooth margin, possibly hypoechoic-dilated gland duct | Hyperplasia of the Brunner gland | None |
| Lymphangioma | 3rd | Anechoic, occasionally multiloculated | No solid components | None |
| Varices | 2nd or 3rd | Anechoic, serpiginous structure with doppler signal | No solid components | None |
| Granular cell tumor | 2nd, 3rd, or 4th | Hypo- or isoechoic, oval, homogeneous, smooth margins | PAS (+), S-100 (+), and NSE (+) | Extremely low risk of malignancy (2–4%) |
| Glomus tumor | 3rd or 4th | Round, hypoechoic, homogeneous, may have a halo | α-SMA (+), vimentin (+), laminin (+), CD34 (rarely), and KIT (-) | Rare |
| GIST | 4th | Benign features: small (≤2 cm), oval or round, hypoechoic but relatively hyperechoic compared to muscle layer, homogeneous Malignant features: large (>3 cm), irregular margins, heterogeneous echotexture, cystic spaces, hypervascularity, marginal halo, hyperechoic spots/echogenic foci | KIT (+), CD34 (+), desmin (+), S-100 (-), DOG1 (+), or a mutation search of the KIT or PDGFRA gene | 10–30% clinically malignant |
| NET | 2nd or 3rd | Oval to round, hypo- or isoechoic, homogeneous, regular margins | Synaptophysin (+), chromogranin (+), INSM1 (+) | Depending on type, size, and location |
| Lymphoma | 2nd, 3rd, or 4th | Hypoechoic | Atypical lymphocyte | Always |
| metastasis | Any layer | Heterogeneous or hypoechoic | Depending on a primary | Always |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, A.; Ryozawa, S.; Mizuide, M.; Tanisaka, Y.; Ogawa, T.; Suzuki, M.; Katsuda, H.; Saito, Y.; Tashima, T.; Miyaguchi, K.; et al. Diagnosis of Pancreatic Solid Lesions, Subepithelial Lesions, and Lymph Nodes Using Endoscopic Ultrasound. J. Clin. Med. 2021, 10, 1076. https://doi.org/10.3390/jcm10051076
Fujita A, Ryozawa S, Mizuide M, Tanisaka Y, Ogawa T, Suzuki M, Katsuda H, Saito Y, Tashima T, Miyaguchi K, et al. Diagnosis of Pancreatic Solid Lesions, Subepithelial Lesions, and Lymph Nodes Using Endoscopic Ultrasound. Journal of Clinical Medicine. 2021; 10(5):1076. https://doi.org/10.3390/jcm10051076
Chicago/Turabian StyleFujita, Akashi, Shomei Ryozawa, Masafumi Mizuide, Yuki Tanisaka, Tomoya Ogawa, Masahiro Suzuki, Hiromune Katsuda, Yoichi Saito, Tomoaki Tashima, Kazuya Miyaguchi, and et al. 2021. "Diagnosis of Pancreatic Solid Lesions, Subepithelial Lesions, and Lymph Nodes Using Endoscopic Ultrasound" Journal of Clinical Medicine 10, no. 5: 1076. https://doi.org/10.3390/jcm10051076
APA StyleFujita, A., Ryozawa, S., Mizuide, M., Tanisaka, Y., Ogawa, T., Suzuki, M., Katsuda, H., Saito, Y., Tashima, T., Miyaguchi, K., Arai, E., Kawasaki, T., & Mashimo, Y. (2021). Diagnosis of Pancreatic Solid Lesions, Subepithelial Lesions, and Lymph Nodes Using Endoscopic Ultrasound. Journal of Clinical Medicine, 10(5), 1076. https://doi.org/10.3390/jcm10051076







