Incidence, Diagnosis and Repair of a Diaphragmatic Hernia Following Hepatic Surgery: A Single Center Analysis of 3107 Consecutive Liver Resections
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Katukuri, G.R.; Madireddi, J.; Agarwal, S.; Kareem, H.; Devasia, T. Delayed Diagnosis of Left-Sided Diaphragmatic Hernia in an Elderly Adult with no History of Trauma. J. Clin. Diagn. Res. 2016, 10, PD04. [Google Scholar] [CrossRef]
- Johnson, C.D.; Ellis, H. Acquired hernias of the diaphragm. Postgrad. Med. J. 1988, 64, 317–321. [Google Scholar] [CrossRef][Green Version]
- Rubikas, R. Diaphragmatic injuries. Eur. J. Cardio-Thoracic Surg. 2001, 20, 53–57. [Google Scholar] [CrossRef]
- Manzini, G.; Kuemmerli, C.; Reiner, C.S.; Petrowsky, H.; Gutschow, C.A. Enterothorax After Hepatic Surgery: A Single-Center Experience. World J. Surg. 2019, 43, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Tabrizian, P.; Jibara, G.; Shrager, B.; Elsabbagh, A.M.; Roayaie, S.; Schwartz, M.E. Diaphragmatic Hernia After Hepatic Resection: Case Series at a Single Western Institution. J. Gastrointest. Surg. 2012, 16, 1910–1914. [Google Scholar] [CrossRef] [PubMed]
- Kousoulas, L.; Richter, N.; Emmanouilidis, N.; Schrem, H.; Klempnauer, J.; Barg-Hock, H.; Becker, T.; Lehner, F. Living donor liver transplantation: Effect of the type of liver graft donation on donor mortality and morbidity. Transpl. Int. 2010, 24, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S.; Takahashi, T.; Funaki, S.; Tanaka, K.; Miyazaki, Y.; Makino, T.; Kurokawa, Y.; Yamasaki, M.; Nakajima, K.; Okumura, M.; et al. Laparoscopic repair of an incarcerated diaphragmatic hernia after right hepatectomy for hepatic injury: A case report. Surg. Case Rep. 2018, 4, 135. [Google Scholar] [CrossRef] [PubMed]
- Vernadakis, S.; Paul, A.; Kykalos, S.; Fouzas, I.; Kaiser, G.; Sotiropoulos, G. Incarcerated Diaphragmatic Hernia After Right Hepatectomy for Living Donor Liver Transplantation: Case Report of an Extremely Rare Late Donor Complication. Transplant. Proc. 2012, 44, 2770–2772. [Google Scholar] [CrossRef] [PubMed]
- Dieter, R.A.; Spitz, J.; Kuzycz, G. Incarcerated Diaphragmatic Hernia with Intrathoracic Bowel Obstruction After Right Liver Donation. Int. Surg. 2011, 96, 239–244. [Google Scholar] [CrossRef]
- Hawxby, A.M.; Mason, D.P.; Klein, A.S. Diaphragmatic hernia after right donor and hepatectomy: A rare donor complication of partial hepatectomy for transplantation. Hepatobiliary Pancreat. Dis. Int. 2006, 5, 459–461. [Google Scholar]
- Matthews, J.; Bhanderi, S.; Mitchell, H.; Whiting, J.; Vohra, R.; Hodson, J.; Griffiths, E. Diaphragmatic herniation following esophagogastric resectional surgery: An increasing problem with minimally invasive techniques? Surg. Endosc. 2016, 30, 5419–5427. [Google Scholar] [CrossRef]
- Singh, T.P.; Rizvi, S.A.; Pretorius, C.F. Post-menopausal acquired diaphragmatic herniation in the context of endometriosis. Int. J. Surg. Case Rep. 2018, 53, 154–156. [Google Scholar] [CrossRef]
- Saito, T.; Chiba, T.; Ogasawara, S.; Inoue, M.; Wakamatsu, T.; Motoyama, T.; Kanogawa, N.; Suzuki, E.; Ooka, Y.; Tawada, A.; et al. Fatal Diaphragmatic Hernia following Radiofrequency Ablation for Hepatocellular Carcinoma: A Case Report and Literature Review. Case Rep. Oncol. 2015, 8, 238–245. [Google Scholar] [CrossRef]
- Koda, M.; Ueki, M.; Maeda, N.; Murawaki, Y. Diaphragmatic Perforation and Hernia After Hepatic Radiofrequency Ablation. Am. J. Roentgenol. 2003, 180, 1561–1562. [Google Scholar] [CrossRef]
- Esposito, F.; Lim, C.; Salloum, C.; Osseis, M.; Lahat, E.; Compagnon, P.; Azoulay, D. Diaphragmatic hernia following liver resection: Case series and review of the literature. Ann. Hepato-Biliary-Pancreat. Surg. 2017, 21, 114–121. [Google Scholar] [CrossRef]
- Matz, D.; Kirchhoff, P.; Kocher, T.M.; Heizmann, O. Consecutive cecum perforation due to incarcerated diaphragmatic hernia after liver surgery. Int. J. Color. Dis. 2009, 24, 1353–1354. [Google Scholar] [CrossRef][Green Version]
- Schmelzle, M.; Wabitsch, S.; Haber, P.K.; Krenzien, F.; Kästner, A.; Biebl, M.; Öllinger, R.; Pratschke, J. Laparoskopische Leberchirurgie—Berliner Zentrumserfahrungen aus 250 konsekutiven Fällen. Zent. Chir. Z. Allg. Visz. Thorax Gefäßchir. 2018, 144, 145–152. [Google Scholar] [CrossRef]
- Raakow, J.; Schulte-Mäter, J.; Andreou, A.; Biebl, M.; Pratschke, J.; Kilian, M. Der postoperative Enterothorax—Fallserie und Literaturanalyse. Zent. Chir. 2017, 142, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-W.; Oh, S.N.; Jung, S.E.; Byun, J.Y. Diaphragmatic Hernia After Living-Donor Right Hepatectomy. J. Comput. Assist. Tomogr. 2017, 41, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Emamaullee, J.A.; Nekrasov, V.; Gilmour, S.; Kneteman, N.; Yanni, G.; Kohli, R.; Thomas, D.; Genyk, Y. Case series and systematic review of acquired diaphragmatic hernia after liver transplantation. Pediatr. Transplant. 2018, 22, e13296. [Google Scholar] [CrossRef] [PubMed]
- Jeng, K.-S.; Huang, C.-C.; Lin, C.-K.; Lin, C.-C.; Wu, J.-M.; Chen, K.-H.; Chu, S.-H. Early Incarcerated Diaphragmatic Hernia Following Right Donor Hepatectomy: A Case Report. Transplant. Proc. 2015, 47, 815–816. [Google Scholar] [CrossRef]
- Mizuno, S.; Tanemura, A.; Isaji, S. Incarcerated left diaphragmatic hernia following left hepatectomy for living donor liver transplantation. Transpl. Int. 2014, 27, e65–e67. [Google Scholar] [CrossRef]
- Eren, S.; Çiriş, F. Diaphragmatic hernia: Diagnostic approaches with review of the literature. Eur. J. Radiol. 2005, 54, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Hirano, E.S.; Silva, V.G.; Bortoto, J.B.; Barros, R.H.D.O.; Caserta, N.M.G.; Fraga, G.P. Exame radiográfico convencional do tórax no diagnóstico de hérnia diafragmática pós-traumática. Rev. Colégio Bras. Cir. 2012, 39, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, S.M.; Andres, A.; Shapiro, A.J.; Kneteman, N.N.; Bigam, D.L. Diaphragmatic Hernia After Living Donor Right Hepatectomy: Proposal for a Screening Protocol. Transplant. Direct 2016, 2, e84. [Google Scholar] [CrossRef]
- Karmazyn, B.; Shold, A.J.; Delaney, L.R.; Brown, B.P.; Marine, M.B.; Jennings, S.G.; Gray, B.W. Ultrasound evaluation of right diaphragmatic eventration and hernia. Pediatr. Radiol. 2019, 49, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Corsini, I.; Parri, N.; Coviello, C.; Leonardi, V.; Dani, C. Lung ultrasound findings in congenital diaphragmatic hernia. Eur. J. Nucl. Med. Mol. Imaging 2019, 178, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, T.; Takahashi, H.; Tanami, Y.; Sato, Y.; Hosokawa, M.; Kato, R.; Kawashima, H.; Oguma, E. Usefulness of Ultrasound in Evaluating the Diaphragm in Neonates and Infants with Congenital Diaphragmatic Hernias. J. Ultrasound Med. 2019, 38, 1109–1113. [Google Scholar] [CrossRef]
- Hosokawa, T.; Tanami, Y.; Sato, Y.; Oguma, E.; Omata, K.; Kawashima, H.; Yamada, Y. Postnatal ultrasonography for evaluation of hernia sac of neonate with congenital diaphragmatic hernia. Radiol. Case Rep. 2019, 14, 683–686. [Google Scholar] [CrossRef]
- Reber, P.U.; Schmied, B.; Seiler, C.A.; Baer, H.U.; Patel, A.G.; Buchler, M.W. Missed Diaphragmatic Injuries and Their Long-Term Sequelae. J. Trauma Inj. Infect. Crit. Care 1998, 44, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Al-Nouri, O.; Hartman, B.; Freedman, R.; Thomas, C.; Esposito, T. Diaphragmatic rupture: Is management with biological mesh feasible? Int. J. Surg. Case Rep. 2012, 3, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Schmelzle, M.; Krenzien, F.; Schöning, W.; Pratschke, J. Laparoscopic liver resection: Indications, limitations, and economic aspects. Langenbecks Arch. Surg. 2020, 405, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Schmelzle, M.; Krenzien, F.; Schöning, W.; Pratschke, J. Möglichkeiten und Grenzen der robotischen Leberchirurgie—Aktueller Stand 2020. Der Chir. 2021, 92, 107–114. [Google Scholar] [CrossRef] [PubMed]

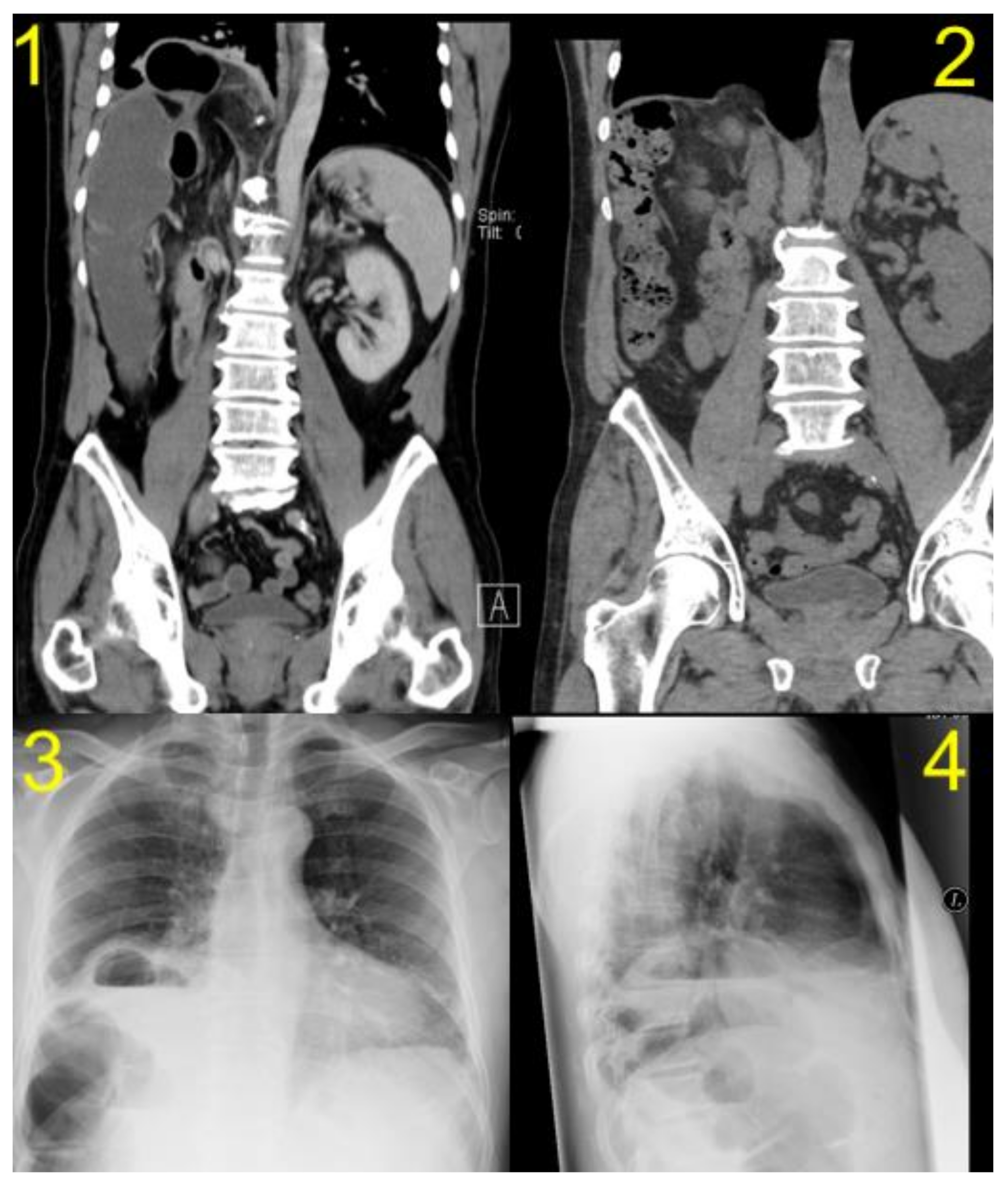
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Age | 54 | 56 | 58 | 49 | 69 |
| Gender | m | f | f | f | f |
| BMI | 26.1 kg/cm2 | 27.9 kg/cm2 | 35.7 kg/cm2 | 20 kg/cm2 | 29.4 kg/cm2 |
| Etiology of LR | CRLM | CCC | CRLM | HCC | cholecystitis |
| Procedure | ext. right hepatectomy | Right hepatectomy | Right hepatectomy | ext. right hepatectomy | ext. right hepatectomy |
| Open or laparoscopic approach | open | open | open | open | open |
| Size Of Tumor | multiple leasons | 10 cm | multiple leasons | 18 cm | parenchymal abscess |
| Resection Of Diahpragm in the Course Of The LR | no | no | no | no | no |
| DH Occurence: Time after Liver Resection | 21 months | 15 months | 34 months | 44 months | 36 months |
| Symptoms/Reason of Presentation | ileus | shortness of breath | colon stenosis during coloscopy | HCC recurrence in Follow-up, asymptomatic in respect of DH | enterothorax with jejunal perforation and peritonitis |
| Diagnostic Study | CT | CT | CT | CT/MRI | CT |
| Herniated organ | right colon flexure, omentum | colon and small bowell | colon and omentum majus | colon | colon |
| Side of hernia | right-sided | right-sided | right-sided | right-sided | right-sided |
| Size of Hernia | 4 cm | <5 cm | 4 cm | 5 cm | 7 cm |
| Elective/Emergent | emergent | elective | elective | elective | emergent/elective |
| DH Repair Approach | Open | Open | Lap. | Open | Open |
| DH Procedure | primary repair | repair with mesh BioA 10 × 7 cm | repair with composite IPOM | primary repair | primary repair/BioA Mesh at recurrence |
| Chest Drain During DH Repair | yes | yes | yes | yes | yes |
| Complications after DH Repair | recurrence | recurrence | none | none with regard to DH | recurrence |
| Hospital Stay after DH Repair | 9 days | 8 days | 5 days | 15 days | 21 days |
| Follow up after DH Repair | 36 months | 41 months | 52 months | 14 months | 62 months |
| Recurrence after DH Repair | yes | yes | no | no | yes |
| Time After DH Repair Till Diagnosis of Recurrence | 12 months | 12 months | - | - | 22 months |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raakow, J.; Megas, I.-F.; Schmelzle, M.; Schoening, W.; Lurje, G.; Biebl, M.; Pratschke, J.; Fikatas, P. Incidence, Diagnosis and Repair of a Diaphragmatic Hernia Following Hepatic Surgery: A Single Center Analysis of 3107 Consecutive Liver Resections. J. Clin. Med. 2021, 10, 1011. https://doi.org/10.3390/jcm10051011
Raakow J, Megas I-F, Schmelzle M, Schoening W, Lurje G, Biebl M, Pratschke J, Fikatas P. Incidence, Diagnosis and Repair of a Diaphragmatic Hernia Following Hepatic Surgery: A Single Center Analysis of 3107 Consecutive Liver Resections. Journal of Clinical Medicine. 2021; 10(5):1011. https://doi.org/10.3390/jcm10051011
Chicago/Turabian StyleRaakow, Jonas, Ioannis-Fivos Megas, Moritz Schmelzle, Wenzel Schoening, Georg Lurje, Matthias Biebl, Johann Pratschke, and Panagiotis Fikatas. 2021. "Incidence, Diagnosis and Repair of a Diaphragmatic Hernia Following Hepatic Surgery: A Single Center Analysis of 3107 Consecutive Liver Resections" Journal of Clinical Medicine 10, no. 5: 1011. https://doi.org/10.3390/jcm10051011
APA StyleRaakow, J., Megas, I.-F., Schmelzle, M., Schoening, W., Lurje, G., Biebl, M., Pratschke, J., & Fikatas, P. (2021). Incidence, Diagnosis and Repair of a Diaphragmatic Hernia Following Hepatic Surgery: A Single Center Analysis of 3107 Consecutive Liver Resections. Journal of Clinical Medicine, 10(5), 1011. https://doi.org/10.3390/jcm10051011








