Detection of Inflammatory and Homeostasis Biomarkers after Selective Removal of Carious Dentin—An In Vivo Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
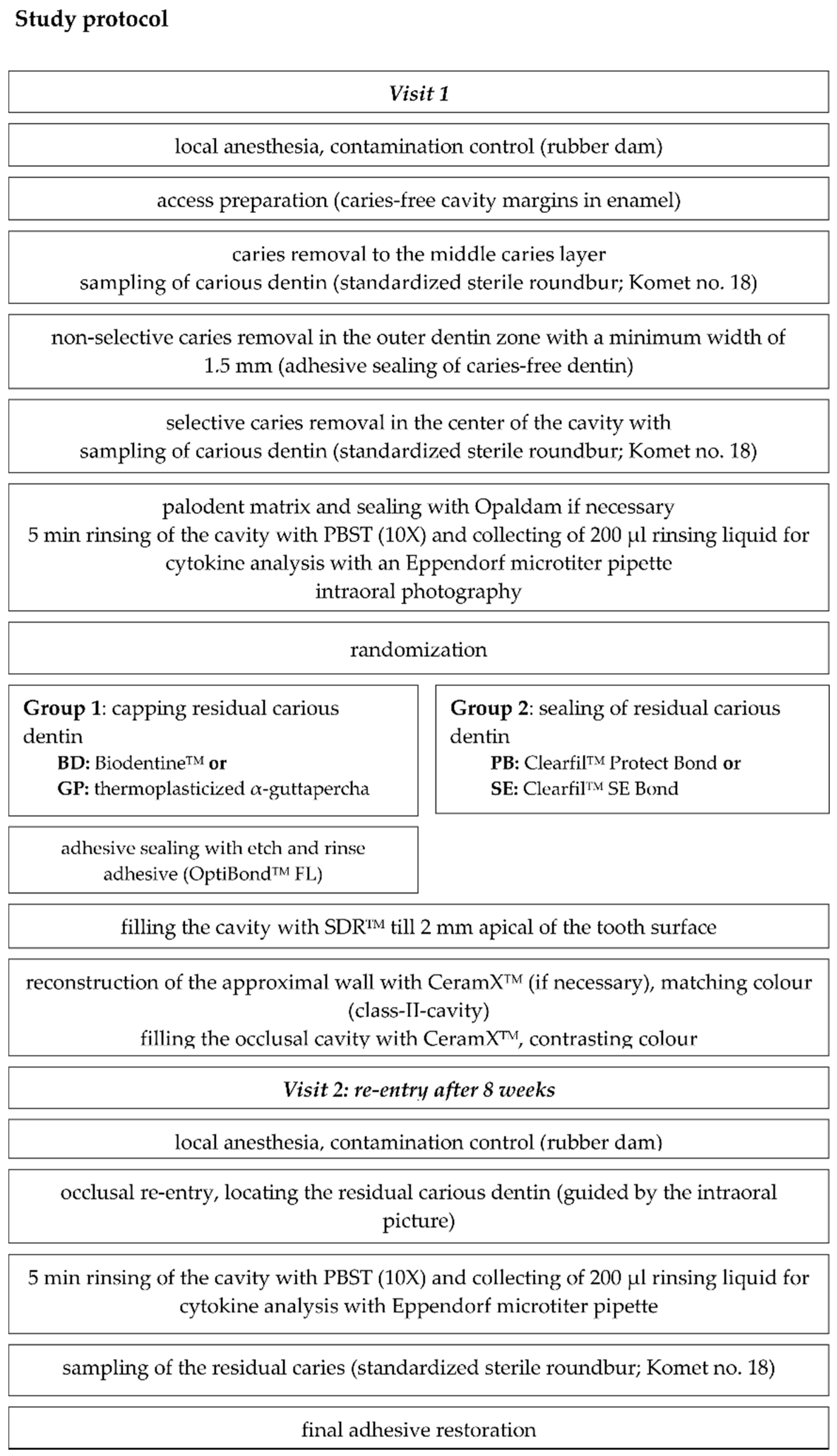
2.1. Origin of Samples: Study Design, Patient Recruitment, and Randomization
2.2. Sample Processing and Cytokine Analysis
2.3. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Study Participants and Teeth
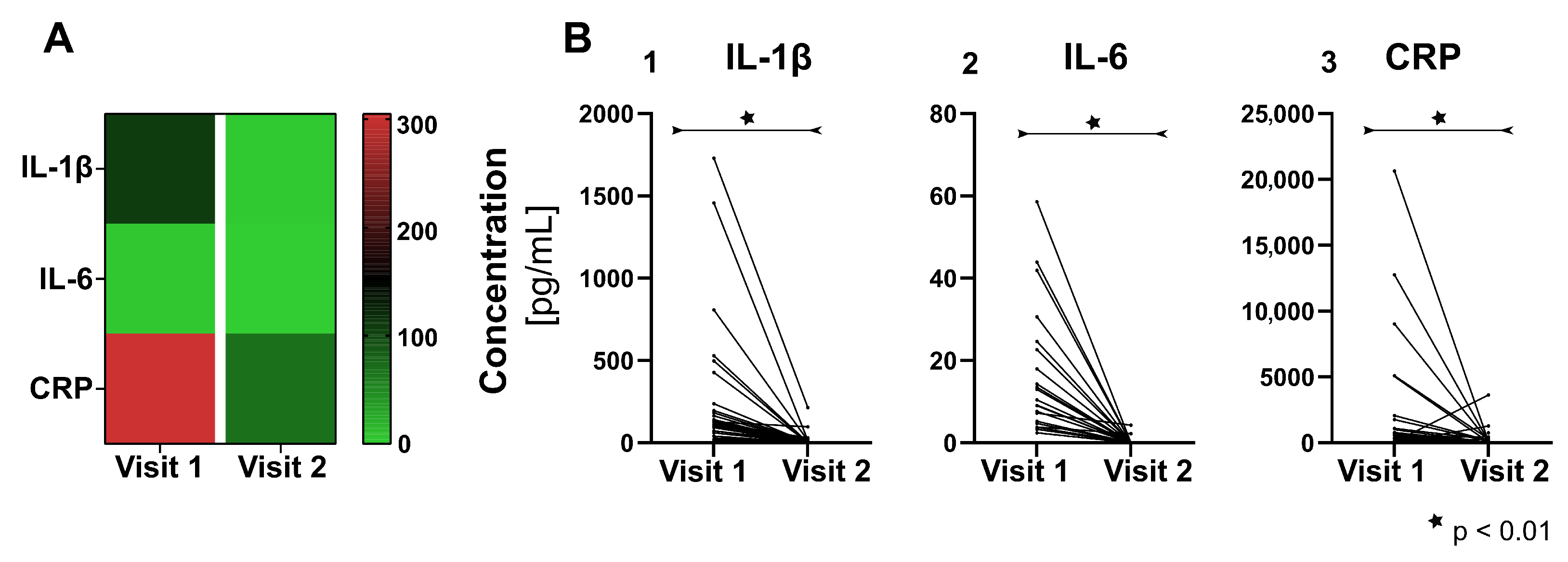
3.2. Pro-Inflammatory Cytokines
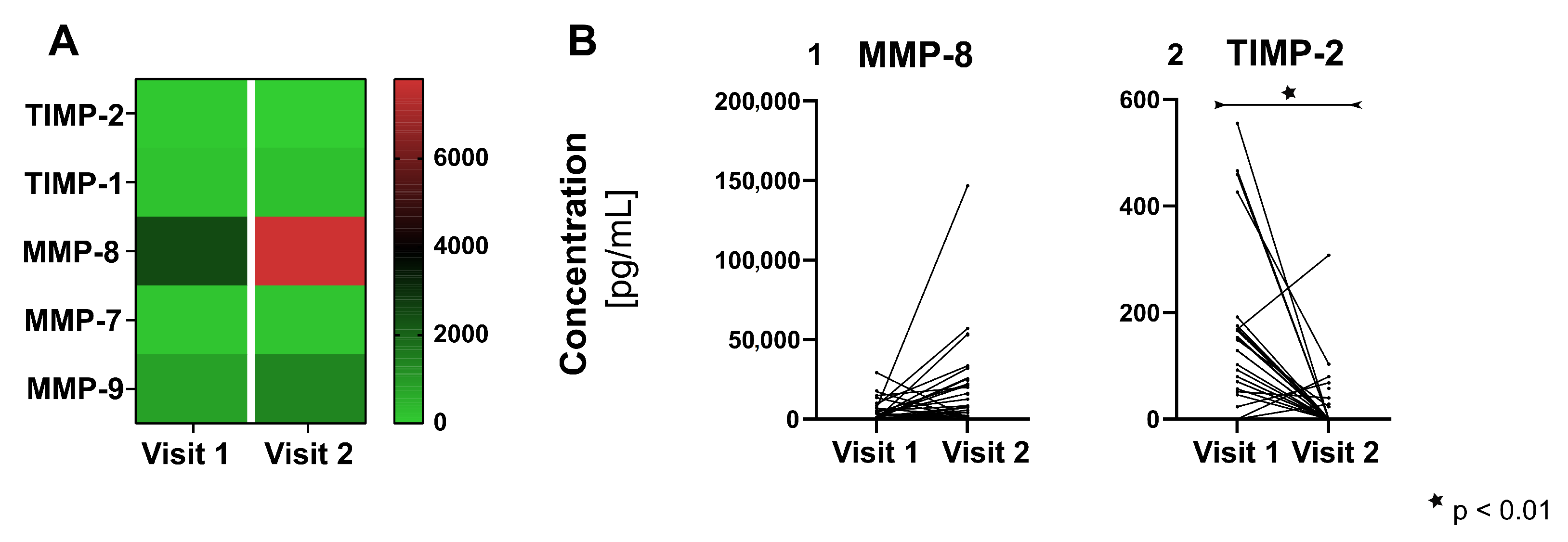
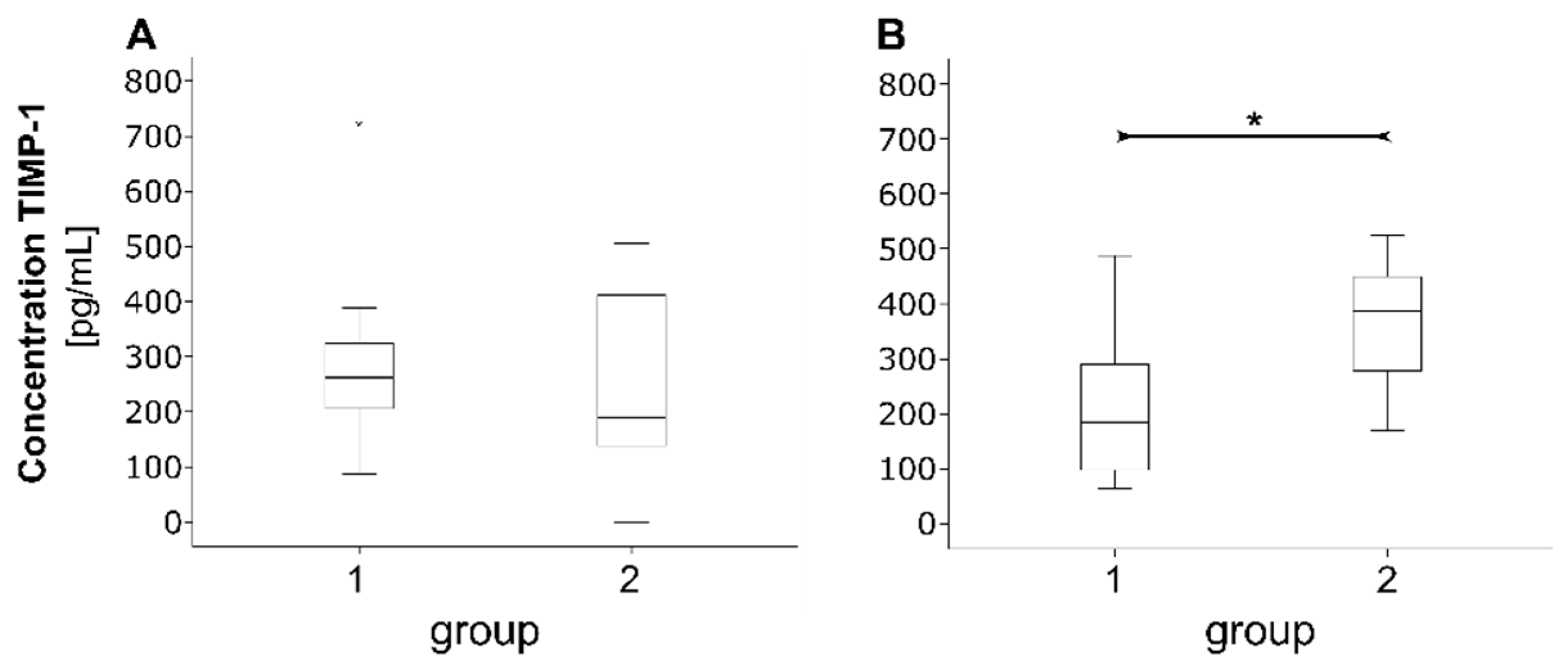
3.3. Proteins of Tissue Homeostasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Philip, N.; Suneja, B.; Walsh, L. Beyond Streptococcus Mutans: Clinical Implications of the Evolving Dental Caries Aetiological Paradigms and its Associated Microbiome. Br. Dent. J. 2018, 224, 219–225. [Google Scholar] [CrossRef]
- Takahashi, N.; Nyvad, B. Ecological Hypothesis of Dentin and Root Caries. Caries Res. 2016, 50, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, V.; Wolgin, M.; Mitronin, A.V.; Kielbassa, A.M. Inflammatory Cytokines in Normal and Irreversibly Inflamed Pulps: A Systematic Review. Arch. Oral Biol. 2017, 82, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Farges, J.-C.; Alliot-Licht, B.; Renard, E.; Ducret, M.; Gaudin, A.; Smith, A.J.; Cooper, P.R. Dental Pulp Defence and Repair Mechanisms in Dental Caries. Mediat. Inflamm. 2015, 230251. [Google Scholar] [CrossRef] [PubMed]
- Maltz, M.; Garcia, R.; Jardim, J.J.; De Paula, L.M.; Yamaguti, P.M.; Moura, M.S.; Garcia, F.; Nascimento, C.; Oliveira, A.; Mestrinho, H.D. Randomized Trial of Partial vs. Stepwise Caries Removal: 3-Year Follow-Up. J. Dent. Res. 2012, 91, 1026–1031. [Google Scholar] [CrossRef]
- Schwendicke, F.; Dörfer, C.E.; Paris, S. Incomplete Caries Removal: A Systematic Review and Meta-Analysis. J. Dent. Res. 2013, 92, 306–314. [Google Scholar] [CrossRef]
- Neves, V.C.M.; Sharpe, P.T. Regulation of Reactionary Dentine Formation. J. Dent. Res. 2018, 97, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Giraud, T.; Jeanneau, C.; Rombouts, C.; Bakhtiar, H.; Laurent, P.; About, I. Pulp Capping Materials Modulate the Balance between Inflammation and Regeneration. Dent. Mater. 2019, 35, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Rechenberg, D.-K.; Galicia, J.C.; Peters, O.A. Biological Markers for Pulpal Inflammation: A Systematic Review. PLoS ONE 2016, 11, e0167289. [Google Scholar] [CrossRef]
- Zehnder, M.; Wegehaupt, F.J.; Attin, T. A First Study on the Usefulness of Matrix Metalloproteinase 9 from Dentinal Fluid to Indicate Pulp Inflammation. J. Endod. 2011, 37, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Meyer-Lueckel, H.; Dörfer, C.; Paris, S. Failure of Incompletely Excavated Teeth—A Systematic Review. J. Dent. 2013, 41, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Frencken, J.E.; Bjørndal, L.; Maltz, M.; Manton, D.J.; Ricketts, D.; van Landuyt, K.; Banerjee, A.; Campus, G.; Doméjean, S.; et al. Managing Carious Lesions: Consensus Recommendations on Carious Tissue Removal. Adv. Dent. Res. 2016, 28, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Hanks, C.T.; Strawn, S.E.; Wataha, J.C.; Craig, R.G. Cytotoxic Effects of Resin Components on Cultured Mammalian Fibroblasts. J. Dent. Res. 1991, 70, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.A.; Vaerten, M.A.; Edwards, C.A.; Hanks, C.T. Cytotoxic Effects of Current Dental Adhesive Systems on Immortalized Odontoblast Cell Line MDPC-23. Dent. Mater. 1999, 15, 434–441. [Google Scholar] [CrossRef]
- Da Silva, A.F.; Marques, M.R.; Da Rosa, W.L.D.O.; Tarquinio, S.B.C.; Rosalen, P.L.; Barros, S.P. Biological Response to Self-Etch Adhesive After Partial Caries Removal in Rats. Clin. Oral Investig. 2018, 22, 2161–2173. [Google Scholar] [CrossRef]
- Koliniotou-Koumpia, E.; Papadimitrio, S.; Tziafas, D. Pulpal Responses after Application of Current Adhesive Systems to Deep Cavities. Clin. Oral Investig. 2007, 11, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Yamashita, Y.; Ichijo, T.; Fusayama, T. The Ultrastructure and Hardness of the Transparent Layer of Human Carious Dentin. J. Dent. Res. 1983, 62, 7–10. [Google Scholar] [CrossRef]
- Fusayama, T. Two Layers of Carious Dentin; Diagnosis and Treatment. Oper. Dent. 1979, 4, 63–70. [Google Scholar]
- Bouillaguet, S.; Wataha, J.C.; Hanks, C.T.; Ciucchi, B.; Holz, J. In Vitro Cytotoxicity and Dentin Permeability of HEMA. J. Endod. 1996, 22, 244–248. [Google Scholar] [CrossRef]
- Graham, L.; Cooper, P.R.; Cassidy, N.; Nor, J.E.; Sloan, A.J.; Smith, A.J. The Effect of Calcium Hydroxide on Solubilisation of Bio-Active Dentine Matrix Components. Biomaterials 2006, 27, 2865–2873. [Google Scholar] [CrossRef]
- Leye Benoist, F.; Gaye Ndiaye, F.; Kane, A.W.; Benoist, H.M.; Farge, P. Evaluation of Mineral Trioxide Aggregate (MTA) versus Calcium Hydroxide Cement (Dycal(®) in the Formation of a Dentine Bridge: A Randomised Controlled Trial. Int. Dent. J. 2012, 62, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hashem, D.; Mannocci, F.; Patel, S.; Manoharan, A.; Watson, T.F.; Banerjee, A. Evaluation of the Efficacy of Calcium Silicate vs. Glass Ionomer Cement Indirect Pulp Capping and Restoration Assessment Criteria: A Randomised Controlled Clinical Trial-2-Year Results. Clin. Oral Investig. 2018, 23, 1931–1939. [Google Scholar] [CrossRef]
- Schmidt, J.; Buenger, L.; Krohn, S.; Kallies, R.; Zeller, K.; Schneider, H.; Ziebolz, D.; Berg, T.; Haak, R. Effect of a Bioactive Cement on the Microbial Community in Carious Dentin after Selective Caries Removal—An In-Vivo Study. J. Dent. 2020, 92, 103264. [Google Scholar] [CrossRef] [PubMed]
- Laurent, P.; Camps, J.; About, I. Biodentine(TM) Induces TGF-β1 Release from Human Pulp Cells and Early Dental Pulp Mineralization. Int. Endod. J. 2012, 45, 439–448. [Google Scholar] [CrossRef]
- Laurent, P.; Camps, J.; De Méo, M.; Déjou, J.; About, I. Induction of Specific Cell Responses to a Ca(3)SiO(5)-Based Posterior Restorative Material. Dent. Mater. 2008, 24, 1486–1494. [Google Scholar] [CrossRef]
- Meraji, N.; Camilleri, J. Bonding over Dentin Replacement Materials. J. Endod. 2017, 43, 1343–1349. [Google Scholar] [CrossRef]
- Palma, P.J.; Marques, J.A.; Antunes, M.; Falacho, R.I.; Sequeira, D.; Roseiro, L.; Santos, J.M.; Ramos, J.C. Effect of Restorative Timing on Shear Bond Strength of Composite Resin/Calcium Silicate-Based Cements Adhesive Interfaces. Clin. Oral Investig. 2020. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.; Marques, J.A.; Falacho, R.I.; Vinagre, A.; Santos, J.M.; Ramos, J.C. Does Delayed Restoration Improve Shear Bond Strength of Different Restorative Protocols to Calcium Silicate-Based Cements? Materials 2018, 11, 2216. [Google Scholar] [CrossRef] [PubMed]
- Fröb, L.; Rüttermann, S.; Romanos, G.E.; Herrmann, E.; Gerhardt-Szép, S. Cytotoxicity of Self-Etch Versus Etch-and-Rinse Dentin Adhesives: A Screening Study. Materials 2020, 13, 452. [Google Scholar] [CrossRef]
- Lanza, C.R.M.; De Souza Costa, C.A.; Furlan, M.; Alécio, A.; Hebling, J. Transdentinal Diffusion and Cytotoxicity of Self-Etching Adhesive Systems. Cell Biol. Toxicol. 2009, 25, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Caldas, I.P.; Alves, G.G.; Barbosa, I.B.; Scelza, P.; De Noronha, F.; Scelza, M.Z. In Vitro Cytotoxicity of Dental Adhesives: A Systematic Review. Dent. Mater. 2019, 35, 195–205. [Google Scholar] [CrossRef]
- Falster, C.A.; Araujo, F.B.; Straffon, L.H.; Nör, J.E. Indirect Pulp Treatment: In Vivo Outcomes of an Adhesive Resin System vs Calcium Hydroxide for Protection of the Dentin-Pulp Complex. Pediatric Dent. 2002, 24, 241–248. [Google Scholar]
- Hebling, J.; Giro, E.M.; Costa, C.A. Human Pulp Response after an Adhesive System Application in Deep Cavities. J. Dent. 1999, 27, 557–564. [Google Scholar] [CrossRef]
- Jiang, R.D.; Lin, H.; Zheng, G.; Zhang, X.M.; Du, Q.; Yang, M. In Vitro Dentin Barrier Cytotoxicity Testing of Some Dental Restorative Materials. J. Dent. 2017, 58, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Krohn, S.; Kallies, R.; Schneider, H.; Zeller, K.; Ziebolz, D.; Berg, T.; Haak, R. Antibacterial Effect of a Brominated Self-Etch Adhesive on Carious Dentin—An In Vivo Study. J. Dent. 2021, 105, 103555. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Kuramoto, A.; Takahashi, Y.; Ebisu, S.; Peters, M.C. In Vitro Antibacterial Effects of the Dentin Primer of Clearfil Protect Bond. Dent. Mater. 2006, 22, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Tjäderhane, L.; Checchi, V.; Di Lenarda, R.; Salo, T.; Tay, F.R.; Pashley, D.H.; Breschi, L. Role of Dentin MMPs in Caries Progression and Bond Stability. J. Dent. Res. 2015, 94, 241–251. [Google Scholar] [CrossRef]
- Pezelj-Ribaric, S.; Anic, I.; Brekalo, I.; Miletic, I.; Hasan, M.; Simunovic-Soskic, M. Detection of Tumor Necrosis Factor Alpha in Normal and Inflamed Human Dental Pulps. Arch. Med. Res. 2002, 33, 482–484. [Google Scholar] [CrossRef]
- Abd-Elmeguid, A.; Abdeldayem, M.; Kline, L.W.; Moqbel, R.; Vliagoftis, H.; Yu, D.C. Osteocalcin Expression in Pulp Inflammation. J. Endod. 2013, 39, 865–872. [Google Scholar] [CrossRef]
- Elsalhy, M.; Azizieh, F.; Raghupathy, R. Cytokines as Diagnostic Markers of Pulpal Inflammation. Int. Endod. J. 2013, 46, 573–580. [Google Scholar] [CrossRef]
- Geraldeli, S.; Li, Y.; Hogan, M.M.B.; Tjaderhane, L.S.; Pashley, D.H.; Morgan, T.A.; Zimmerman, M.B.; Brogden, K.A. Inflammatory Mediators in Fluid Extracted from the Coronal Occlusal Dentine of Trimmed Teeth. Arch. Oral Biol. 2012, 57, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Farges, J.-C.; Carrouel, F.; Keller, J.-F.; Baudouin, C.; Msika, P.; Bleicher, F.; Staquet, M.J. Cytokine Production by Human Odontoblast-Like Cells upon Toll-Like Receptor-2 Engagement. Immunobiology 2011, 216, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Renard, E.; Gaudin, A.; Bienvenu, G.; Amiaud, J.; Farges, J.C.; Cuturi, M.C.; Moreau, A.; Alliot-Licht, B. Immune Cells and Molecular Networks in Experimentally Induced Pulpitis. J. Dent. Res. 2016, 95, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Barkhordar, R.A.; Hayashi, C.; Hussain, M.Z. Detection of Interleukin-6 in Human Dental Pulp and Periapical Lesions. Endod. Dent. Traumatol. 1999, 15, 26–27. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.O.; Faria, M.R.; Fontes, A.; Campos, M.S.; Cavalcanti, B.N. Interleukin-1 Beta and Interleukin-8 in Healthy and Inflamed Dental Pulps. J. Appl. Oral Sci. 2009, 17, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Tjäderhane, L.; Larjava, H.; Sorsa, T.; Uitto, V.J.; Larmas, M.; Salo, T. The Activation and Function of Host Matrix Metalloproteinases in Dentin Matrix Breakdown in Caries Lesions. J. Dent. Res. 1998, 77, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.D.; Minciotti, C.L.; Geraldeli, S.; Carrilho, M.R.; Pashley, D.H.; Tay, F.R.; Nader, H.B.; Salo, T.; Tjäderhane, L.; Tersariol, I.L. Cysteine Cathepsins in Human Carious Dentin. J. Dent. Res. 2011, 90, 506–511. [Google Scholar] [CrossRef]
- Kuhn, E.; Reis, A.; Campagnoli, E.B.; Chibinski, A.C.R.; Carrilho, M.R.D.O.; Wambier, D.S. Effect of Sealing Infected Dentin with Glass Ionomer Cement on the Abundance and Localization of MMP-2, MMP-8, and MMP-9 in Young Permanent Molars In Vivo. Int. J. Paediatr. Dent. 2016, 26, 125–133. [Google Scholar] [CrossRef]
- Palosaari, H.; Pennington, C.J.; Larmas, M.; Edwards, D.R.; Tjäderhane, L.; Salo, T. Expression Profile of Matrix Metalloproteinases (MMPs) and Tissue Inhibitors of MMPs in Mature Human Odontoblasts and Pulp Tissue. Eur. J. Oral Sci. 2003, 111, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, K.; Yamashita, K.; Nakagaki, H.; Iwata, K.; Hayakawa, T. Identification of Tissue Inhibitor of Metalloproteinases-1 (TIMP-1) in Human Teeth and its Distribution in Cementum and Dentine. Arch. Oral Biol. 1994, 39, 345–349. [Google Scholar] [CrossRef]
- Alves, M.J.; Grenho, L.; Lopes, C.; Borges, J.; Vaz, F.; Vaz, I.P.; Fernandes, M.H. Antibacterial Effect and Biocompatibility of a Novel Nanostructured ZnO-Coated Gutta-percha Cone for Improved Endodontic Treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Krohn, S.; Buenger, L.; Zeller, K.; Schneider, H.; Treuheit, M.; Kaiser, T.; Ziebolz, D.; Berg, T.; Haak, R. Molecular Characterization of Intact Cell-Derived and Cell-Free Bacterial DNA from Carious Dentine Samples. J. Microbiol. Methods 2019. [Google Scholar] [CrossRef] [PubMed]
| Group 1 (GP and BD) 1 (n = 11) | Group 2 (PB and SE) 2 (n = 10) | |||
|---|---|---|---|---|
| Sex, n (%) | ||||
| Males | 8 (72.7) | 7 (70.0) | ||
| Females | 3 (27.3) | 3 (30.0) | ||
| Median age, y (min–max) | 26 (19–40) | 23.5 (19–39) | ||
| GP | BD | PB | SE | |
| Tooth type, n (%) | ||||
| Premolars | 8 (72.7) | 7 (63.6) | 2 (20) | 5 (50) |
| Molars | 3 (27.3) | 4 (36.4) | 8 (80) | 5 (50) |
| Restorations, n (%) | ||||
| Class I | 1 (9.1) | 1 (9.1) | 0 (0) | 0 (0) |
| Class II | 10 (90.9) | 10 (90.9) | 10 (100) | 10 (100) |
| Carious dentin consistency | ||||
| visit I, n (%) | ||||
| soft | 5 (45.5) | 2 (18.2) | 2 (20) | 8 (80) |
| medium | 3 (27.3) | 7 (63.6) | 8 (80) | 2 (20) |
| hard | 3 (27.3) | 2 (18.2) | 0 (0) | 0 (0) |
| Carious dentin consistency | ||||
| visit II, n (%) | ||||
| soft | 3 (27.3) | 1 (9.1) | 0 (0) | 0 (0) |
| medium | 2 (18.2) | 4 (36.4) | 9 (90) | 7 (70) |
| hard | 6 (54.5) | 6 (54.5) | 1 (10) | 2 (20) |
| Postoperative symptoms, n (%) | 0 (0) | 0 (0) | 0 (0) | 1 (10) |
| Drop-outs, n (%) | 0 (0) | 0 (0) | 0 (0) | 1 (10) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, J.; Hübler, C.; Krohn, S.; Schmalz, G.; Schneider, H.; Berg, T.; Haak, R.; Ziebolz, D. Detection of Inflammatory and Homeostasis Biomarkers after Selective Removal of Carious Dentin—An In Vivo Feasibility Study. J. Clin. Med. 2021, 10, 1003. https://doi.org/10.3390/jcm10051003
Schmidt J, Hübler C, Krohn S, Schmalz G, Schneider H, Berg T, Haak R, Ziebolz D. Detection of Inflammatory and Homeostasis Biomarkers after Selective Removal of Carious Dentin—An In Vivo Feasibility Study. Journal of Clinical Medicine. 2021; 10(5):1003. https://doi.org/10.3390/jcm10051003
Chicago/Turabian StyleSchmidt, Jana, Clemens Hübler, Sandra Krohn, Gerhard Schmalz, Hartmut Schneider, Thomas Berg, Rainer Haak, and Dirk Ziebolz. 2021. "Detection of Inflammatory and Homeostasis Biomarkers after Selective Removal of Carious Dentin—An In Vivo Feasibility Study" Journal of Clinical Medicine 10, no. 5: 1003. https://doi.org/10.3390/jcm10051003
APA StyleSchmidt, J., Hübler, C., Krohn, S., Schmalz, G., Schneider, H., Berg, T., Haak, R., & Ziebolz, D. (2021). Detection of Inflammatory and Homeostasis Biomarkers after Selective Removal of Carious Dentin—An In Vivo Feasibility Study. Journal of Clinical Medicine, 10(5), 1003. https://doi.org/10.3390/jcm10051003








