Ketoanalogs’ Effects on Intestinal Microbiota Modulation and Uremic Toxins Serum Levels in Chronic Kidney Disease (Medika2 Study)
Abstract
1. Introduction
2. Materials and Methods
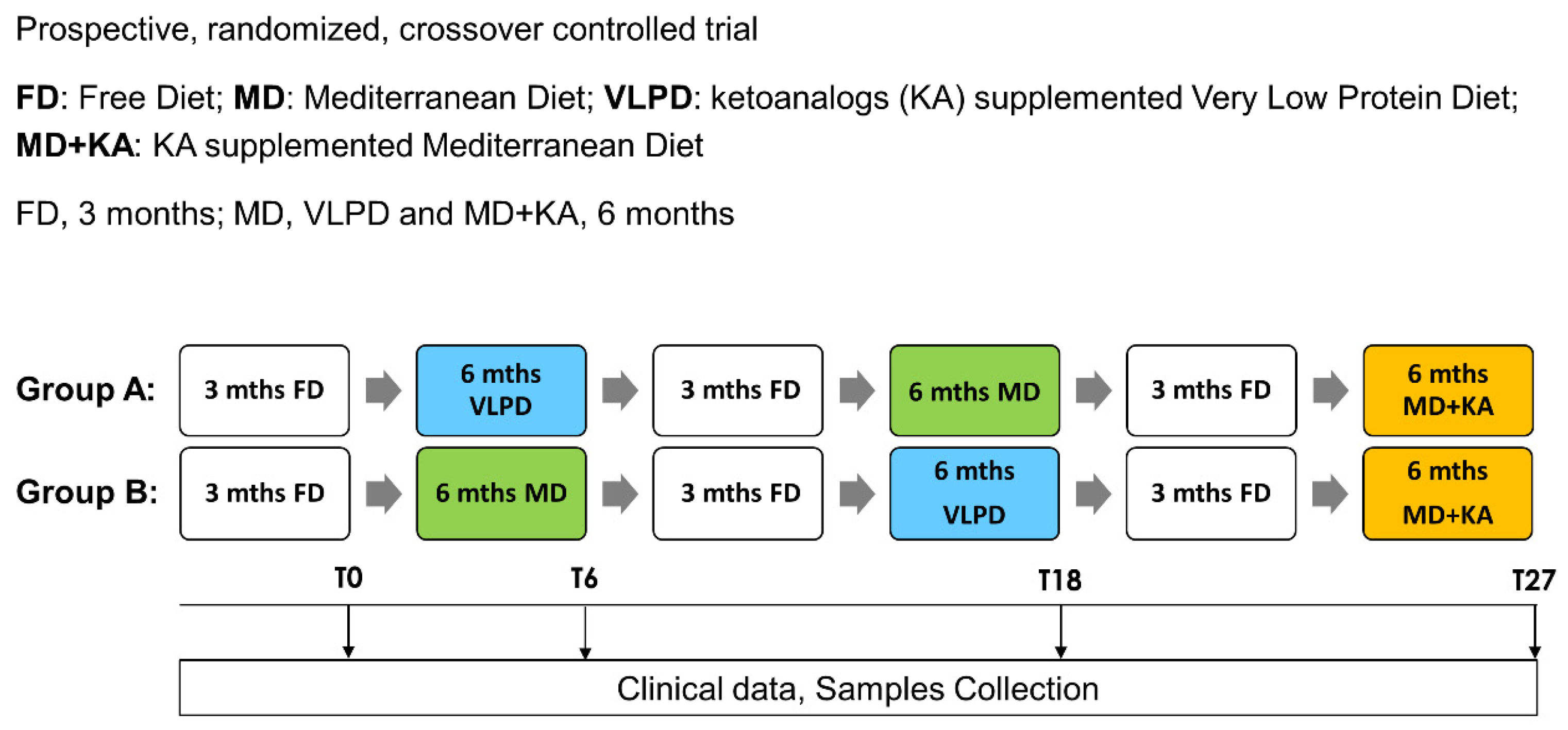
2.1. Study Design
2.2. Collection of Serum and Fecal Samples
2.3. Reagents
2.4. LC-MS/MS for Quantification of PCS and IS
2.5. Serum D-Lactate Assay
2.6. RNA Extraction from Fecal Samples and 16S cDNA Sequencing
2.7. Statistics
3. Results
3.1. Patients
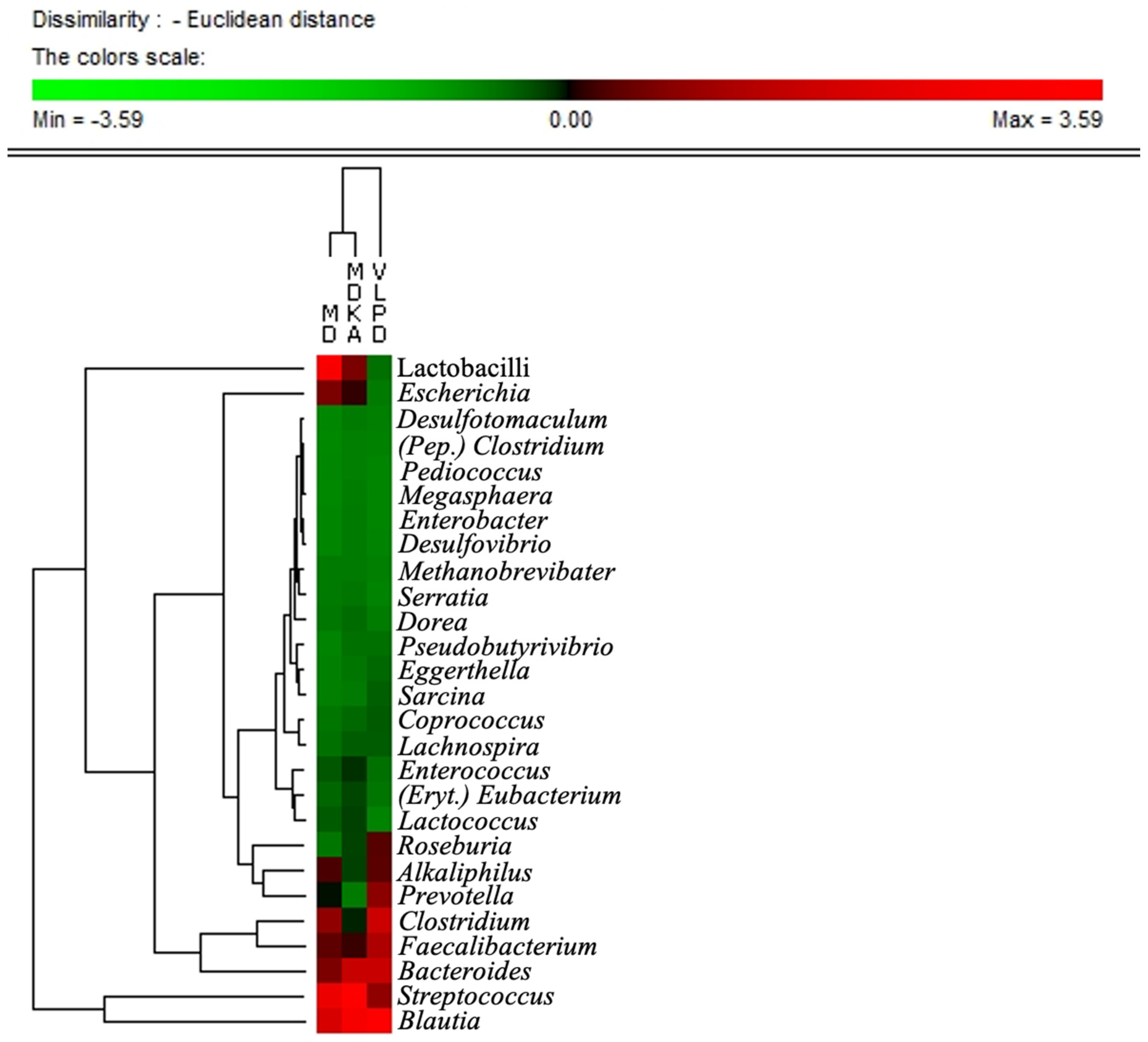
3.2. Microbial Components Linked to Specific Dietary Intake
3.3. Microbial Pattern Linked to Ketoanalogs Administration
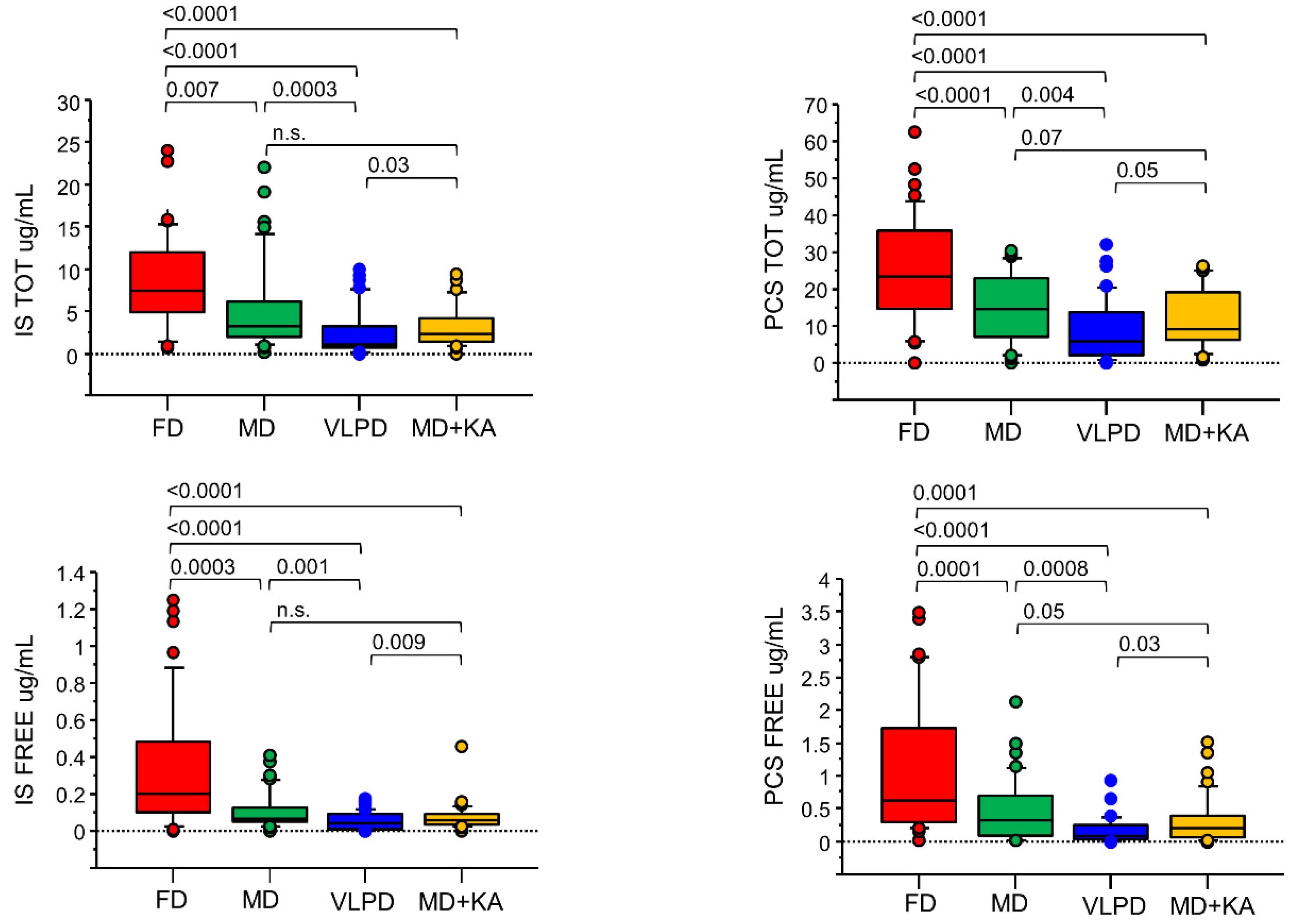
3.4. Uremic Toxins
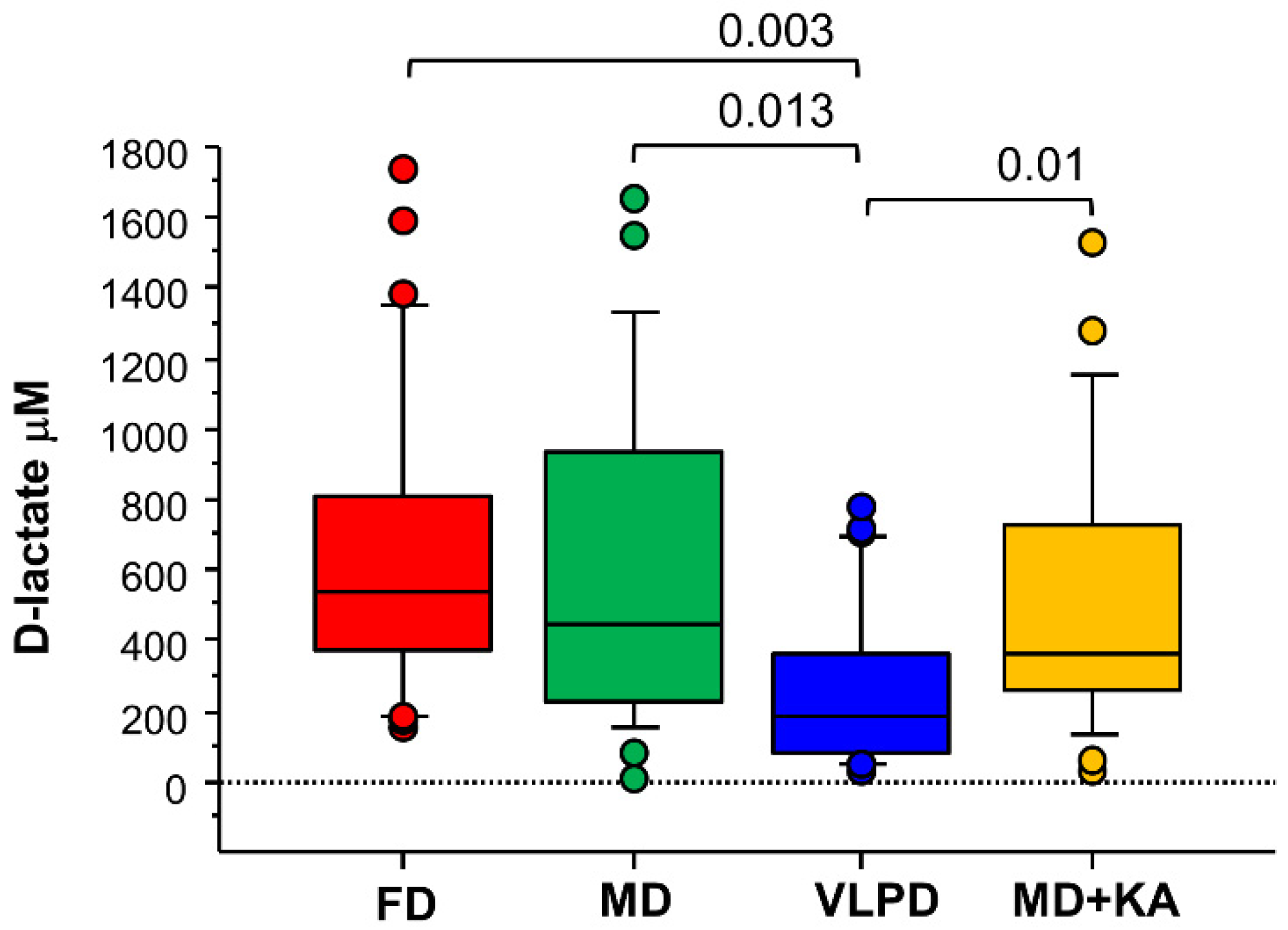
3.5. KA Supplementation Does Not Reduce Intestinal Permeability Compared to the MD
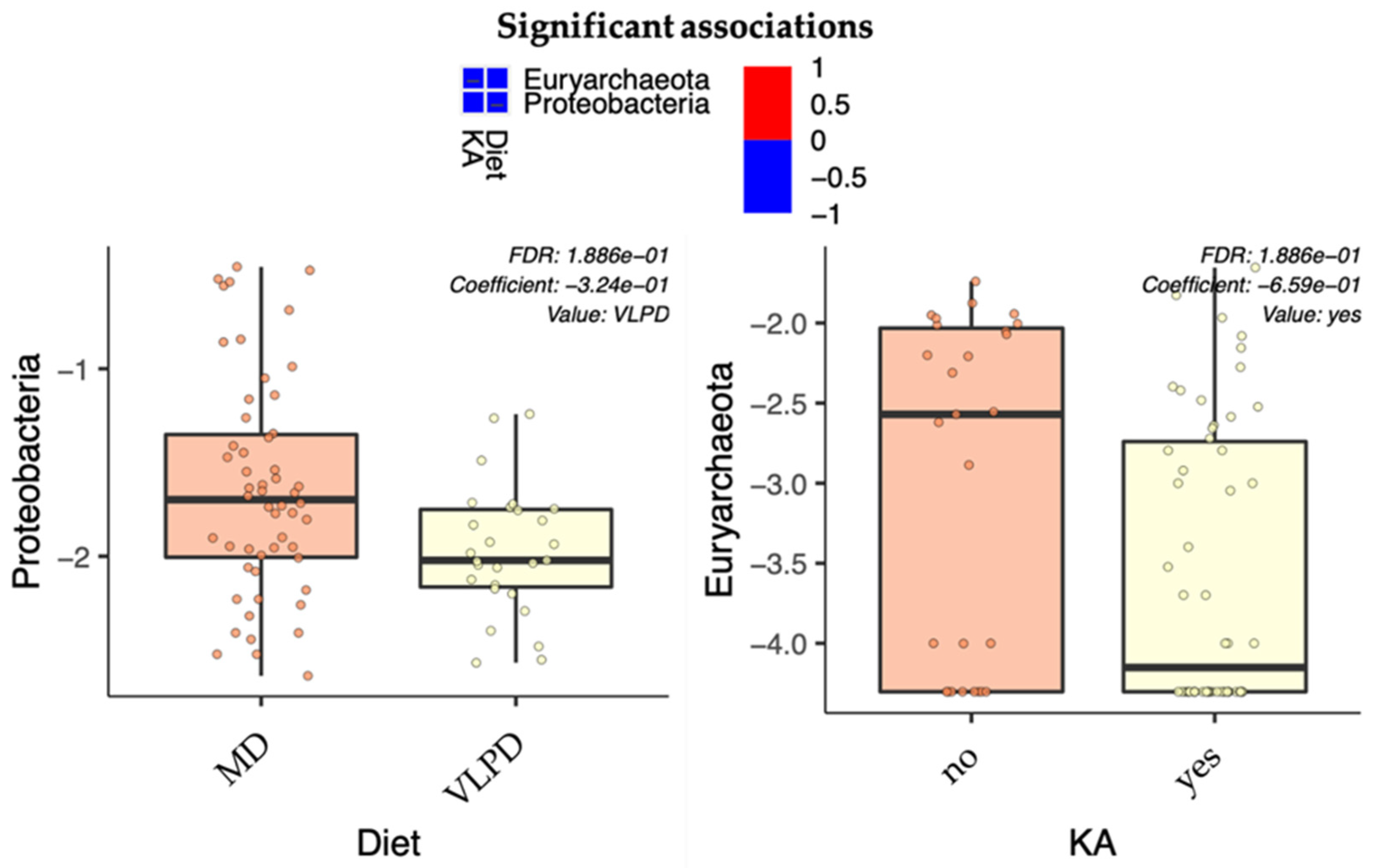
3.6. Correlations between Dietary Intake and Metabolome with Microbiome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Di Iorio, B.; De Santo, N.G.; Anastasio, P.; Perna, A.; Pollastro, M.R.; Di Micco, L.; Cirillo, M. The Giordano-Giovannetti diet. J. Nephrol. 2013, 26 (Suppl. S22), 143–152. [Google Scholar] [CrossRef]
- Giovannetti, S.; Maggiore, Q. A low-nitrogen diet with proteins of high biological value for severe chronic uremia. Lancet 1964, 1, 1000–1003. [Google Scholar] [CrossRef]
- Kopple, J.D.; Fouque, D. PRO: The rationale for dietary therapy for patients with advanced chronic kidney disease. Nephrol. Dial. Transpl. 2018, 33, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; Brunori, G.; Di Iorio, B.R.; D’Alessandro, C.; Pasticci, F.; Cosola, C.; Bellizzi, V.; Bolasco, P.; Capitanini, A.; Fantuzzi, A.L.; et al. Nutritional treatment of advanced CKD: Twenty consensus statements. J. Nephrol. 2018, 31, 457–473. [Google Scholar] [CrossRef]
- Di Iorio, B.R.; Di Micco, L.; Marzocco, S.; De Simone, E.; De Blasio, A.; Sirico, M.L.; Nardone, L.; UBI Study Group. Very Low-Protein Diet (VLPD) Reduces Metabolic Acidosis in Subjects with Chronic Kidney Disease: The “Nutritional Light Signal” of the Renal Acid Load. Nutrients 2017, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-Supplemented Vegetarian Very Low-Protein Diet and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, M.; Bellizzi, V.; Chauveau, P.; Cupisti, A.; Ecder, T.; Fouque, D.; Garneata, L.; Lin, S.; Mitch, W.; Teplan, V.; et al. Do ketoanalogues still have a role in delaying dialysis initiation in CKD predialysis patients? Semin. Dial. 2013, 26, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, B.R.; Marzocco, S.; Bellasi, A.; De Simone, E.; Dal Piaz, F.; Rocchetti, M.T.; Cosola, C.; Di Micco, L.; Gesualdo, L. Nutritional therapy reduces protein carbamylation through urea lowering in chronic kidney disease. Nephrol. Dial. Transpl. 2018, 33, 804–813. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Yuan, J.; Norris, K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am. J. Nephrol. 2013, 37, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cosola, C.; Rocchetti, M.T.; Cupisti, A.; Gesualdo, L. Microbiota metabolites: Pivotal players of cardiovascular damage in chronic kidney disease. Pharmacol. Res. 2018, 130, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Fouque, D. Nutritional Management of Chronic Kidney Disease. N. Engl. J. Med. Engl. 2017, 377, 1765–1776. [Google Scholar] [CrossRef]
- Gallieni, M.; Cupisti, A. DASH and Mediterranean Diets as Nutritional Interventions for CKD Patients. Am. J. Kidney Dis. 2016, 68, 828–830. [Google Scholar] [CrossRef]
- Chauveau, P.; Aparicio, M.; Bellizzi, V.; Campbell, K.; Hong, X.; Johansson, L.; Kolko, A.; Molina, P.; Sezer, S.; Wanner, C.; et al. Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol. Dial. Transpl. 2018, 33, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Capizzi, I.; Vigotti, F.N.; Leone, F.; D’Alessandro, C.; Giuffrida, D.; Nazha, M.; Roggero, S.; Colombi, N.; Mauro, G.; et al. Low protein diets in patients with chronic kidney disease: A bridge between mainstream and complementary-alternative medicines? BMC Nephrol. 2016, 17, 76. [Google Scholar] [CrossRef][Green Version]
- Zoccali, C.; Mallamaci, F. Moderator’s view: Low-protein diet in chronic kidney disease: Effectiveness, efficacy and precision nutritional treatments in nephrology. Nephrol. Dial. Transpl. 2018, 33, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, B.R.; Rocchetti, M.T.; De Angelis, M.; Cosola, C.; Marzocco, S.; Di Micco, L.; di Bari, I.; Accetturo, M.; Vacca, M.; Gobbetti, M.; et al. Nutritional Therapy Modulates Intestinal Microbiota and Reduces Serum Levels of Total and Free Indoxyl Sulfate and P-Cresyl Sulfate in Chronic Kidney Disease (Medika Study). J. Clin. Med. 2019, 8, 1424. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, V.; Di Iorio, B.R.; De Nicola, L.; Minutolo, R.; Zamboli, P.; Trucillo, P.; Catapano, F.; Cristofano, C.; Scalfi, L.; Conte, G.; et al. Very low protein diet supplemented with ketoanalogs improves blood pressure control in chronic kidney disease. Kidney Int. 2007, 71, 245–251. [Google Scholar] [CrossRef]
- Panichi, V.; Rocchetti, M.T.; Scatena, A.; Rosati, A.; Migliori, M.; Pizzarelli, F.; Gesualdo, L.; REDERT Study group. Long term variation of serum levels of uremic toxins in patients treated by post-dilution high volume on-line hemodiafiltration in comparison to standard low-flux bicarbonate dialysis: Results from the REDERT study. J. Nephrol. 2017, 30, 583–591. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Montemurno, E.; Piccolo, M.; Vannini, L.; Lauriero, G.; Maranzano, V.; Gozzi, G.; Serrazanetti, D.; Dalfino, G.; Gobbetti, M.; et al. Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN). PLoS ONE 2014, 9, e99006. [Google Scholar] [CrossRef]
- Gondalia, S.V.; Palombo, E.A.; Knowles, S.R.; Cox, S.B.; Meyer, D.; Austin, D.W. Molecular characterisation of gastrointestinal microbiota of children with autism (with and without gastrointestinal dysfunction) and their neurotypical siblings. Autism Res. 2012, 5, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Serino, M.; Luche, E.; Grès, S.; Baylac, A.; Bergé, M.; Cenac, C.; Waget, A.; Klopp, P.; Iacovoni, J.; Klopp, C.; et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 2012, 6, 543–553. [Google Scholar] [CrossRef]
- Di Iorio, B.R.; Minutolo, R.; De Nicola, L.; Bellizzi, V.; Catapano, F.; Iodice, C.; Rubino, R.; Conte, G. Supplemented very low protein diet ameliorates responsiveness to erythropoietin in chronic renal failure. Kidney Int. 2003, 64, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Marzocco, S.; Fazely, G.; Di Micco, L.; Autore, G.; Adesso, S.; Dal Piaz, F.; Heidland, A.; Di Iorio, B. Supplementation of short-chain fatty acid, sodium propionate, in patients on maintenance hemodialysis—Beneficial effects on inflammatory parameters and gut-derived uremic toxins—A pilot study (PLAN Study). J. Clin. Med. 2018, 7, 315. [Google Scholar] [CrossRef]
- Milovanova, L.; Fomin, V.; Moiseev, S.; Taranova, M.; Milovanov, Y.; Kozlovskaya, L.L.; Kozlov, V.; Kozevnikova, E.; Milovanova, S.; Lebedeva, M.; et al. Effect of essential amino acid ketoanalogues and protein restriction diet on morphogenetic proteins (FGF-23 and Klotho) in 3b–4 stages chronic kidney disease patients: A randomized pilot study. Clin. Exp. Nephrol. 2018, 22, 1351–1359. [Google Scholar] [CrossRef]
- Di Iorio, B.; Di Micco, L.; Torraca, S.; Sirico, M.L.; Russo, L.; Pota, A.; Mirenghi, F.; Russo, D. Acute effects of very-low-protein diet on FGF23 levels: A randomized study. Clin. J. Am. Soc. Nephrol. 2012, 7, 581–587. [Google Scholar] [CrossRef]
- Marzocco, S.; Dal Piaz, F.; Di Micco, L.; Torraca, S.; Sirico, M.L.; Tartaglia, D.; Autore, G.; Di Iorio, B. Very low protein diet reduces indoxyl sulfate levels in chronic kidney disease. Blood Purif. 2013, 35, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Feiten, S.F.; Draibe, S.A.; Watanabe, R.; Duenhas, M.R.; Baxmann, A.C.; Nerbass, F.B.; Cuppari, L. Short-term effects of a very-low-protein diet supplemented with ketoacids in nondialyzed chronic kidney disease patients. Eur. J. Clin. Nutr. 2005, 59, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Adler, S.; Caggiula, A.W.; England, B.K.; Greene, T.; Hunsicker, L.G.; Kusek, J.W.; Rogers, N.L.; Teschan, P.E. Effects of dietary protein restriction on the progression of advanced renal disease in the Modification of Diet in Renal Disease Study. Am. J. Kidney Dis. 1996, 27, 652–663. [Google Scholar] [CrossRef]
- Teplan, V.; Schück, O.; Racek, J.; Mareckova, O.; Stollova, M.; Hanzal, V.; Malý, J. Reduction of plasma asymmetric dimethylarginine in obese patients with chronic kidney disease after three years of a low-protein diet supplemented with keto-amino acids: A randomized controlled trial. Wien. Klin. Wochenschr. 2008, 120, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Teplan, V.; Schück, O.; Knotek, A.; Hajný, J.; Horácková, M.; Kvapil, M. Czech multicenter study. Enhanced metabolic effect of erythropoietin and keto acids in CRF patients on low-protein diet: Czech multicenter study. Am. J. Kidney Dis. 2003, 41, 26–30. [Google Scholar] [CrossRef]
- Pollock, C.A.; Wyndham, R.; Collett, P.V.; Elder, G.; Field, M.J.; Kalowski, S.; Lawrence, J.R.; Waugh, D.A.; George, C.R. Effects of erythropoietin therapy on the lipid profile in end-stage renal failure. Kidney Int. 1994, 45, 897–902. [Google Scholar] [CrossRef]
- Requena, T.; Martínez-Cuesta, P.M. Diet and microbiota linked in health and disease. Food Funct. 2018, 9, 688–704. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- De Angelis, M.; Ferrocino, I.; Maria Calabrese, F.; De Filippis, F.; Cavallo, N.; Siragusa, S.; Rampelli, S.; Di Cagno, R.; Rantsiou, K.; Vannini, L.; et al. Diet influences the functions of the human intestinal microbiome. Sci. Rep. 2020, 10, 4247. [Google Scholar] [CrossRef]
- Cosola, C.; Rocchetti, M.T.; Sabatino, A.; Fiaccadori, E.; Di Iorio, B.R.; Gesualdo, L. Microbiota issue in CKD: How promising are gut-targeted approaches? J. Nephrol. 2019, 32, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Lun, H.; Yang, W.; Zhao, S.; Jiang, M.; Xu, M.; Liu, F.; Wang, Y. Altered gut microbiota and microbial biomarkers associated with chronic kidney disease. Microbiologyopen 2019, 8, e00678. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Gryp, T.; Huys, G.R.B.; Joossens, M.; Biesen, W.; Glorieux, G.; Vaneechoutte, M. Isolation and quantification of uremic toxin precursor-generating gut bacteria in chronic kidney disease patients. Int. J. Mol. Sci. 2020, 21, 1986. [Google Scholar] [CrossRef]
- Buck, S.S.; Hansen, E.E.; Manchester, J.K.; Coutinho, P.M.; Henrissat, B.; Fulton, R.; Latreille, P.; Kim, K.; Wilson, R.K.; Gordon, J.I. Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proc. Natl. Acad. Sci. USA 2007, 104, 10643–10648. [Google Scholar] [CrossRef]
- Gaci, N.; Borrel, G.; Tottey, W.; O’Toole, P.W.; Brugère, J.F. Archaea and the human gut: New beginning of an old story. World J. Gastroenterol. 2014, 20, 16062–16078. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, J.R.; Cai, H.; Larsen, R.L.; Hughes, J.E.; Welker, D.L.; De Carvalho, V.G.; Tompkins, T.A.; Ardö, Y.; Vogensen, F.; De Lorentiis, A.; et al. Genetic diversity in proteolytic enzymes and amino acid metabolism among Lactobacillus helveticus strains. J. Dairy Sci. 2011, 94, 4313–4328. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, R.; Cristofori, F.; Vacca, M.; Barone, M.; De Angelis, M. Advances in understanding the potential therapeutic applications of gut microbiota and probiotic mediated therapies in celiac disease. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Vukotić, G.; Strahinić, I.; Begović, J.; Lukić, J.; Kojić, M.; Fira, D. Survey on proteolytic activity and diversity of proteinase genes in mesophilic lactobacilli. Microbiology 2016, 85, 33–41. [Google Scholar] [CrossRef]
- Salvetti, E.; O’Toole, P.W. The Genomic Basis of Lactobacilli as Health-Promoting Organisms. Microbiol Spectr 2017, 5, 49–71. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Q.; Wei, Y.; et al. A Novel Postbiotic From Lactobacillus rhamnosus GG With a Beneficial Effect on Intestinal Barrier Function. Front. Microbiol. 2019, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Shokryazdan, P.; Sieo, C.C.; Kalavathy, R.; Liang, J.B.; Alitheen, N.B.; Jahromi, M.F.; Ho, Y.W. Probiotic Potential of Lactobacillus Strains with Antimicrobial Activity against Some Human Pathogenic Strains. BioMed Res. Int. 2014, 927268. [Google Scholar] [CrossRef]
- Yang, X.; Twitchell, E.; Li, G.; Wen, K.; Weiss, M.; Kocher, J.; Lei, S.; Ramesh, A.; Ryan, E.P.; Yuan, L. High protective efficacy of rice bran against human rotavirus diarrhea via enhancing probiotic growth, gut barrier function, and innate immunity. Sci. Rep. 2015, 5, 15004. [Google Scholar] [CrossRef]
- Bunešová, V.; Joch, M.; Musilová, S.; Rada, V. Bifidobacteria, Lactobacilli, and Short Chain Fatty Acids of Vegetarians and Omnivores. Sci. Agric. Bohem. 2017, 48, 47–54. [Google Scholar] [CrossRef]
- Wu, P.; Lin, T.; Ho, H.J.; Tseng, C.; Lin, Y.; Liang, S.; Lee, H.; Kuo, M.; Hung, S.; Chiu, Y.; et al. Differences in Gut Microbiota Proles and Functions between End-stage Renal Disease and Healthy Populations. Available online: https://www.researchsquare.com/article/rs-82652/v1 (accessed on 30 September 2020). [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, L.; Dou, X.; Wang, C.; Zhang, W.; Gao, K.; Liu, J.; Wang, H. Lactobacillus reuteri ZJ617 maintains intestinal integrity via regulating tight junction, autophagy and apoptosis in mice challenged with lipopolysaccharide. Oncotarget 2017, 8, 77489–77499. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ajuwon, K.M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS ONE 2017, 12, e0179586. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.S.; Ki, S.J.; Thanavel, R.; Kim, J.J.; Kim, J.; Lee, M.; Kim, J.; Jung, C.; Han, T.; Cho, H.; et al. Maturation of human intestinal organoids in vitro facilitates colonization by commensal lactobacilli by reinforcing the mucus layer. FASEB J. 2020, 34, 9899–9910. [Google Scholar] [CrossRef] [PubMed]
- De Cal, M.; Cazzavillan, S.; Rassu, M.; Ronco, C. Residual of Bacterial Dna in Hemodialyzers: The Proof of Subclinical Infection Sustaining Chronic Inflammation. Int. J. Artif. Organs 2008, 31, 395–404. [Google Scholar] [CrossRef]
- Szeto, C.C.; McIntyre, C.W.; Li, P.K. Circulating Bacterial Fragments as Cardiovascular Risk Factors in CKD. J. Am. Soc. Nephrol. 2018, 29, 1601–1608. [Google Scholar] [CrossRef]
- Singh, S.; Bhatia, R.; Khare, P.; Sharma, S.; Rajarammohan, S.; Bishnoi, M.; Bhadada, S.K.; Sharma, S.S.; Kaur, J.; Kondepudi, K.K. Anti-inflammatory Bifidobacterium strains prevent dextran sodium sulfate induced colitis and associated gut microbial dysbiosis in mice. Sci. Rep. 2020, 10, 18597. [Google Scholar] [CrossRef]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef]
- Tuomainen, M.; Lindström, J.; Lehtonen, M.; Auriola, S.; Pihlajamäki, J.; Peltonen, M.; Tuomilehto, J.; Uusitupa, M.; de Mello, V.D.; Hanhineva, K. Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutr. Diabetes 2018, 8, 35. [Google Scholar] [CrossRef]
- Sun, C.; Lin, C.; Pan, H.; Lee, C.; Lu, S.; Hsieh, Y.; Huang, S.; Huang, H. Clinical association between the metabolite of healthy gut microbiota, 3-indolepropionic acid and chronic kidney disease. Clin. Nutr. 2019, 38, 2945–2948. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Huć, T.; Nowinski, A.; Drapala, A.; Konopelski, P.; Ufnal, M. Indole and indoxyl sulfate, gut bacteria metabolites of tryptophan, change arterial blood pressure via peripheral and central mechanisms in rats. Pharmacol. Res. 2017, 130, 172–179. [Google Scholar] [CrossRef]
- Van Treuren, W.; Dodd, D. Microbial Contribution to the Human Metabolome: Implications for Health and Disease. Annu. Rev. Pathol. 2020, 15, 345–369. [Google Scholar] [CrossRef] [PubMed]

| Mediterranean Diet | Very-Low-Protein Diet + KA | Mediterranean Diet + KA | |
|---|---|---|---|
| Protein Intake, (g/kg bw/day) | 0.8 | 0.3 | 0.8 |
| Animal Protein (g/day) | 30–40 | zero | 30–40 |
| Vegetal Protein (g/day) | 40–50 | 30–40 | 30–40 |
| Total Fat (Saturated) | 34% (4%) | 32% (4%) | 34% (4%) |
| Carbohydrates | 58% | 62% | 58% |
| Sugar | 11% | 11% | 11% |
| Fibres (g/day) | 20 | 22 | 20 |
| Energy (kcal/kg bw/day) | 30–35 | 30–35 | 30–35 |
| Sodium (g/day) | 5–6 | 5–6 | 5–6 |
| Potassium (g/day) | 2–4 | 3–5 | 3–5 |
| Calcium (g/day) | 1.1–1.3 | 1.1–1.3 | 1.1–1.2 |
| Phosphorus (g/day) | 1.2–1.5 | 0.6–0.8 | 1.0–1.2 |
| Fe (mg/day) | 9 | 5 | 9 |
| Ketoanalogs (cpr/5 kg bw/day) | zero | 1 | 1 |
| Free Diet | Mediterranean Diet–MD | VLPD + KA | MD + KA | p Value | |
|---|---|---|---|---|---|
| Number | 43 | ||||
| Sex (M), number | 32 | ||||
| Age, years | 65 ± 15 | ||||
| Diabetic, n (%) | 18 (41.8) | ||||
| Weight, kg | 73 ± 14 | 73 ± 14 | 72 ± 13 | 72 ± 13 | n.s |
| SBP, mm Body | 137 ± 20 | 136 ± 16 | 127 ± 13 * | 134 ± 15 | <0.001 |
| DBP, mm Hg | 77 ± 12 | 77 ± 7 | 73 ± 6 § | 80 ± 7 ** | 0.02 |
| Creatinine, mg/dL | 3.6 ± 1.8 | 3.5 ± 1.4 | 3.2 ± 1.3 * | 3.5 ± 1.5 | 0.006 |
| eGFR, ml/min/1.73 m2 | 20 ± 11 | 20.2 ± 10 | 22.3 ± 12.6 * | 20.5 ± 11.2 | 0.0001 |
| Urea, mg/dL | 172 ± 23 | 133 ± 30 # | 62 ± 20 * | 130 ± 31 # | <0.001 |
| Glycaemia, mg/dL | 118 ± 32 | 115 ± 30 | 112 ± 21 | 113 ± 23 | n.s. |
| Glycated HB (%) | 7.4 ± 1.1 | 7.4 ± 1.0 | 7.2 ± 0.8 | 7.1 ± 0.8 # | 0.05 |
| Uricemia, mg/dL | 6.1 ± 2.3 | 5.8 ± 1.5 | 5.0 ± 1.1 $ | 5.2 ± 1.0 $ | 0.03 |
| Natrium, mmol/L | 140 ± 2 | 141 ± 2 | 139 ± 2 $ | 140 ± 2 | 0.001 |
| Cloro, mmol/L | 104 ± 6 | 102 ± 5 | 100 ± 5 | 103 ± 5 | n.s. |
| Potassium, mmol/L | 4.9 ± 0.6 | 4.8 ± 0.5 | 4.9 ± 0.5 | 4.8 ± 0.4 | n.s. |
| Calcium, mmol/L | 9.1 ± 0.6 | 9.3 ± 0.5 | 9.2 ± 0.4 | 9.4 ± 0.4 | n.s. |
| Phosphate, mg/dl | 4.7 ± 1.0 | 4.3 ± 0.8 # | 3.7 ± 0.5 * | 4.3 ± 0.6 ° | <0.001 |
| Bicarbonates, mmol/L | 21 ± 3 | 23 ± 3 # | 25 ± 2 * | 23.6 ± 2 ° | 0.001 |
| Cholesterol, mg/dL | 166 ± 39 | 163 ± 37 | 166 ± 37 | 167 ± 29 | n.s. |
| Triglycerides, mg/dL | 143 ± 78 | 147 ± 37 | 149 ± 62 | 167 ± 29 £ | 0.03 |
| Sideremia | 80 ± 33 | 71 ± 24 | 80 ± 26 £ | 82 ± 32 | 0.02 |
| Trasferrin | 214 ± 42 | 211 ± 46 | 201 ± 46 | 220 ± 52 δ | 0.01 |
| Ferritin, ng/ml | 135 ± 93 | 155 ± 295 # | 111 ± 68 | 116 ± 83 | 0.03 |
| PTH, pg/ml | 239 ± 170 | 241 ± 199 | 170 ± 87 $ | 215 ± 177 | 0.001 |
| Haemoglobin, g/dL | 11.6 ± 1.8 | 11.8 ± 1.5 | 12.0 ± 1.3 | 11.8 ± 1.8 | n.s. |
| Albumin, g/dL | 3.7 ± 0.5 | 3.8 ± 0.4 | 3.8 ± 0.3 | 3.8 ± 0.4 | n.s. |
| CRP, mg/L | 7.0 ± 12 | 4.3 ± 4.8 | 2.4 ± 2.0 * | 4.3 ± 6.2 ° | 0.01 |
| Diuresis, ml/day | 2120 ± 410 | 2086 ± 511 | 2028 ± 359 | 2124 ± 392 | n.s. |
| Urinary natrium, mmol/day | 163 ± 47 | 137 ± 36 | 122 ± 28 * | 144 ± 50 | 0.001 |
| Urinary potassium, mmol/day | 43 ± 13 | 47 ± 14 # | 55 ± 14 * | 49 ± 16 ° | 0.001 |
| Urinary phosphate, mmol/day | 679 ± 150 | 521 ± 174 # | 304 ± 104 * | 490 ± 178 | 0.001 |
| Urinary Cl, mmol/day | 130 ± 43 | 114 ± 26 | 103 ± 17 * | 121 ± 40 | <0.001 |
| Prot-u, mg/day | 1156 ± 1263 | 1083 ± 980 | 936 ± 814 $ | 1035 ± 964 | 0.02 |
| Creatinine clearance, mL/min | 24 ± 14.3 | 22.6 ± 13.0 | 23.6 ± 12.8 § | 20.8 ± 12.5 £ | 0.003 |
| P intake, mg/day | 1013 ± 224 | 797 ± 242 # | 484 ± 141 * | 746 ± 263 | <0.001 |
| Na intake, g/day | 9.6 ± 1.9 | 8.0 ± 1.6 | 6.8 ± 1.4 * | 8.8 ± 2.6 | <0.001 |
| Protein intake | 1.14 ± 0.2 | 0.96 ± 0.15 ° | 0.49 ± 0.12 * | 1.0 ± 0.2 ° | <0.001 |
| Phylum | Family | Species | MD | MD + KA | VLPD | * p MD vs. MD + KA | p MD vs. VLPD | p MD + KA vs. VLPD |
|---|---|---|---|---|---|---|---|---|
| Actinobacteria | Bifidobacteriaceae | Bifidobacterium adolescentis | 1.25 | 1.01 | 3.39 | ** ns | 0.038 | 0.022 |
| Bifidobacterium stercoris | 0.91 | 0.92 | 1.75 | ns | 0.036 | 0.014 | ||
| Bacteroidetes | Bacteroidaceae | Bacteroides eggerthii | 0.2 | 1.06 | 0.01 | ns | ns | 0.024 |
| Bacteroides stercoris | 0.74 | 0.7 | 1.73 | ns | 0.019 | 0.004 | ||
| Bacteroides uniformis | 0.31 | 1.14 | 0.84 | 0.030 | ns | ns | ||
| Prevotellaceae | Prevotella copri | 2.51 | 0.06 | 5.34 | 0.036 | ns | 0.010 | |
| Firmicutes | Clostridiaceae | Alkaliphilus crotonatoxidans | 0.91 | 0.37 | 0.71 | 0.028 | ns | ns |
| Clostridium cadaveris | 2.23 | 0.37 | 7.17 | ns | 0.019 | 0.000 | ||
| Sarcina maxima | 0.24 | 0.18 | 1.23 | ns | 0.021 | 0.014 | ||
| Coriobacteriaceae | Eggerthella sinensis | 0.3 | 0.23 | 0.89 | ns | 0.001 | 0.002 | |
| Enterococcaceae | Enterococcus lactis | 1.31 | 1.7 | 0.19 | ns | ns | 0.024 | |
| Erysipelotrichaceae | Erysipelothrix inopinata | 0.3 | 0.4 | 0.57 | ns | 0.047 | ns | |
| Eubacterium biforme | 1.12 | 1.84 | 0.46 | ns | ns | 0.008 | ||
| Lachnospiraceae | Blautia coccoides | 3.71 | 6.52 | 6.59 | 0.001 | 0.013 | ns | |
| Blautia hydrogenotrophica | 0.01 | 0.27 | 0.55 | ns | 0.027 | ns | ||
| Blautia obeum | 1.29 | 1.24 | 2.79 | ns | 0.008 | 0.001 | ||
| Blautia wexlerae | 2.92 | 6.62 | 6.39 | ns | 0.021 | ns | ||
| Coprococcus eutactus | 0.28 | 0.62 | 0.98 | ns | 0.004 | ns | ||
| Dorea formicigenerans | 0.63 | 0.71 | 0.28 | ns | 0.005 | 0.001 | ||
| Lachnospira pectinoschiza | 0.83 | 1.26 | 1.35 | 0.020 | 0.021 | ns | ||
| Pseudobutyrivibrio xylanivorans | 0.24 | 0.56 | 0.71 | ns | 0.036 | ns | ||
| Roseburia faecis | 0.68 | 1.95 | 3.87 | 0.029 | 0.007 | 0.022 | ||
| Lactobacillaceae | Limosilactobacillus fermentum | 0.6 | 0.4 | 0 | ns | 0.001 | 0.005 | |
| Lactobacillus gasseri | 0.73 | 0.49 | 0.1 | ns | 0.045 | ns | ||
| Limosilactobacillus oris | 0.49 | 0.5 | 0.03 | ns | 0.023 | ns | ||
| Ligilactobacillus salivarius | 8.91 | 1.91 | 0.11 | ns | 0.041 | ns | ||
| Ruminococcaceae | Faecalibacterium prausnitzii | 3.99 | 3.38 | 7.3 | ns | 0.007 | 0.001 | |
| Streptococcaceae | Streptococcus parasanguinis | 0.64 | 1.06 | 0.05 | ns | 0.002 | 0.000 | |
| Streptococcus sobrinus | 0.78 | 0.04 | 0 | ns | 0.043 | ns | ||
| Streptococcus vestibularis | 7.56 | 11.89 | 2.62 | ns | 0.043 | 0.000 | ||
| Proteobacteria | Enterobacteriaceae | Escherichia albertii | 4.85 | 3.18 | 0.4 | ns | 0.029 | 0.033 |
| Serratia entomophila | 0.52 | 0.38 | 0.05 | ns | 0.022 | 0.022 |
| Metadata | Taxon | Value | Coef | Stderr | Pval | Qval | |
|---|---|---|---|---|---|---|---|
| Family | Diet | Streptococcaceae | VLPD | −0.93 | 0.16 | 6.04 × 10−8 | 3.62 × 10−6 |
| Diet | Clostridiaceae | VLPD | 0.38 | 0.11 | 5.18 × 10−4 | 1.55 × 10−2 | |
| Diet | Lactobacillaceae | VLPD | −0.78 | 0.22 | 8.21 × 10−4 | 1.64 × 10−2 | |
| KA | Lachnospiraceae | yes | 0.22 | 0.07 | 2.85 × 10−3 | 4.27 × 10−2 | |
| Genus | Diet | Streptococcus | VLPD | −0.89 | 0.16 | 2.43 × 10−7 | 2.28 × 10−5 |
| Diet | Dorea | VLPD | −0.48 | 0.11 | 5.92 × 10−5 | 2.78 × 10−3 | |
| Diet | Clostridium | VLPD | 0.47 | 0.12 | 2.08 × 10−4 | 6.51 × 10−3 | |
| Diet | lactobacilli | VLPD | −0.79 | 0.23 | 8.83 × 10−4 | 2.08 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocchetti, M.T.; Di Iorio, B.R.; Vacca, M.; Cosola, C.; Marzocco, S.; di Bari, I.; Calabrese, F.M.; Ciarcia, R.; De Angelis, M.; Gesualdo, L. Ketoanalogs’ Effects on Intestinal Microbiota Modulation and Uremic Toxins Serum Levels in Chronic Kidney Disease (Medika2 Study). J. Clin. Med. 2021, 10, 840. https://doi.org/10.3390/jcm10040840
Rocchetti MT, Di Iorio BR, Vacca M, Cosola C, Marzocco S, di Bari I, Calabrese FM, Ciarcia R, De Angelis M, Gesualdo L. Ketoanalogs’ Effects on Intestinal Microbiota Modulation and Uremic Toxins Serum Levels in Chronic Kidney Disease (Medika2 Study). Journal of Clinical Medicine. 2021; 10(4):840. https://doi.org/10.3390/jcm10040840
Chicago/Turabian StyleRocchetti, Maria Teresa, Biagio Raffaele Di Iorio, Mirco Vacca, Carmela Cosola, Stefania Marzocco, Ighli di Bari, Francesco Maria Calabrese, Roberto Ciarcia, Maria De Angelis, and Loreto Gesualdo. 2021. "Ketoanalogs’ Effects on Intestinal Microbiota Modulation and Uremic Toxins Serum Levels in Chronic Kidney Disease (Medika2 Study)" Journal of Clinical Medicine 10, no. 4: 840. https://doi.org/10.3390/jcm10040840
APA StyleRocchetti, M. T., Di Iorio, B. R., Vacca, M., Cosola, C., Marzocco, S., di Bari, I., Calabrese, F. M., Ciarcia, R., De Angelis, M., & Gesualdo, L. (2021). Ketoanalogs’ Effects on Intestinal Microbiota Modulation and Uremic Toxins Serum Levels in Chronic Kidney Disease (Medika2 Study). Journal of Clinical Medicine, 10(4), 840. https://doi.org/10.3390/jcm10040840








