Management of Anticoagulant-Related Nephropathy: A Single Center Experience
Abstract
1. Introduction
2. Presentation of the Representative Case
3. Patients and Methods
4. Results
4.1. Clinical Features
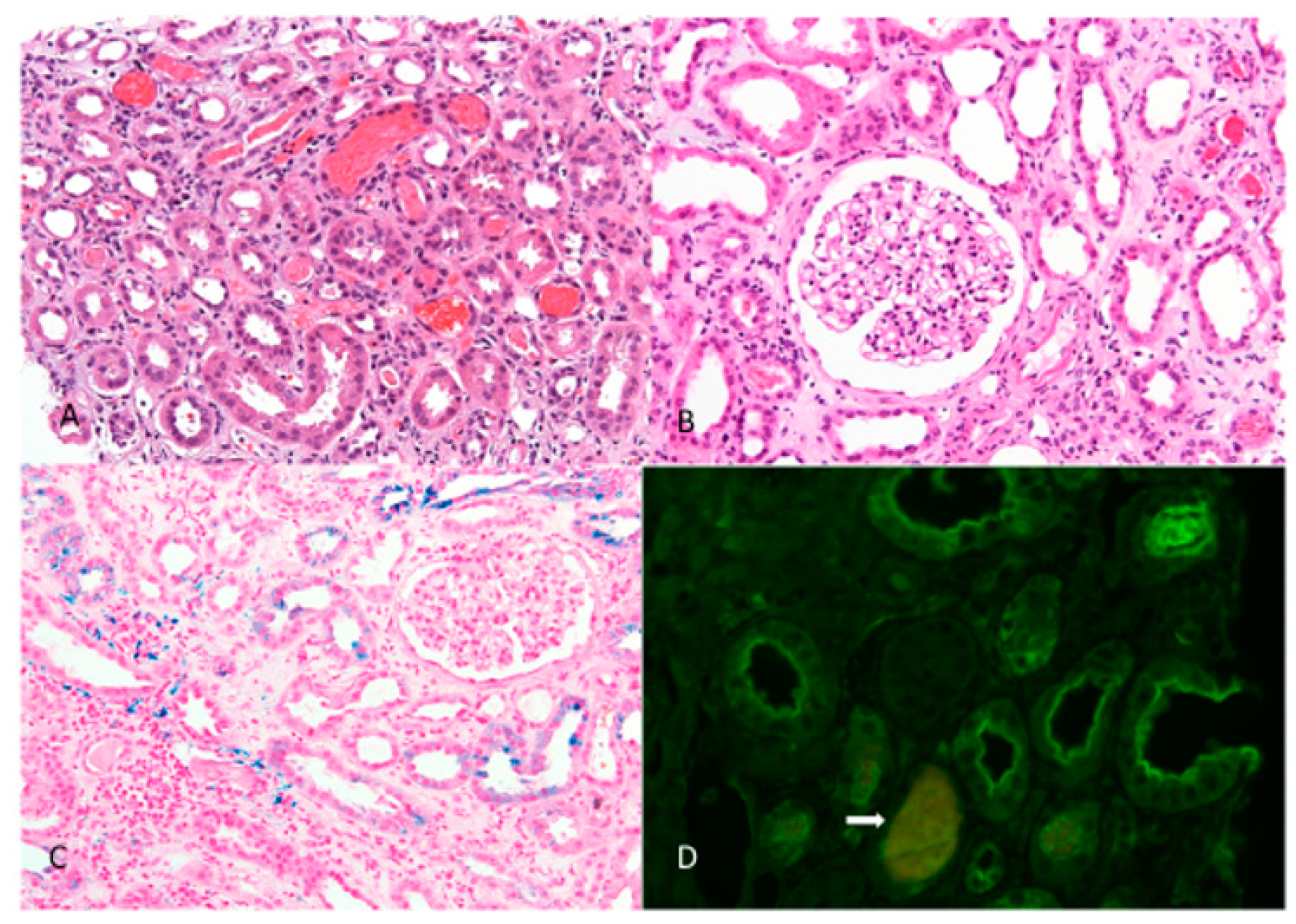
4.2. Histopathological Features
4.3. Treatment and Outcome
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brodsky, S.V.; Collins, M.; Park, E.; Rovin, B.H.; Satoskar, A.A.; Nadasdy, G.; Wu, H.; Bhatt, U.; Nadasdy, T.; Hebert, L.A. Warfarin therapy that results in an International Normalization Ratio above the therapeutic range is associated with accelerated progression of chronic kidney disease. Nephron Clin. Pract. 2010, 115, c142–c146. [Google Scholar] [CrossRef]
- Brodsky, S.V.; Nadasdy, T.; Rovin, B.H.; Satoskar, A.A.; Nadasdy, G.M.; Wu, H.M.; Bhatt, U.Y.; Hebert, L.A. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011, 80, 181–189. [Google Scholar] [CrossRef]
- Brodsky, S.V.; Satoskar, A.; Chen, J.; Nadasdy, G.; Eagen, J.W.; Hamirani, M.; Hebert, L.; Calomeni, E.; Nadasdy, T. Acute Kidney Injury During Warfarin Therapy Associated With Obstructive Tubular Red Blood Cell Casts: A Report of 9 Cases. Am. J. Kidney Dis. 2009, 54, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, S.V. Anticoagulants and acute kidney injury: Clinical and pathology considerations. Kidney Res. Clin. Pr. 2014, 33, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzidis, R.G.; Duni, A.; Liapis, G.; Balafa, O.; Xiromeriti, S.; Rapsomanikis, P.K.; Elisaf, M. Anticoagulant-related nephropathy: A case report and review of the literature of an increasingly recognized entity. Int. Urol. Nephrol. 2017, 49, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, S.V.; Mhaskar, N.S.; Thiruveedi, S.; Dhingra, R.; Reuben, S.C.; Calomeni, E.; Ivanov, I.; Satoskar, A.; Hemminger, J.; Nadasdy, G.; et al. Acute kidney injury aggravated by treatment initiation with apixaban: Another twist of anticoagulant-related nephropathy. Kidney Res. Clin. Pr. 2017, 36, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Fujino, Y.; Takahashi, C.; Mitsumoto, K.; Uzu, T. Rivaroxaban-related acute kidney injury in a patient with IgA vasculitis. BMJ Case Rep. 2019, 12, e227756. [Google Scholar] [CrossRef]
- Suzuki, K.; Honda, K.; Tanabe, K.; Toma, H.; Nihei, H.; Yamaguchi, Y. Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int. 2003, 63, 2286–2294. [Google Scholar] [CrossRef]
- L’Imperio, V.; Guarnieri, A.; Pieruzzi, F.; Sinico, R.A.; Pagni, F. Anticoagulant-related nephropathy: A pathological note. J. Thromb. Thrombolysis 2018, 46, 260–263. [Google Scholar] [CrossRef]
- Glassock, R.J. Anticoagulant-Related Nephropathy: It’s the Real McCoy. Clin. J. Am. Soc. Nephrol. 2019, 14, 935–937. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.S.; Giugliano, R.P.; Rangaswami, J. Anticoagulation-related nephropathy. J. Thromb. Haemost. 2016, 14, 461–467. [Google Scholar] [CrossRef]
- Woo, K.T.; Lina, H.L.C.; Lee Grace, S.L.; Chan, C.M.; Ng, C.Y.; Tan, C.S.; Marjorie, W.Y.F.; Thye, K.W. Use of Warfarin as a Component of Melbourne Cocktail in Chronic Kidney Disease and the Association with Acute Kidney Injury—A Historical Perspective. Ann. Med. Chem. Res. 2017, 3, 1018. [Google Scholar]
- Walker, R.G.; Yu, S.H.; Owen, J.E.; Kincaid-Smith, P. The treatment of mesangial IgA nephropathy with cyclophosphamide, dipyridamole and warfarin: A two-year prospective trial. Clin. Nephrol. 1990, 34, 103–107. [Google Scholar] [PubMed]
- Kratka, K.; Havrda, M.; Honsová, E.; Rychlík, I. Bioptically Proven “Anticoagulation-Related Nephropathy” Induced by Dual Antiplatelet Therapy. Case Rep. Nephrol. Dial. 2018, 8, 216–222. [Google Scholar] [CrossRef]
- Marcelino, G.; Hemett, O.M.; Descombes, E. Acute Renal Failure in a Patient with Rivaroxaban-Induced Hypersensitivity Syndrome: A Case Report with a Review of the Literature and of Pharmacovigilance Registries. Case Rep. Nephrol. 2020, 2020, 6940183. [Google Scholar] [CrossRef] [PubMed]
- An, J.N.; Ahn, S.Y.; Yoon, C.H.; Youn, T.-J.; Han, M.; Kim, S.; Chin, H.J.; Na, K.Y.; Chae, D.-W. The Occurrence of Warfarin-Related Nephropathy and Effects on Renal and Patient Outcomes in Korean Patients. PLoS ONE 2013, 8, e57661. [Google Scholar] [CrossRef] [PubMed]
- Moura, K.B.D.A.; Behrens, P.M.P.; Pirolli, R.; Sauer, A.; Melamed, D.; Veronese, F.; Da Silva, A.L.F.A. Anticoagulant-related nephropathy: Systematic review and meta-analysis. Clin. Kidney J. 2019, 12, 400–407. [Google Scholar] [CrossRef]
- Escoli, R.; Santos, P.; Andrade, S.; Carvalho, F. Dabigatran-Related Nephropathy in a Patient with Undiagnosed IgA Nephropathy. Case Rep. Nephrol. 2015, 2015, 298261. [Google Scholar] [CrossRef]
- Ware, K.; Brodsky, P.; Satoskar, A.A.; Nadasdy, T.; Nadasdy, G.; Wu, H.; Rovin, B.H.; Bhatt, U.; Von Visger, J.; Hebert, L.A.; et al. Warfarin-Related Nephropathy Modeled by Nephron Reduction and Excessive Anticoagulation. J. Am. Soc. Nephrol. 2011, 22, 1856–1862. [Google Scholar] [CrossRef]
- Patel, S.; Hossain, M.A.; Ajam, F.; Patel, M.; Nakrani, M.; Patel, J.; Alhillan, A.; Hammoda, M.; Alrefaee, A.; Levitt, M.; et al. Dabigatran-Induced Acute Interstitial Nephritis: An Important Complication of Newer Oral Anticoagulation Agents. J. Clin. Med. Res. 2018, 10, 791–794. [Google Scholar] [CrossRef]
- Abdulhadi, B.; Mulki, R.; Goyal, A.; Rangaswami, J. Novel oral anticoagulant and kidney injury: Apixaban-related acute interstitial nephritis. BMJ Case Rep. 2017, 2017. [Google Scholar] [CrossRef]
- Basetty, P.; Bellovich, K. A cautionary tale: Dabigatran toxic-ity associated with allergic interstitial nephritis. Am. J. Kidney Dis. 2013, 61, B24. [Google Scholar]
- Monahan, R.C.; Suttorp, M.M.; Gabreëls, B.A.T.F. A case of rivaroxaban-associated acute tubulointerstitial nephritis. Neth. J. Med. 2017, 75, 169–171. [Google Scholar]
- Kapoor, K.G.; Bekaii-Saab, T. Warfarin-induced allergic interstitial nephritis and leucocytoclastic vasculitis. Intern. Med. J. 2008, 38, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Moeckel, G.W.; Luciano, R.L.; Brewster, U.C. Warfarin-related nephropathy in a patient with mild IgA nephropathy on dabigatran and aspirin. Clin. Kidney J. 2013, 6, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Raju, D.S.B.; Golla, A.; Goli, R.; Nagalla, V.K.; Kiran, B.V.; Uppin, M.S. Warfarin-related nephropathy. Indian J. Nephrol. 2018, 28, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Kveder, R.; Lindic, J.; Ales, A.; Kovac, D.; Vizjak, A.; Ferluga, D. Acute Kidney Injury in Immunoglobulin A Nephropathy: Potential Role of Macroscopic Hematuria and Acute Tubulointerstitial Injury. Ther. Apher. Dial. 2009, 13, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.A.; Leaf, D.E.; Dieleman, J.M.; Van Dijk, D.; Nierich, A.P.; Rosseel, P.M.; Van Der Maaten, J.M.; Hofland, J.; Diephuis, J.C.; De Lange, F.; et al. Intraoperative High-Dose Dexamethasone and Severe AKI after Cardiac Surgery. J. Am. Soc. Nephrol. 2015, 26, 2947–2951. [Google Scholar] [CrossRef] [PubMed]
- Moonen, L.; Geryl, H.; D’Haese, P.C.; Vervaet, B.A. Short-term dexamethasone treatment transiently, but not permanently, attenuates fibrosis after acute-to-chronic kidney injury. BMC Nephrol. 2018, 19, 343. [Google Scholar] [CrossRef]
- Ozkok, A. Cholesterol-embolization syndrome: Current perspectives. Vasc. Health Risk Manag. 2019, 15, 209–220. [Google Scholar] [CrossRef]

| Laboratory Analysis | |
|---|---|
| Sodium | 119 mmol/L |
| Potassium | 5.2 mmol/L |
| Urea | 15.2 mmol/L |
| Creatinine | 373 µmol/L |
| eGFR (CKD-EPI) | 10 mL/min/1.73m2 |
| pH | 7.33 |
| Sodium bicarbonate | 18.3 mmol/L |
| Hemoglobin | 78 g/L |
| Mean corpuscular volume | 88.5 |
| aPTT | 141 s |
| TT | >150 s |
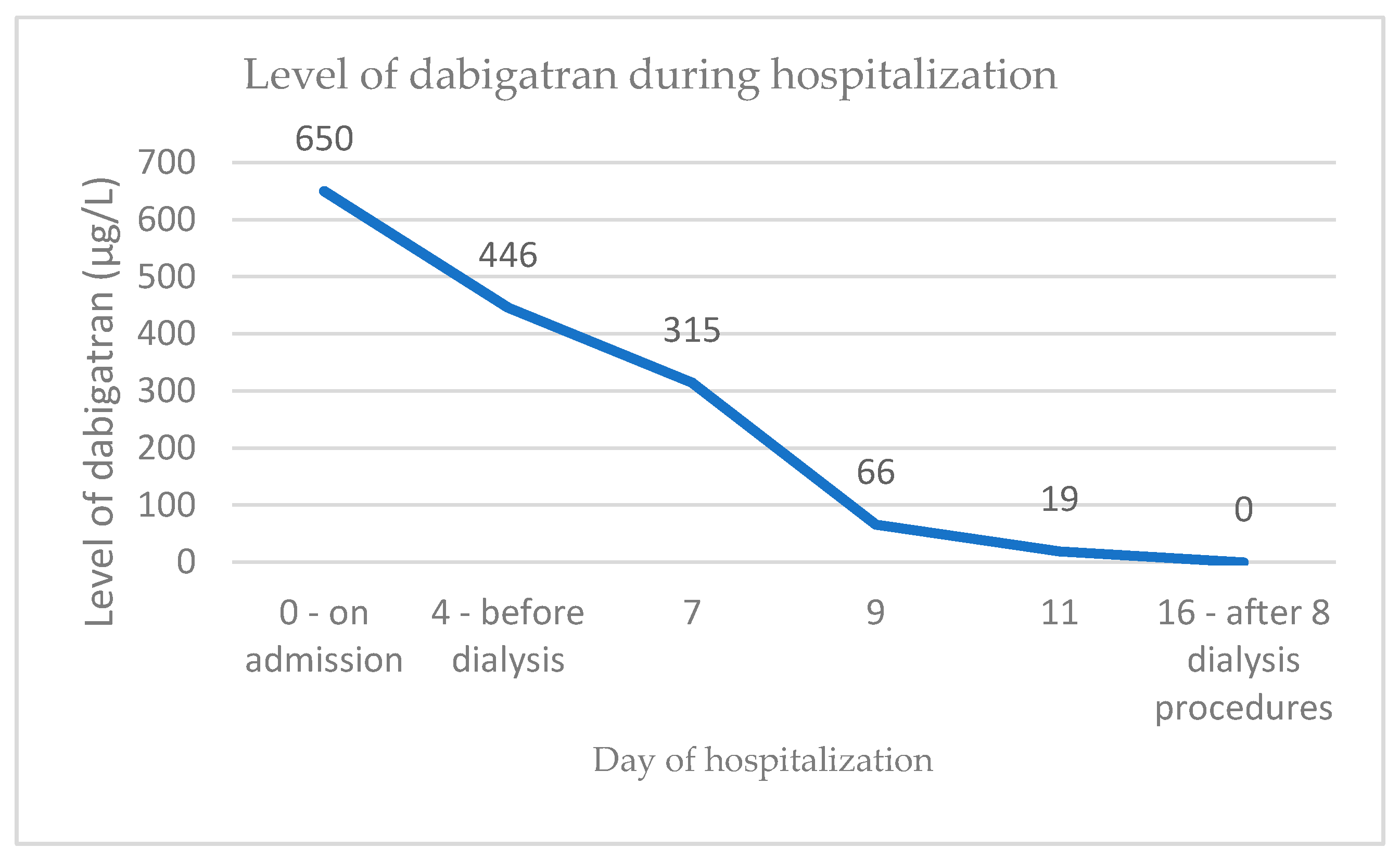
| TT-dabigatran | 650 µg/L |
| Urine analysis | |
| Urine sediment | |
| Leukocytes | >100 per HPF |
| Bacteria | 0 |
| Red blood cells | >100 per HPF |
| Casts | Rare per HPF |
| Immunologic findings | |
| ANCA, anti-GBM Ab, ANA | All negative |
| Doppler ultrasound | |
| Imaging result | Normal-sized kidney with preserved parenchymal thickness, increased echogenicity and increased RI, no signs of obstruction |
| Pt | Age | Sex | AC | SCr Baseline (µmol/L) | SCr at Biopsy (µmol/L)/eGF (mL/min) | Increase in Serum SCr from Basal Level (%) | SCr at Discharge (µmol/L)/eGF (mL/min) | SCr (µmol/L)/eGF (mL/min) at Time of Last Follow up/Follow up Time | Time to ARN from Introduction of AC | Symptoms at Biopsy | INR/aPTT (s)/Dabigatran level (µg/L)/Anti-Xa-Rivaroxaban (µg/L) | Relevant Comorbidities | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 77 | f | warfarin | not known | 448 (10) | >25 | 285 (13) | 127 (35)/17 months | 20 months | gross hematuria | supra-therapeutic INR was never recorded | mitral and aortic valve stenosis, post CVI, meningeoma, recent breast cancer | discontinuation of warfarin, methyl-prednisolone 3 × 125 mg iv, followed by 0.5 mg/kg bw for 1 month, then taper down until discontinuation in six weeks | improvement of kidney function, no rea-appearance of gross hematuria, later aceno-coumarol was introduced with no ARN |
| 2 | 74 | m | warfarin | 150 | 510 (9) | 240 | 337 (16) | 137 (43)/21 months | 2 years | gross hematuria | INR 3.8 | atrial fibrillation, aortic stenosis, congestive heart failure, Parkinson’s disease | discontinuation of warfarin; methyl-prednisolone 0.5 mg/kg bw for 1 month, then taper down until discontinuation in 6 months | improvement of kidney function, no hematuria |
| 3 | 66 | f | warfarin | 110 | first episode 129 (37), second episode 160 (28) | first episode 17, second episode 45.4 | 120 (39) | 126 (37)/43 months | 3 months | gross hematuria | INR 1.63 | atrial fibrillation, Sjogren’s syndrome, fibromyalgia | discontinuation of warfarin; on re-introducement gross hematuria re-appeared with an INR 3.6, after that event warfarin was stopped | improvement of kidney function, persistent micro-hematuria |
| 4 | 51 | m | warfarin | 170–200 | 249 (24) | 46.5 | 253 (23) | 174 (36)/15 months | 15 years | gross hematuria | INR 5.0 | mitral valve replacement, atrial fibrillation, CVI, diabetes mellitus type 2, cystostoma due to urethral stenosis | temporary discontinuation of warfarin, LMWH introduced; steroids 0.7 mg/kg bw 1 month, then taper down until discontinuation in 3 months; strict control of anticoagulant therapy | improvement of kidney function, hematuria persisted; recurrence of gross hematuria after reintroduction of warfarin (at INR 5.0), improvement after correction of INR |
| 5 | 76 | m | warfarin | 430 | 487 (9) | 13.30 | on dialysis | on dialysis/12 months | 10 years | gross hematuria | INR 4.4 | mechanical aortic valve, severe atherosclerosis, arterial hypertension, diabetes mellitus type 2, HBV infection | dialysis, better control of INR | no improvement of kidney function |
| 6 | 69 | m | dabigatran, 2 × 150 mg | 88 | 194 (30) | 120.5 | 181 (32) | 164 (36)/6 months | 2 months | microscopic hematuria | dabigatran level 293 µg/L | atrial fibrillation, arterial hypertension, diabetes mellitus type 2, ethylic liver cirrhosis | discontinuation of dabigatran, methyl-prednisolone, 0.6 mg/kg bw 14 days, then taper down until discontinuation in 3 months | improvement of kidney function, persistent microhematuria |
| 7 | 67 | m | rivaroxaban (previously on apixaban and dabigatran which caused GI bleeding) | 75 | 442 (12) | 489.3 | 386 (13) | 82 (86)/12 months | 2.5 months | gross hematuria | INR 1.24, aPTT 45.1 s | atrial fibrillation, IgA vasculitis with skin involvement | discontinuation of rivaroxaban, methyl-prednisolone, 3 × 250 mg iv, followed by 0.8 mg/kg bw 1 month, then taper down until discontinuation in 4 months | improvement of kidney function |
| 8 | 82 | f | rivaroxaban (previously on apixaban for 5 days which was stopped due to skin rash) | 119 | 315 (12) | 164.7 | 267 (15) | 179 (22)/15 months | 1 week | gross hematuria | INR 1.65, aPTT 41.4 s, antiXa- rivaroxaban 28 µg/L -performed 21 days after rivaroxaban discontinuation | atrial fibrillation, arterial hypertension, ischemic heart disease | discontinuation of rivaroxaban, methyl-prednisolone, three pulses 500 mg, followed by 0.7 mg/kg bw 1 month, then taper down until discontinuation in 3 months | improvement of kidney function, no hematuria |
| 9 | 82 | f | dabigatran, 2 × 110 mg | 124 | 373 (10) | 200.8 | 401 (9) | transient improvement of SCr 201 µmol/L, however numerous further complications (please see case report for details) | 18 days | gross hematuria | 650 µg/L | atrial fibrillation, diabetes mellitus type 2, arterial hypertension | discontinuation of dabigatran, methylprednisolone.4 mg/kg bw 1 month, then taper down until discontinuation in 3 months | improvement of kidney function, later re-bleeding on warfarin, death due to sepsis |
| 10 | 56 | f | acenocoumarol (skin rash on warfarin) | 72 (82) | 81 (70) | 12.5 | 95 (53) | 80 (71) | 11 months | microscopic hematuria | 2.42 | mechanical aortic valve, atrial fibrillation, systemic lupus, Sjogren’s syndrome, IgA vasculitis with isolated skin involvement, kidney stone | better control of INR in the lower level of therapeutic range | persistence of microscopic hematuria, stable kidney function |
| 11 | 66 | m | gross hematuria appeared after 8 days of LMWH therapeuric dose -previously aceno-coumarol | 149 (42) | 669 (7) | 349 | 350 (15) | unknown, the patient refused further treatment | 26 years aceno-coumarol, LMWH 8 days | gross hematuria, epistaxis | INR 1.36, anti-Xa LMWH 0.69 | mechanical aortic valve, IgA vasculitis with predominant skin involvement, SCr 108 µmol/L and micro-hematuria treated with steroid prior to current hospitalization, infection of aortic stent graft, post CVI | patient transiently converted to heparin; due to aortic stent graft infection steroid treatment was contraindicated | gross hematuria resolved after LMWH was discontinued and patient was converted to heparin, kidney function slightly improved, acenocoumarol was reintroduced with no additionalbleeding episodes |
| 12 | 60 | m | warfarin | 74 | 372 (14) | 402.7 | 274 (21) | 206 (29)/1 month | 3 months | gross hematuria | INR 3.46 | arterial hypertension, portal vein thrombosis | methyl-prednisolone i.v. pulses 3 × 500 mg, then 0.8 mg/kg bw for 1 month (still on therapy), anticoagulant stopped | improvement of kidney function |
| 13 | 81 | m | warfarin 13 years converted to dabigatran 3 weeks prior to biopsy | 117 (53) | 141 (40) | 20.5 | 114 (52) | 117 (50)/12 months | 3 weeks post introduction of dabigatran | gross hematuria | INR 1.31, aPTT 78.1 s, dabigatran level 65 µg/L | chronic heart failure, chronic Budd Chiarri, post cerebrovascular insult | discontinuation of dabigatran | improvement to baseline function in three weeks |
| Patient No. | Kidney Biopsy | Oxford Classification | EM–GBM | Podocyte Effacement | RBC Casts | Global Glomerulo-Sclerosis | ATI | ATI Degree | Interstitial Edema | Interstitial Infiltrate | Perls | IFTA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ARN, IgAN | M0, E0, S1, T0, C0 | 185–370 nm, average 280 nm | no | 5.70% | 7/20 | 100% | moderate | 100% | none | tubuli ++ int. + | 15% |
| 2 | ARN, IgAN | M0, E0, S1, T0, C0 | 230–505 nm, average 350 nm | no | 9.30% | 5/23 | 90% | moderate/severe | 90% | 5% | tubuli + int. + | 15% |
| 3 | ARN, IgAN | M0, E0, S1, T0, C0 | 230–385 nm, average 325 nm | no | 6.40% | 0/6 | 50% | mild | 10% | none | tubuli ± int. − | 10% |
| 4 | ARN, IgAN | M0, E0, S1, T2, C0 | 220–400 nm, average 300 nm | 30% | 7.80% | 4/29 | 70% | mild | 70% | up to 10% | tubuli ++ int. ± | 20% |
| 5 | ARN, 50% global glomerular sclerosis, unclassified | / | 230–515 nm, average 370 nm | no | 3.20% | 7/14 | 70% | mild/moderate | 40% | none | tubuli ± int. − | 20% |
| 6 | ARN, thin GBM, latent IgA deposits | M0, E0, S0, T0, C0 | 155–265 nm, average 190 nm | 20% | 7% | 2/12 | 50% | mild | 25% | up to 5% | tubuli +/++ int. − | 15% |
| 7 | ARN, IgAN | M0, E0, S0, T0, C0 | 165–425 nm, average 285 nm | 15% | 10% | 1/18 | 90% | moderate | 90% | up to 10% | tubuli + int. + | up to 10% |
| 8 | ARN, IgAN | M0, E0, S0, T1, C1 | 210–440 nm, average 325 nm | not aplicable due to glomerular colapse | 4.80% | 3/17 | 50% | mild | 20% | up to 10% | tubuli ± int. + | 10% |
| 9 -case report | ARN, IgAN | M0, E0, S0, T0, C0 | 165–475 nm, average 320 nm | not applicable due to glomerular collapse | 8.10% | 1/11 | 100% | severe | 100% | 5% | tubuli +++ int. ++ | 10% |
| 10 | ARN, IgAN | M0, E0, S0, T0, C0 | 120–305 nm, average 200 nm | 20% | 2% | 1/10 | 25% | mild | 25% | none | tubuli ± int. − | 10% |
| 11 | ARN, IgAN, benign nephrosclerosis | M0, E0, S0, T0, C0 | 210–415 nm, average 290 nm | 10% | 20.30% | 1/8 | 100% | mild/moderate | 40% | none | tubuli ± int. − | up to 5% |
| 12 | ARN, IgAN | M0, E0, S0, T0, C0 | 165–335 nm, average 260 nm | 15% | 4.90% | 3/17 | 80% | moderate | 45% | up to 10% | tubuli + int. ± | 5–10% |
| 13 | ARN, IgAN | M0, E0, S0, T0, C0 | 225–400 nm, average 305 nm | not applicable due to glomerular collapse | 3,3% | 2/22 | 20% | mild | 5% | up to 10% | not performed | 20% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belčič Mikič, T.; Kojc, N.; Frelih, M.; Aleš-Rigler, A.; Večerić-Haler, Ž. Management of Anticoagulant-Related Nephropathy: A Single Center Experience. J. Clin. Med. 2021, 10, 796. https://doi.org/10.3390/jcm10040796
Belčič Mikič T, Kojc N, Frelih M, Aleš-Rigler A, Večerić-Haler Ž. Management of Anticoagulant-Related Nephropathy: A Single Center Experience. Journal of Clinical Medicine. 2021; 10(4):796. https://doi.org/10.3390/jcm10040796
Chicago/Turabian StyleBelčič Mikič, Tanja, Nika Kojc, Maja Frelih, Andreja Aleš-Rigler, and Željka Večerić-Haler. 2021. "Management of Anticoagulant-Related Nephropathy: A Single Center Experience" Journal of Clinical Medicine 10, no. 4: 796. https://doi.org/10.3390/jcm10040796
APA StyleBelčič Mikič, T., Kojc, N., Frelih, M., Aleš-Rigler, A., & Večerić-Haler, Ž. (2021). Management of Anticoagulant-Related Nephropathy: A Single Center Experience. Journal of Clinical Medicine, 10(4), 796. https://doi.org/10.3390/jcm10040796






