Patients with Proliferative Lupus Nephritis Have Autoantibodies That React to Moesin and Demonstrate Increased Glomerular Moesin Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Glomerular and Podocyte Protein Extracts
2.3. Western Blot Analysis
2.4. Mass Spectrometry Analysis
2.5. Enzyme-Linked Immunosorbent Assay
2.6. Immunohistochemistry
2.7. Statistical Analyses
3. Results
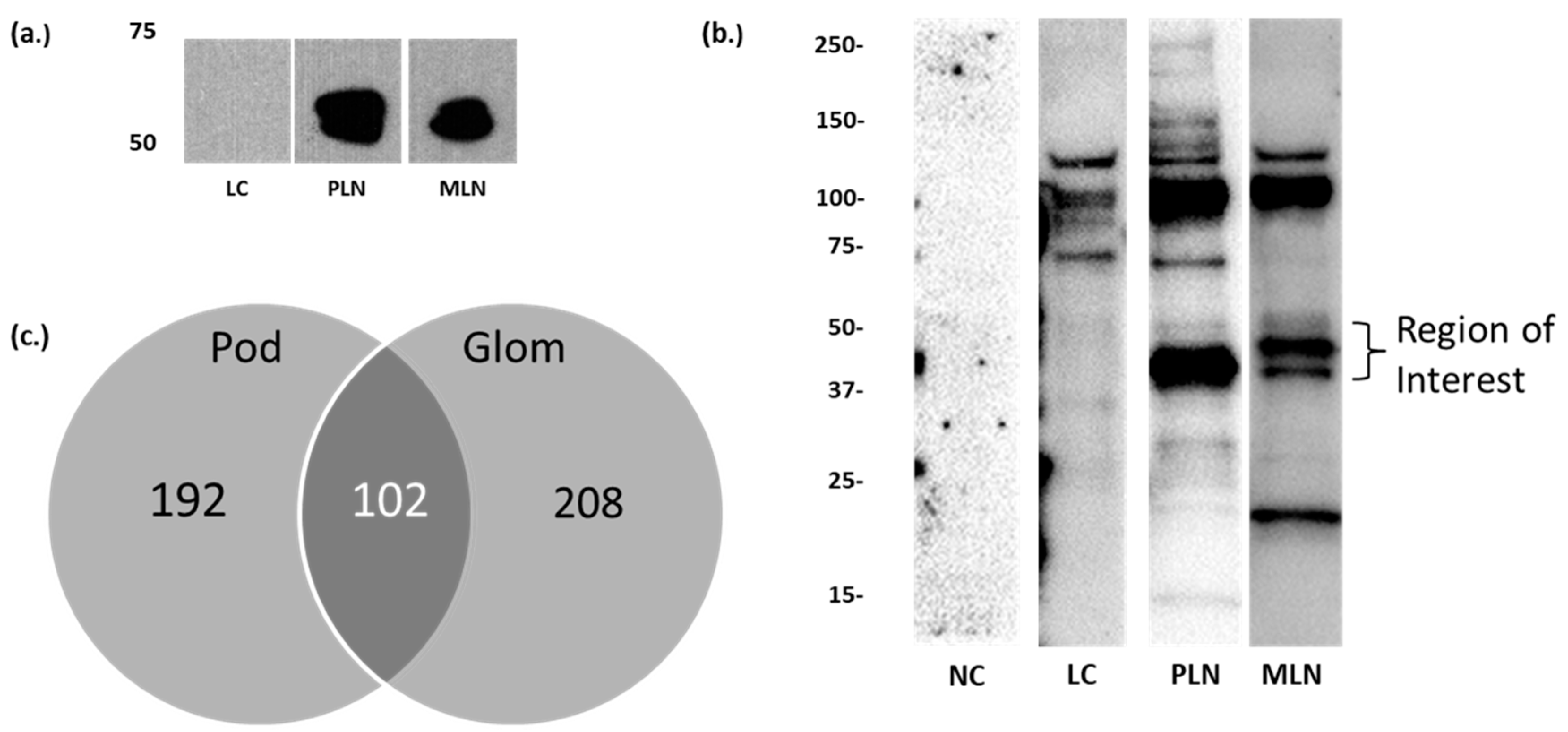
3.1. Identification of Candidate LN Target Antigens
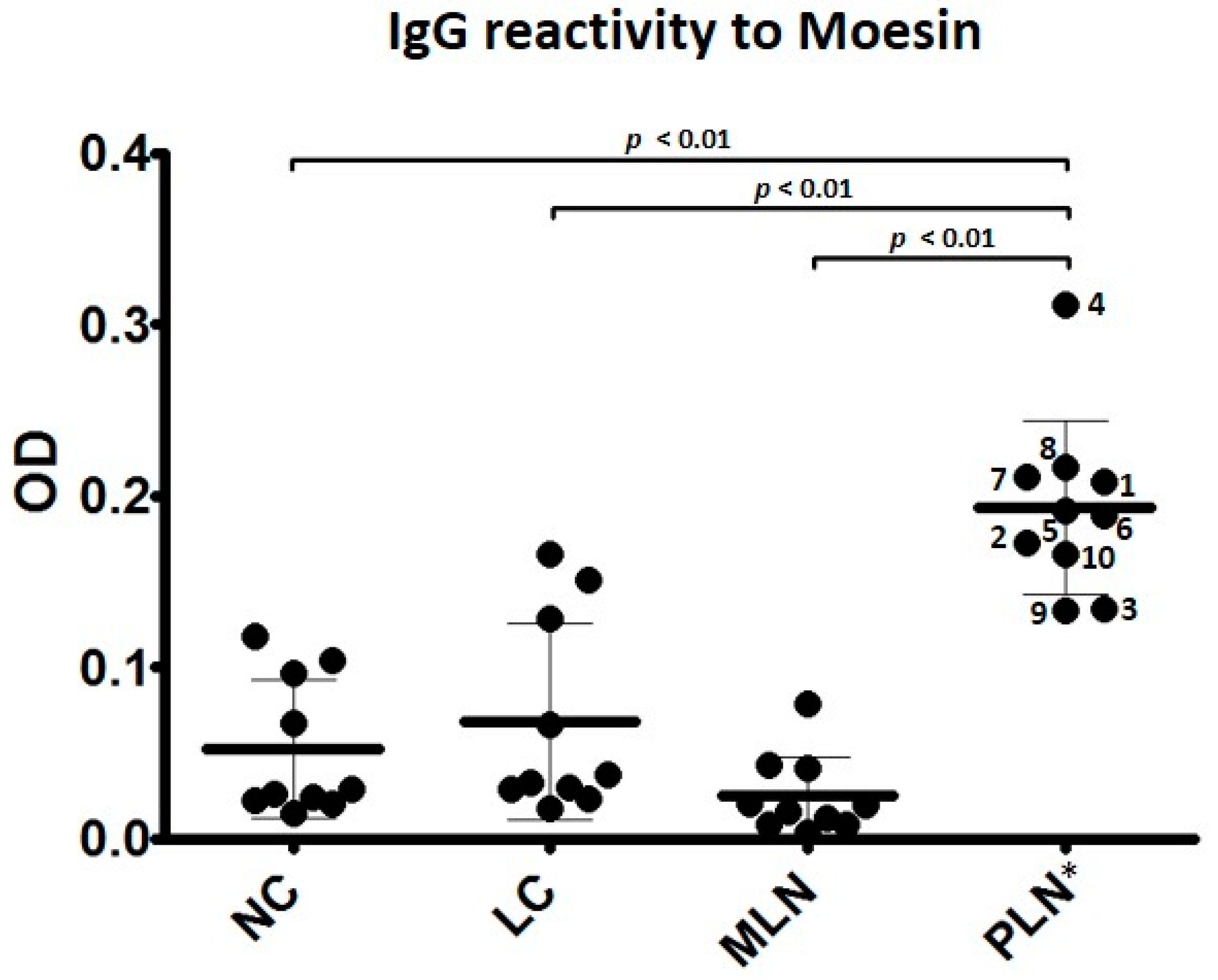
3.2. Autoantibodies to Moesin Are Elevated in PLN, Not MLN
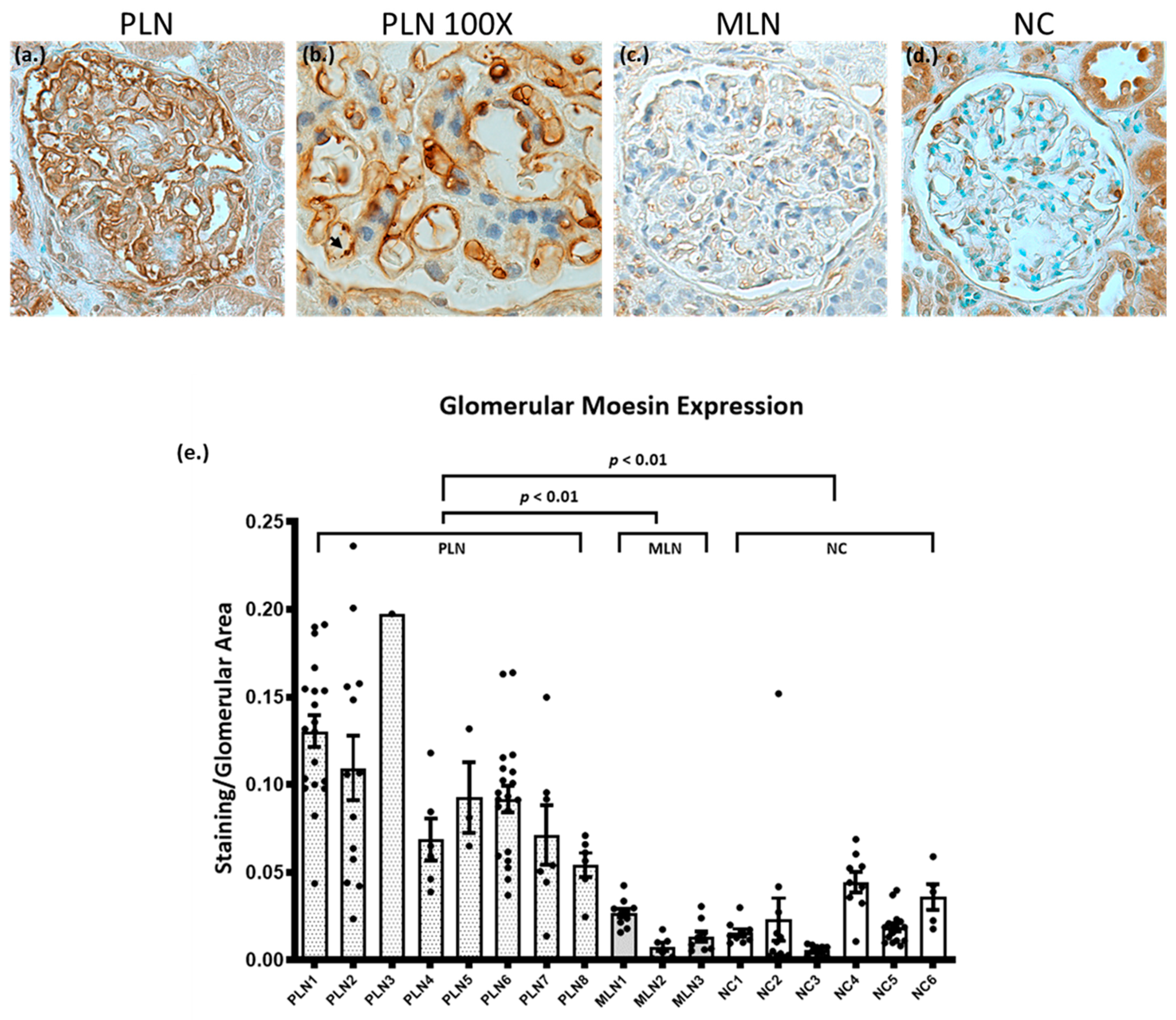
3.3. Biopsies from Patients with PLN Demonstrate Increased Glomerular Expression of Moesin
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsokos, G.C. Systemic Lupus Erythematosus. N. Engl. J. Med. 2011, 365, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- Cozzani, E.; Drosera, M.; Gasparini, G.; Parodi, A. Serology of Lupus Erythematosus: Correlation between Immunopathological Features and Clinical Aspects. Autoimmune Dis. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.V.; Almaani, S.; Brodsky, S.; Rovin, B.H. Update on Lupus Nephritis: Core Curriculum 2020. Am. J. Kidney Dis. 2020, 76, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Weening, J.J.; D’Agati, V.D.; Schwartz, M.M.; Seshan, S.V.; Alpers, C.E.; Appel, G.B.; Balow, J.E.; Bruijn, J.A.; Cook, T.; Ferrario, F.; et al. The Classification of Glomerulonephritis in Systemic Lupus Erythematosus Revisited. J. Am. Soc. Nephrol. 2004, 15, 241–250. [Google Scholar] [CrossRef]
- Bhinder, S.; Singh, A.; Majithia, V. Membranous (Class V) Renal Disease in Systemic Lupus Erythematosus May Be More Common Than Previously Reported: Results of a 6-Year Retrospective Analysis. Am. J. Med. Sci. 2010, 339, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Hanrotel-Saliou, C.; Segalen, I.; Le Meur, Y.; Youinou, P.; Renaudineau, Y. Glomerular Antibodies in Lupus Nephritis. Clin. Rev. Allergy Immunol. 2010, 40, 151–158. [Google Scholar] [CrossRef]
- Mannik, M.; Merrill, C.E.; Stamps, L.D.; Wener, M.H. Multiple autoantibodies form the glomerular immune deposits in patients with systemic lupus erythematosus. J. Rheumatol. 2003, 30, 1495–1504. [Google Scholar] [PubMed]
- Seret, G.; Le Meur, Y.; Renaudineau, Y.; Youinou, P. Mesangial Cell-Specific Antibodies Are Central to the Pathogenesis of Lupus Nephritis. Clin. Dev. Immunol. 2011, 2012, 1–8. [Google Scholar] [CrossRef]
- Olson, S.W.; Lee, J.J.; Prince, L.K.; Baker, T.P.; Papadopoulos, P.; Edison, J.; Abbott, K.C. Elevated Subclinical Double-Stranded DNA Antibodies and Future Proliferative Lupus Nephritis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1702–1708. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kalaaji, M.; Mortensen, E.; Jørgensen, L.; Olsen, R.; Rekvig, O.P. Nephritogenic Lupus Antibodies Recognize Glomerular Basement Membrane-Associated Chromatin Fragments Released from Apoptotic Intraglomerular Cells. Am. J. Pathol. 2006, 168, 1779–1792. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, D.J.; Hebert, L.A.; Song, H.; Noonan, W.T.; Rovin, B.H.; Nagaraja, H.N.; Yu, C.Y. Evidence that abnormally large seasonal declines in vitamin D status may trigger SLE flare in non-African Americans. Lupus 2012, 21, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.; Sevier, S.; Kelly, J.A.; Glenn, S.B.; Aberle, T.; Cooney, C.M.; Grether, A.; James, E.; Ning, J.; Tesiram, J.; et al. The Lupus Family Registry and Repository. Rheumatology 2010, 50, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Caster, D.J.; Korte, E.A.; Merchant, M.L.; Klein, J.B.; Wilkey, D.W.; Rovin, B.H.; Birmingham, D.J.; Harley, J.B.; Cobb, B.L.; Namjou, B.; et al. Autoantibodies targeting glomerular annexin A2 identify patients with proliferative lupus nephritis. Proteom. Clin. Appl. 2015, 9, 1012–1020. [Google Scholar] [CrossRef]
- Yamamoto, T. Isolation and Enrichment of Glomeruli Using Sieving Techniques. In Renal and Urinary Proteomics; Wiley-VCH Verlag: Weinheim, Germany, 2010; pp. 1–7. [Google Scholar]
- Saleem, M.A.; O’Hare, M.J.; Reiser, J.; Coward, R.J.; Inward, C.D.; Farren, T.; Xing, C.Y.; Ni, L.; Mathieson, P.W.; Mundel, P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 2002, 13, 630–638. [Google Scholar]
- Beck, L.H.; Bonegio, R.G.; Lambeau, G.; Beck, D.M.; Powell, D.W.; Cummins, T.D.; Klein, J.B.; Salant, D.J. M-Type Phospholipase A2Receptor as Target Antigen in Idiopathic Membranous Nephropathy. New Engl. J. Med. 2009, 361, 11–21. [Google Scholar] [CrossRef]
- Powell, D.W.; Rane, M.J.; Joughin, B.A.; Kalmukova, R.; Hong, J.-H.; Tidor, B.; Dean, W.L.; Pierce, W.M.; Klein, J.B.; Yaffe, M.B.; et al. Proteomic identification of 14-3-3zeta as a mitogen-activated protein kinase-activated protein kinase 2 substrate: Role in dimer formation and ligand binding. Mol. Cell. Biol. 2003, 23, 5376–5387. [Google Scholar] [CrossRef]
- Cummins, T.D.; Barati, M.T.; Coventry, S.C.; Salyer, S.A.; Klein, J.B.; Powell, D.W. Quantitative mass spectrometry of diabetic kidney tubules identifies GRAP as a novel regulator of TGF-β signaling. Biochim. et Biophys. Acta (BBA) Proteins Proteom. 2010, 1804, 653–661. [Google Scholar] [CrossRef]
- Baba, S.P.; Hoetker, J.D.; Merchant, M.; Klein, J.B.; Cai, J.; Barski, O.A.; Conklin, D.J.; Bhatnagar, A. Role of Aldose Reductase in the Metabolism and Detoxification of Carnosine-Acrolein Conjugates. J. Biol. Chem. 2013, 288, 28163–28179. [Google Scholar] [CrossRef]
- Seiler, C.Y.; Park, J.G.; Sharma, A.; Hunter, P.; Surapaneni, P.; Sedillo, C.; Field, J.; Algar, R.; Price, A.; Steel, J.; et al. DNASU plasmid and PSI:Biology-Materials repositories: Resources to accelerate biological research. Nucleic Acids Res. 2014, 42, D1253–D1260. [Google Scholar] [CrossRef] [PubMed]
- Barati, M.T.; Powell, D.W.; Kechavarzi, B.D.; Isaacs, S.M.; Zheng, S.; Epstein, P.N.; Cai, L.; Coventry, S.; Rane, M.J.; Klein, J.B. Differential expression of endoplasmic reticulum stress-response proteins in different renal tubule subtypes of OVE26 diabetic mice. Cell Stress Chaperon 2015, 21, 155–166. [Google Scholar] [CrossRef]
- Tomas, N.M.; Beck, L.H.; Meyer-Schwesinger, C.; Seitz-Polski, B.; Ma, H.; Zahner, G.; Dolla, G.; Hoxha, E.; Helmchen, U.; Dabert-Gay, A.-S.; et al. Thrombospondin Type-1 Domain-Containing 7A in Idiopathic Membranous Nephropathy. N. Engl. J. Med. 2014, 371, 2277–2287. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Akiyama, K.; Morita, H.; Suetsugu, S.; Kuraba, S.; Numata, Y.; Yamamoto, Y.; Inui, K.; Ideura, T.; Wakisaka, N.; Nakano, K.; et al. Actin -related protein 3 (Arp3) is mutated in proteinuric BUF/Mna rats. Mamm. Genome 2008, 19, 41–50. [Google Scholar] [CrossRef]
- Bruschi, M.; Sinico, R.A.; Moroni, G.; Pratesi, F.; Migliorini, P.; Galetti, M.; Murtas, C.; Tincani, A.; Madaio, M.; Radice, A.; et al. Glomerular Autoimmune Multicomponents of Human Lupus Nephritis In Vivo: α-Enolase and Annexin AI. J. Am. Soc. Nephrol. 2014, 25, 2483–2498. [Google Scholar] [CrossRef]
- Bruschi, M.; Carnevali, M.L.; Murtas, C.; Candiano, G.; Petretto, A.; Prunotto, M.; Gatti, R.; Argentiero, L.; Magistroni, R.; Garibotto, G.; et al. Direct characterization of target podocyte antigens and auto-antibodies in human membranous glomerulonephritis: Alfa-enolase and borderline antigens. J. Proteom. 2011, 74, 2008–2017. [Google Scholar] [CrossRef] [PubMed]
- Zha, D.; Chen, C.; Liang, W.; Chen, X.; Ma, T.; Yang, H.; Van Goor, H.; Ding, G. Nephrin phosphorylation regulates podocyte adhesion through the PINCH-1-ILK-α-parvin complex. BMB Rep. 2013, 46, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Yung, S.; Cheung, K.F.; Zhang, Q.; Chan, T.M. Anti-dsDNA Antibodies Bind to Mesangial Annexin II in Lupus Nephritis. J. Am. Soc. Nephrol. 2010, 21, 1912–1927. [Google Scholar] [CrossRef]
- Wasik, A.A.; Koskelainen, S.; Hyvönen, M.E.; Musante, L.; Lehtonen, S.; Koskenniemi, K.; Tienari, J.; Vaheri, A.; Kerjaschki, N.; Szalay, C.; et al. Ezrin Is Down-Regulated in Diabetic Kidney Glomeruli and Regulates Actin Reorganization and Glucose Uptake via GLUT1 in Cultured Podocytes. Am. J. Pathol. 2014, 184, 1727–1739. [Google Scholar] [CrossRef]
- Ostalska-Nowicka, D.; Zachwieja, J.; Nowicki, M.; Kaczmarek, E.; Siwinska, A.; Witt, M. Ezrin--a useful factor in the prognosis of nephrotic syndrome in children: An immunohistochemical approach. J. Clin. Pathol. 2006, 59, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, T.; Maeda, K.; Sato, W.; Maruyama, S.; Kadomatsu, K. CD147 (EMMPRIN/Basigin) in kidney diseases: From an inflammation and immune system viewpoint. Nephrol. Dial. Transplant. 2014, 30, 1097–1103. [Google Scholar] [CrossRef]
- Hugo, C.; Pichler, R.; Gordon, K.; Schmidt, R.; Amieva, M.; Couser, W.G.; Furthmayr, H.; Johnson, R.J. The cytoskeletal linking proteins, moesin and radixin, are upregulated by platelet-derived growth factor, but not basic fibroblast growth factor in experimental mesangial proliferative glomerulonephritis. J. Clin. Investig. 1996, 97, 2499–2508. [Google Scholar] [CrossRef]
- Suzuki, K.; Nagao, T.; Itabashi, M.; Hamano, Y.; Sugamata, R.; Yamazaki, Y.; Yumura, W.; Tsukita, S.; Wang, P.C.; Nakayama, T. A novel autoantibody against moesin in the serum of patients with MPO-ANCA-associated vasculitis. Nephrol. Dial. Transplant. 2013, 29, 1168–1177. [Google Scholar] [CrossRef][Green Version]
- Hsu, H.-H.; Hoffmann, S.; Endlich, N.; Velic, A.; Schwab, A.; Weide, T.; Schlatter, E.; Pavenstädt, H. Mechanisms of angiotensin II signaling on cytoskeleton of podocytes. J. Mol. Med. 2008, 86, 1379–1394. [Google Scholar] [CrossRef]
- Bonanni, A.; Vaglio, A.; Bruschi, M.; Sinico, R.A.; Cavagna, L.; Moroni, G.; Franceschini, F.; Allegri, L.; Pratesi, F.; Migliorini, P.; et al. Multi-antibody composition in lupus nephritis: Isotype and antigen specificity make the difference. Autoimmun. Rev. 2015, 14, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.P.; Networks, O.B.O.T.B.A.G.; Adrianto, I.; Lessard, C.J.; Kelly, J.A.; Kaufman, K.M.; Guthridge, J.M.; Freedman, B.I.; Anaya, J.-M.; Alarcón-Riquelme, M.E.; et al. Role of MYH9 and APOL1 in African and non-African populations with lupus nephritis. Genes Immun. 2011, 13, 232–238. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, X.; Shi, S.; Su, H.; Bai, X.; Cai, G.; Yang, F.; Xie, Z.; Zhu, Y.; Zhang, Y.; et al. Bioinformatics Analysis of Proteomic Profiles During the Process of Anti-Thy1 Nephritis. Mol. Cell. Proteom. 2012, 11. [Google Scholar] [CrossRef]
- Lang, A.; Benke, D.; Eitner, F.; Engel, D.; Ehrlich, S.; Breloer, M.; Hamilton-Williams, E.; Specht, S.; Hoerauf, A.; Floege, J.; et al. Heat Shock Protein 60 Is Released in Immune-Mediated Glomerulonephritis and Aggravates Disease: In Vivo Evidence for an Immunologic Danger Signal. J. Am. Soc. Nephrol. 2004, 16, 383–391. [Google Scholar] [CrossRef]
- Pierchala, B.A.; Muñoz, M.R.; Tsui, C.C. Proteomic analysis of the slit diaphragm complex: CLIC5 is a protein critical for podocyte morphology and function. Kidney Int. 2010, 78, 868–882. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Suzuki, K.; Nagao, T.; Nakayama, T. Proposal of anti-moesin as a novel biomarker for ANCA-associated vasculitis. Clin. Exp. Nephrol. 2013, 17, 638–641. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Zhou, T.; Kim, H.; Barnes, S.; Yang, P.; Wu, Q.; Zhou, J.; Freeman, B.A.; Luo, M.; Mountz, J.D. Production of a novel class of polyreactive pathogenic autoantibodies in BXD2 mice causes glomerulonephritis and arthritis. Arthritis Rheum. 2005, 54, 343–355. [Google Scholar] [CrossRef]
- Beck, L.H.; Salant, D.J. Membranous nephropathy: From models to man. J. Clin. Investig. 2014, 124, 2307–2314. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Debiec, H.; Madden, B.; Charlesworth, M.C.; Morelle, J.; Gross, L.; Ravindran, A.; Buob, D.; Jadoul, M.; Fervenza, F.C.; et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 2020, 97, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Seret, G.; Cañas, F.; Costanzo, L.P.-D.; Hanrotel-Saliou, C.; Jousse-Joulin, S.; Le Meur, Y.; Saraux, A.; Valeri, A.; Putterman, C.; Youinou, P.; et al. Anti-alpha-actinin antibodies are part of the anti-cell membrane antibody spectrum that characterize patients with lupus nephritis. J. Autoimmun. 2015, 61, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-H.; Pan, H.-F.; Zhao, X.-F.; Ye, N.-Q.; Li, X.-P.; Xu, J.-H. Anti-α-actinin antibodies in relation to new-onset systemic lupus erythematosus and lupus nephritis. Mol. Biol. Rep. 2009, 37, 1341–1345. [Google Scholar] [CrossRef]
- Becker-Merok, A.; Kalaaji, M.; Haugbro, K.; Nikolaisen, C.; Nilsen, K.; Rekvig, O.P.; Nossent, J.C. Alpha-actinin-binding antibodies in relation to systemic lupus erythematosus and lupus nephritis. Arthritis Res. Ther. 2006, 8, R162. [Google Scholar] [CrossRef]
- Cheung, K.F.; Yung, S.; Chau, M.K.; Yap, D.Y.; Chan, K.W.; Lee, C.K.; Tang, C.S.; Chan, T.M. Annexin II-binding immunoglobulins in patients with lupus nephritis and their correlation with disease manifestations. Clin. Sci. 2017, 131, 653–671. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Hong, Y.-H.; Kim, Y.-J.; Kim, H.-S.; Park, J.-W.; Do, J.-Y.; Kim, K.-J.; Bae, S.-W.; Kim, C.-W.; Lee, C.-K. Anti-heparan sulfate antibody and functional loss of glomerular heparan sulfate proteoglycans in lupus nephritis. Lupus 2016, 26, 815–824. [Google Scholar] [CrossRef]
- Krishnan, M.R.; Wang, C.; Marion, T.N. Anti-DNA autoantibodies initiate experimental lupus nephritis by binding directly to the glomerular basement membrane in mice. Kidney Int. 2012, 82, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Olin, A.I.; Mörgelin, M.; Truedsson, L.; Sturfelt, G.; Bengtsson, A.A. Pathogenic Mechanisms in Lupus Nephritis: Nucleosomes Bind Aberrant Laminin β1 With High Affinity and Colocalize in the Electron-Dense Deposits. Arthritis Rheumatol. 2014, 66, 397–406. [Google Scholar] [CrossRef]
- Mjelle, J.E.; Rekvig, O.P.; Fenton, K.A. Nucleosomes possess a high affinity for glomerular laminin and collagen IV and bind nephritogenic antibodies in murine lupus-like nephritis. Ann. Rheum. Dis. 2007, 66, 1661–1668. [Google Scholar] [CrossRef]
- Takamatsu, H.; Espinoza, J.L.; Lu, X.; Qi, Z.; Okawa, K.; Nakao, S. Anti-moesin antibodies in the serum of patients with aplastic anemia stimulate peripheral blood mononuclear cells to secrete TNF-alpha and IFN-gamma. J. Immunol. 2009, 182, 703–710. [Google Scholar] [CrossRef]
- Okano, T.; Takeuchi, S.; Soma, Y.; Suzuki, K.; Tsukita, S.; Ishizu, A.; Kawakami, T. Presence of anti-phosphatidylserine-prothrombin complex antibodies and anti-moesin antibodies in patients with polyarteritis nodosa. J. Dermatol. 2016. [Google Scholar] [CrossRef]
- Lin, X.; Liang, Q.; Lin, L.; Ding, Q.; Wang, X.; Wang, Y. Identification of anti-moesin antibodies in the serums of patients with antiphospholipid syndrome. Thromb. Res. 2015, 135, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hussain, M.; Yang, X.; Chen, P.; Yang, C.; Xun, Y.; Tian, Y.; Du, H. Identification of Moesin as a Novel Autoantigen in Patients with Sjögren’s Syndrome. Protein Pept. Lett. 2018, 25, 350–355. [Google Scholar] [CrossRef]
- Nagao, T.; Suzuki, K.; Utsunomiya, K.; Matsumura, M.; Saiga, K.; Wang, P.-C.; Minamitani, H.; Aratani, Y.; Nakayama, T.; Suzuki, K. Direct activation of glomerular endothelial cells by anti-moesin activity of anti-myeloperoxidase antibody. Nephrol. Dial. Transplant. 2011, 26, 2752–2760. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Wang, C.; Chang, D.-Y.; Hu, N.; Chen, M.; Zhao, M.-H. High mobility group box-1 contributes to anti-myeloperoxidase antibody-induced glomerular endothelial cell injury through a moesin-dependent route. Arthritis Res. 2017, 19, 125. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.J.; Mills, K.; Jury, E.; Mason, L.; D’Cruz, D.P.; Ni, L.; Saleem, M.; Mathieson, P.; Isenberg, D.; Rahman, A. Pathogenic autoantibodies from patients with lupus nephritis cause reduced tyrosine phosphorylation of podocyte proteins, including tubulin. Lupus Sci. Med. 2014, 1, e000013. [Google Scholar] [CrossRef]
| Subject | Sex | Age | Race | WHO Class * | Creatinine mg/dL | eGFR ** | UPCR g/g | Anti-dsDNA ab | C3 | C4 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 56 | W | 4 | 2.5 | 21 | 2.1 | NA | 102 | NA |
| 2 | F | 46 | W | 4 | 0.9 | 77 | 2.91 | + | 64 | 7 |
| 3 | F | 44 | AA | 4 | 1.6 | 45 | 4.8 | NA | 71 | 11 |
| 4 | F | 36 | AA | 3,4 | 1.1 | 75 | 2.3 | NA | 72 | 22 |
| 5 | F | 20 | AA | 4 | 0.5 | 161 | NA | NA | 61 | 3 |
| 6 | F | 23 | AA | 4 | 1 | 92 | NA | NA | 98 | 13 |
| 7 | F | 29 | Asian | 4 | 0.8 | 100 | 2.2 | NA | 74 | 7 |
| 8 | F | 19 | AA | 4 | 4.34 | 16 | NA | NA | 43 | 20 |
| 9 | F | 21 | W,H | 4 | 1.82 | 39 | NA | + | 68 | 8 |
| 10 | F | 34 | W | 4 | 1.53 | 44 | 1.36 | NA | 81 | 13 |
| 11 | F | 26 | AA | 5 | 1.6 | 51 | 16.5 | + | 67 | 17 |
| 12 | F | 24 | AA | 5 | 0.9 | 104 | 4.4 | + | 65 | 15 |
| 13 | F | 60 | W | 5 | 0.68 | 95 | 0.97 | NA | 138 | 20 |
| 14 | F | 37 | W | 5 | 0.63 | 115 | 4.2 | + | 63 | 11 |
| 15 | F | 22 | W | 5 | 1.43 | 52 | 1.1 | − | 93 | 21 |
| Protein | Gene Name | GN | Slit Diaphragm Protein 1 |
|---|---|---|---|
| Actin-related protein 3 | ACTR3 | [24] | |
| Alpha-enolase | ENO1 | [25,26] | |
| Alpha-parvin | PARVA | [27] | + |
| Annexin A2 | ANXA2 | [13,28] | + |
| Ezrin | EZR | [29,30] | + |
| Isoform 2 of basigin | BSG | [31] | |
| Moesin | MSN | [32,33,34,35] | + |
| Myosin-9 | MYH9 | [36] | + |
| V-type proton ATPase subunit B, brain isoform | ATP6V1B2 | [37] | |
| 60 kDa heat shock protein, mitochondrial | HSPD1 | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caster, D.J.; Korte, E.A.; Merchant, M.L.; Klein, J.B.; Barati, M.T.; Joglekar, A.; Wilkey, D.W.; Coventry, S.; Hata, J.; Rovin, B.H.; et al. Patients with Proliferative Lupus Nephritis Have Autoantibodies That React to Moesin and Demonstrate Increased Glomerular Moesin Expression. J. Clin. Med. 2021, 10, 793. https://doi.org/10.3390/jcm10040793
Caster DJ, Korte EA, Merchant ML, Klein JB, Barati MT, Joglekar A, Wilkey DW, Coventry S, Hata J, Rovin BH, et al. Patients with Proliferative Lupus Nephritis Have Autoantibodies That React to Moesin and Demonstrate Increased Glomerular Moesin Expression. Journal of Clinical Medicine. 2021; 10(4):793. https://doi.org/10.3390/jcm10040793
Chicago/Turabian StyleCaster, Dawn J., Erik A. Korte, Michael L. Merchant, Jon B. Klein, Michelle T. Barati, Ami Joglekar, Daniel W. Wilkey, Susan Coventry, Jessica Hata, Brad H. Rovin, and et al. 2021. "Patients with Proliferative Lupus Nephritis Have Autoantibodies That React to Moesin and Demonstrate Increased Glomerular Moesin Expression" Journal of Clinical Medicine 10, no. 4: 793. https://doi.org/10.3390/jcm10040793
APA StyleCaster, D. J., Korte, E. A., Merchant, M. L., Klein, J. B., Barati, M. T., Joglekar, A., Wilkey, D. W., Coventry, S., Hata, J., Rovin, B. H., Harley, J. B., Namjou-Khales, B., McLeish, K. R., & Powell, D. W. (2021). Patients with Proliferative Lupus Nephritis Have Autoantibodies That React to Moesin and Demonstrate Increased Glomerular Moesin Expression. Journal of Clinical Medicine, 10(4), 793. https://doi.org/10.3390/jcm10040793







