Clinical Factors Associated with Reinfection versus Relapse in Infective Endocarditis: Prospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Design and Setting
2.2. Definitions
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Clinical Factors Associated with Recurrence (Reinfection or Relapse)
4.2. Clinical Factors Associated with Relapse
4.3. Clinical Factors Associated with Reinfection
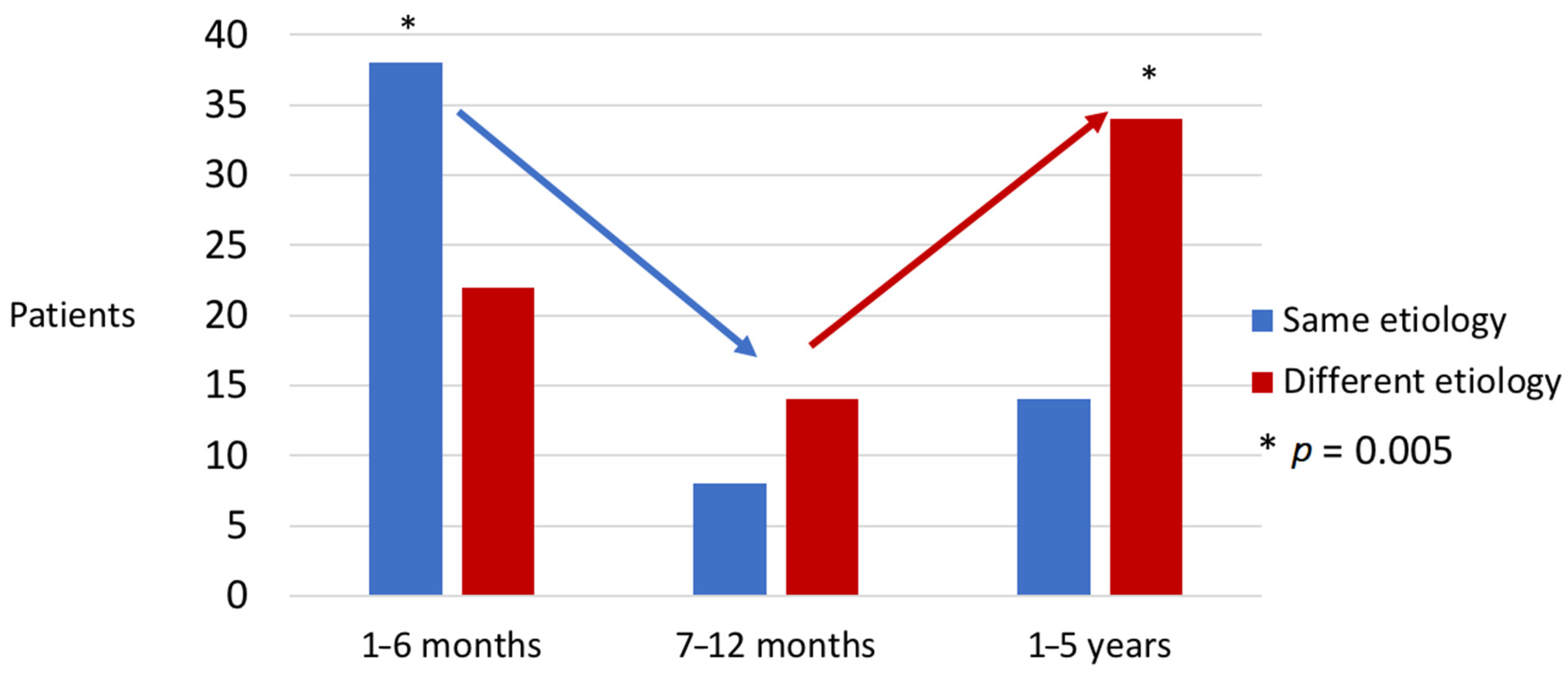
4.4. Relationship between the Microbiology of the First Episode and That of the Second Episode in Patients with Reinfection
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- David, T.E.; Gavra, G.; Feindel, C.M.; Regesta, T.; Armstrong, S.; Maganti, M.D. Surgical treatment of active infective endocarditis: A continued challenge. J. Thorac. Cardiovasc. Surg. 2007, 133, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Anguita Sánchez, M.; Torres Calvo, F.; Castillo Domínguez, J.C.; Delgado Ortega, M.; Mesa Rubio, D.; Ruiz Ortiz, M.; Romo Peña, E.; Arizón del Prado, J.M.; Suarez de Lezo, J. Short- and long-term prognosis of infective endocarditis in non-injection drug users: Improved results over 15 years (1987–2001). Rev. Esp. Cardiol. 2005, 58, 1188–1196. [Google Scholar] [CrossRef]
- Alagna, L.; Park, L.P.; Nicholson, B.P.; Keiger, A.J.; Strahilevitz, J.; Morris, A.; Wray, D.; Gordon, D.; Delahaye, F.; Edathodu, J.; et al. Repeat endocarditis: Analysis of risk factors based on the International Collaboration on Endocarditis—Prospective Cohort Study. Clin. Microbiol. Infect. 2014, 20, 566–575. [Google Scholar] [CrossRef]
- Freitas-Ferraz, A.B.; Tirado-Conte, G.; Vilacosta, I.; Olmos, C.; Sáez, C.; López, J.; Sarriá, C.; Pérez-García, C.N.; García-Arribas, D.; Ciudad, M.; et al. Contemporary epidemiology and outcomes in recurrent infective endocarditis. Heart 2019, 106, 596–602. [Google Scholar] [CrossRef]
- Mansur, A.J.; Bo, C.M.D.; Fukushima, J.T.; Issa, V.S.; Grinberg, M.; Pomerantzeff, P.M. Relapses, recurrences, valve replacements, and mortality during the long-term follow-up after infective endocarditis. Am. Heart J. 2001, 141, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Lossos, I.S.; Oren, R. Recurrent infective endocarditis. Postgrad. Med. J. 1993, 69, 816–818. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rudasill, S.E.; Sanaiha, Y.; Mardock, A.L.; Khoury, H.; Xing, H.; Antonios, J.W.; McKinnell, J.A.; Benharash, P. Clinical Outcomes of Infective Endocarditis in Injection Drug Users. J. Am. Coll. Cardiol. 2019, 73, 559–570. [Google Scholar] [CrossRef]
- Chu, V.H.; Sexton, D.J.; Cabell, C.H.; Barth, R.L.; Pappas, P.A.; Singh, R.K.; Fowler, V.G.; Ralph, C.G.; Aksoy, O.; Woods, C.W. Repeat Infective Endocarditis: Differentiating Relapse from Reinfection. Clin. Infect. Dis. 2005, 41, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, A.; Carozza, A.; Romano, G.; De Feo, M.; Della Corte, A.; Gregorio, R.; Cotrufo, M. Recurrent infective endocarditis: A multivariate analysis of 21 years of experience. Ann. Thorac. Surg. 2001, 72, 39–43. [Google Scholar] [CrossRef]
- Fernández-Hidalgo, N.; Almirante, B.; Tornos, P.; Pigrau, C.; Sambola, A.; Igual, A.; Pahissa, A. Contemporary Epidemiology and Prognosis of Health Care–Associated Infective Endocarditis. Clin. Infect. Dis. 2008, 47, 1287–1297. [Google Scholar] [CrossRef]
- Martínez-Sellés, M.; Muñoz, P.; Arnáiz, A.; Moreno, M.; Gálvez, J.; Rodríguez-Roda, J.; De Alarcón, A.; Cabrera, E.G.; Fariñas, M.C.; Miró, J.M.; et al. Valve surgery in active infective endocarditis: A simple score to predict in-hospital prognosis. Int. J. Cardiol. 2014, 175, 133–137. [Google Scholar] [CrossRef]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, J.V.G.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed Modifications to the Duke Criteria for the Diagnosis of Infective Endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.-P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis. The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 17, 3075–3128. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and by the International Society of Chemotherapy (ISC) for Infection and Cancer; Habib, G.; Hoen, B.; Tornos, P.; Thuny, F.; Prendergast, B.; Vilacosta, I.; Moreillon, P.; Antunes, M.D.J.; Thilen, U.; et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): The Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Eur. Heart J. 2009, 30, 2369–2413. [Google Scholar]
- Fernandez-Hidalgo, N.; Almirante, B.; Tornos, P.; González-Alujas, M.T.; Planes, A.M.; Galiñanes, M.; Pahissa, A. Immediate and long-term outcome of left-sided infective endocarditis. A 12-year prospective study from a contemporary cohort in a referral hospital. Clin. Microbiol. Infect. 2012, 18, E522–E530. [Google Scholar] [CrossRef]
- Tornos, M.-P.; Permanyer-Miralda, G.; Olona, M.; Gil, M.; Galve, E.; Almirante, B.; Soler-Soler, J. Long-Term Complications of Native Valve Infective Endocarditis in Non-Addicts. Ann. Intern. Med. 1992, 117, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Pericàs, J.M.; Cervera, C.; Moreno, A.; Garcia-de-la-Mària, C.; Almela, M.; Falces, C.; Quintana, E.; Vidal, B.; Llopis, J.; Fuster, D.; et al. Outcome of Enterococcus faecalis infective endocarditis according to the length of antibiotic therapy: Preliminary data from a cohort of 78 patients. PLoS ONE 2018, 13, e0192387. [Google Scholar]
- Fernández Guerrero, M.L.; Goyenechea, A.; Verdejo, C.; Roblas, R.F.; de Górgolas, M. Enterococcal endocarditis on native and prosthetic valves: A review of clinical and prognostic factors with emphasis on hospital-acquired infections as a major determinant of outcome. Medicine (Baltimore) 2007, 86, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.R.; Pericàs, J.M.; Fernández-Cruz, A.; Muñoz, P.; Valerio, M.; Kestler, M.; Montejo, M.; Fariñas, M.C.; Sousa, D.; Domínguez, F.; et al. Four weeks versus six weeks of ampicillin plus ceftriaxone in Enterococcus faecalis native valve endocarditis: A prospective cohort study. PLoS ONE 2020, 15, e0237011. [Google Scholar]
- Lin, O.S.; Wu, S.S.; Chen, Y.Y.; Soon, M.S. Bacterial peritonitis after elective endoscopic variceal ligation: A prospective study. Am. J. Gastroenterol. 2000, 95, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.H.; Hsieh, Y.H.; Tseng, K.C.; Tsai, C.C.; Tsai, C.C. The risk for bacterial endocarditis in cirrhotic patients: A population-based 3-year follow-up study. Int. J. Infect. Dis. 2013, 17, e391–e393. [Google Scholar] [CrossRef] [PubMed]
- Allaire, M.; Ollivier-Hourmand, I.; Garioud, A.; Heng, R.; Dao, T.; Cadranel, J.D. Infectious endocarditis in the case of cirrhosis: Where do we stand? Eur. J. Gastroenterol. Hepatol. 2018, 30, 1406–1410. [Google Scholar] [CrossRef] [PubMed]
- Kestler, M.; Muñoz, P.; Marín, M.; Goenaga, M.; Idíforas Viedma, P.; De Alarcón, A.; Lepe, J.; Sousa Regueiro, D.; Bravo-Ferrer, J.; Pajarón, M.; et al. Endocarditis caused by anaerobic bacteria. Anaerobe 2017, 47, 33–38. [Google Scholar] [CrossRef]
- Brook, I. Infective endocarditis caused by anaerobic bacteria. Arch. Cardiovasc. Dis. 2008, 101, 665–676. [Google Scholar] [CrossRef]
- Ramos-Martínez, A.; Cobo, M.; Restrepo, A.; Mucientes, J.; Orden-Martínez, B.; Rodríguez-Alfonso, B. Colonoscopy and Endocarditis: A Compromised Relationship. Rev. Esp. Cardiol. (Engl. Ed.) 2018, 71, 862–864. [Google Scholar] [CrossRef]
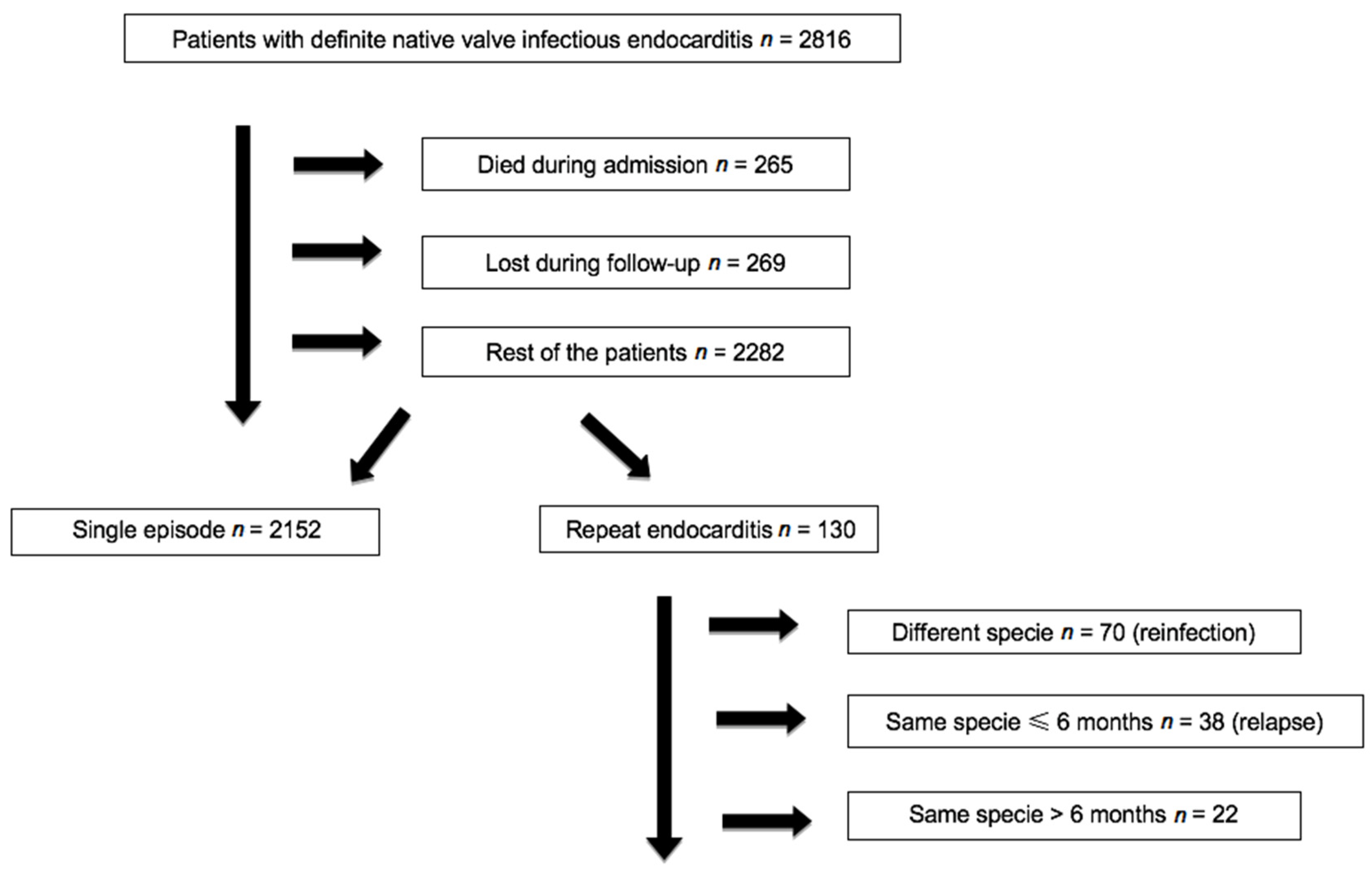
| Recurrent Endocarditis (n = 130) | One Episode (n = 2152) | OR (95% CI) 1 | p | |
|---|---|---|---|---|
| Age (years) | 65 (46–74) | 66 (53–75) | 0.222 | |
| Male gender | 88 (67.7) | 1505 (69.9) | 0.658 | |
| Hospital-acquired | 41 (31.5) | 516 (23.9) | 0.065 | |
| Non-nosocomial healthcare related | 14 (10.7) | 175 (8.1) | 0.370 | |
| Community-acquired | 75 (57.7) | 1461 (67.9) | 0.021 | |
| Diabetes mellitus | 32 (24.6) | 538 (25.0) | 0.995 | |
| Coronary disease | 37 (28.5) | 541 (25.2) | 0.458 | |
| Peripheral arterial disease | 9 (6.9) | 196 (9.1) | 0.491 | |
| Cerebrovascular disease | 22 (16.9) | 240 (11.1) | 0.062 | |
| Previous renal failure | 23 (17.7) | 410 (19.1) | 0.701 | |
| Chronic hemodialysis | 5 (3.8) | 79 (3.6) | 0.891 | |
| Chronic liver disease | 21 (16.3) | 155 (7.2) | 2.34 (1.39–3.9) | <0.01 |
| Injection drug user | 8 (6.2) | 47 (2.2) | 2.06 (0.87–4.93) | 0.004 |
| Neoplasia | 22 (16.9) | 281 (13.0) | 0.259 | |
| Age-adjusted Charlson Comorbidity Index (points) | 4 (2–6) | 4 (2–6) | 0.819 | |
| Site of infection | ||||
| Native valve | 67 (51.5) | 1317 (61.2) | 0.029 | |
| Prosthetic valve | 47 (36.2) | 580 (27.0) | 1.64 (1.12–2.39) | 0.022 |
| Cardiac device | 15 (11.5) | 277 (12.9) | 0.659 | |
| Involved valve | ||||
| Mitral | 69 (53.1) | 1041 (48.4) | 0.297 | |
| Aortic | 52 (40.0) | 862 (40.1) | 0.990 | |
| Tricuspid | 6 (4.6) | 131 (6.1) | 0.493 | |
| Pulmonary | 3 (2.3) | 26 (1.2) | 0.277 | |
| Microbiology | ||||
| Gram-positive bacteria | ||||
| Coagulase-negative staphylococci | 22 (16.9) | 332 (15.4) | 0.647 | |
| S. aureus | 22 (16.9) | 415 (19.3) | 0.506 | |
| Enterococcus spp. | 25 (19.2) | 302 (14.0) | 0.101 | |
| Streptococcus spp. | 30 (23.1) | 654 (30.4) | 0.077 | |
| Gram-negative bacilli | 9 (6.9) | 89 (4.1) | 0.128 | |
| Anaerobic bacteria | 4 (3.1) | 28 (1.3) | 0.094 | |
| Candida | 1 (0.8) | 23 (1.1) | 0.745 | |
| Polymicrobial | 1 (0.8) | 38 (1.8) | 0.395 | |
| Other microorganisms | 18 (13.8) | 224 (10.4) | 0.216 | |
| Negative cultures | 11 (8.5) | 186 (8.6) | 0.943 | |
| Septic shock | 5 (3.8) | 129 (5.9) | 0.412 | |
| Persistent bacteremia | 15 (11.5) | 206 (9.5) | 0.559 | |
| CNS vascular events | 19 (14.6) | 318 (14.7) | 0.938 | |
| Embolism | 28 (21.5) | 435 (20.2) | 0.800 | |
| Heart failure | 43 (33.0) | 651 (30.2) | 0.560 | |
| New or worsening renal insufficiency | 39 (30.0) | 588 (27.3) | 0.573 | |
| Echocardiographic findings | ||||
| Vegetation | 86 (66.2) | 1532 (71.2) | 0.216 | |
| Perivalvular abscess | 17 (13.2) | 272 (12.7) | 0.970 | |
| Valve perforation or rupture | 14 (10.7) | 281 (13.2) | 0.448 | |
| Pseudoaneurysm | 4 (3.1) | 101 (4.7) | 0.692 | |
| Intracardiac fistula | 3 (2.3) | 43 (2.0) | 0.966 | |
| Surgical indication | 75 (57.6) | 1319 (61.2) | 0.468 | |
| Surgery performed | 56 (43.1) | 1119 (52.0) | 0.74 (0.52–1.1) | 0.048 |
| Surgery indicated but not performed | 19 (14.6) | 200 (9.3) | 0.232 | |
| Device extraction | 9 (60) | 233 (84.1) | 0.016 | |
| Duration of antibiotic treatment | 42 (32–50) | 42 (30–47) | 0.323 | |
| Hospital stay (days) | 40 (27–53) | 40 (25–54) | 0.766 |
| One Episode (n = 2152) | OR (95% CI) 1 | p | Relapses (n = 38) | OR (95% CI) 1 | p2 | Reinfection (n = 70) | |
|---|---|---|---|---|---|---|---|
| Age (years) | 66 (53–75) | 0.554 | 67 (59–77) | 0.066 | 64 (45–73) | ||
| Male gender | 1505 (69.9) | 0.469 | 24 (63.1) | 0.712 | 47 (67.1) | ||
| Hospital-acquired | 516 (23.9) | 2.67 (1.37–5.29) | 0.001 | 18 (47.3) | 0.531 | 14 (20.0) | |
| Non-nosocomial healthcare related | 175 (8.1) | 0.805 | 3 (7.8) | 0.233 | 9 (12.8) | ||
| Community-acquired | 1461 (67.9) | <0.001 | 17 (44.7) | 0.986 | 47 (67.1) | ||
| Diabetes mellitus | 538 (25.0) | 0.997 | 10 (26.3) | 0.416 | 14 (20.0) | ||
| Coronary disease | 541 (25.2) | 0.728 | 11 (28.9) | 0.609 | 20 (28.6) | ||
| Peripheral arterial disease | 196 (9.1) | 0.272 | 1 (2.6) | 0.724 | 5 (7.1) | ||
| Cerebrovascular disease | 240 (11.1) | 0.252 | 7 (18.4) | 0.904 | 8 (11.4) | ||
| Previous renal failure | 410 (19.1) | 0.477 | 5 (13.2) | 0.584 | 11 (15.7) | ||
| Chronic hemodialysis | 79 (3.6) | 0.444 | 0 | 0.957 | 3 (4.2) | ||
| Chronic liver disease | 155 (7.2) | 0.090 | 6 (15.8) | 3.1 (1.65–5.83) | 0.009 | 13 (18.6) | |
| Injection drug user | 47 (2.2) | 0.721 | 0 | 0.124 | 4 (5.7) | ||
| Neoplasia | 281 (13.0) | 0.239 | 2 (5.3) | 0.064 | 15 (21.4) | ||
| Age-adjusted Charlson Comorbidity Index (points) | 4 (2–6) | 0.313 | 5 (2–6) | 0.318 | 4 (1–5) | ||
| Site of infection | |||||||
| Native valve | 1317 (61.2) | 0.216 | 19 (50.0) | 0.199 | 37 (52.9) | ||
| Prosthetic valve | 580 (27.0) | 0.124 | 15 (39.5) | 1.71 (1.04–2.82) | 0.044 | 27 (38,6) | |
| Cardiac device | 277 (12.9) | 0.854 | 4 (10.5) | 0.378 | 6 (8,6) | ||
| Involved valve | |||||||
| Mitral | 1041 (48.4) | 0.721 | 20 (52.6) | 0.276 | 39 (55.7) | ||
| Aortic | 862 (40.1) | 0.929 | 16 (42.1) | 0.557 | 31 (44.3) | ||
| Tricuspid | 131 (6.1) | 0.904 | 3 (7.9) | 0.386 | 2 (2.9) | ||
| Pulmonary | 26 (1.2) | 0.962 | 1 (2.6) | 0.697 | 1 (1.4) | ||
| Microbiology | |||||||
| Gram-positive bacteria | |||||||
| Coagulase-negative staphylococci | 332 (15.4) | 0.293 | 3 (7.9) | 0.383 | 14 (20.0) | ||
| S. aureus | 415 (19.3) | 0.198 | 11 (28.9) | 0.036 | 6 (8.6) | ||
| Enterococcus spp. | 302 (14.0) | 3.01 (1.51–6.01) | <0.001 | 14 (36.8) | 0.657 | 8 (11.4) | |
| Streptococcus spp. | 654 (30.4) | 0.077 | 6 (15.8) | 0.652 | 19 (27.1) | ||
| Gram-negative bacilli | 89 (4.1) | 0.125 | 4 (10.5) | 0.353 | 5 (7.1) | ||
| Anaerobic bacteria | 28 (1.3) | 0.983 | 0 | 4.12 (1.4–12.4) | 0.011 | 4 (5.7) | |
| Candida spp. | 23 (1.1) | 0.871 | 0 | 0.763 | 1 (1.4) | ||
| Polymicrobial | 38 (1.8) | 0.408 | 0 | 0.801 | 1 (1.4) | ||
| Other microorganisms | 224 (10.4) | 0.813 | 3 (7.9) | 0.047 | 13 (18.6) | ||
| Negative cultures | 186 (8.6) | 0.109 | 0 | 0.311 | 9 (12.9) | ||
| Septic shock | 129 (5.9) | 0.600 | 1 (2.6) | 0.735 | 3 (4.3) | ||
| Persistent bacteremia | 206 (9.5) | 2.37 (1.05–5.36) | 0.036 | 8 (21.1) | 0.941 | 6 (8.6) | |
| CNS vascular events | 318 (14.7) | 0.615 | 4 (10.5) | 0.706 | 12 (17.1) | ||
| Embolism | 435 (20.2) | 0.128 | 12 (31.6) | 0.439 | 11 (15.7) | ||
| Heart failure | 651 (30.2) | 0.996 | 11 (28.9) | 0.062 | 29 (41.4) | ||
| New or worsening renal insufficiency | 588 (27.3) | 0.066 | 16 (42.1) | 0.918 | 19 (27.1) | ||
| Echocardiographic findings | |||||||
| Vegetation | 1532 (71.2) | 0.985 | 27 (71.1) | 0.934 | 49 (70.0) | ||
| Perivalvular abscess | 272 (12.7) | 0.530 | 3 (7.9) | 0.353 | 12 (17.4) | ||
| Valve perforation or rupture | 281 (13.2) | 0.822 | 5 (13.2) | 0.827 | 8 (11.6) | ||
| Pseudoaneurysm | 101 (4.7) | 0.834 | 1 (2.6) | 0.897 | 3 (4.3) | ||
| Intracardiac fistula | 43 (2.0) | 0.771 | 0 | 0.370 | 3 (4.3) | ||
| Surgical indication | 1319 (61.2) | 0.003 | 14 (36.8) | 0.111 | 50 (71.4) | ||
| Surgery performed | 1119 (52.0) | 0.23 (0.1–0.53) | 0.001 | 7 (18.4) | 0.231 | 42 (60.0) | |
| Surgery indicated not performed | 200 (9.3) | 0.211 | 7 (18.4) | 1.03 (0.49–2.21) | 0.971 | 8 (11.4) | |
| Duration of antibiotic treatment | 40 (25–54) | 0.662 | 40 (27–56) | 0.655 | 42 (28–53) | ||
| Hospital stay (days) | 42 (30–47) | 0.882 | 42 (30–49) | 0.092 | 42 (37–49) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Parra, J.; Kestler, M.; Ramos-Martínez, A.; Bouza, E.; Valerio, M.; de Alarcón, A.; Luque, R.; Goenaga, M.Á.; Echeverría, T.; Fariñas, M.C.; et al. Clinical Factors Associated with Reinfection versus Relapse in Infective Endocarditis: Prospective Cohort Study. J. Clin. Med. 2021, 10, 748. https://doi.org/10.3390/jcm10040748
Calderón-Parra J, Kestler M, Ramos-Martínez A, Bouza E, Valerio M, de Alarcón A, Luque R, Goenaga MÁ, Echeverría T, Fariñas MC, et al. Clinical Factors Associated with Reinfection versus Relapse in Infective Endocarditis: Prospective Cohort Study. Journal of Clinical Medicine. 2021; 10(4):748. https://doi.org/10.3390/jcm10040748
Chicago/Turabian StyleCalderón-Parra, Jorge, Martha Kestler, Antonio Ramos-Martínez, Emilio Bouza, Maricela Valerio, Arístides de Alarcón, Rafael Luque, Miguel Ángel Goenaga, Tomás Echeverría, Mª Carmen Fariñas, and et al. 2021. "Clinical Factors Associated with Reinfection versus Relapse in Infective Endocarditis: Prospective Cohort Study" Journal of Clinical Medicine 10, no. 4: 748. https://doi.org/10.3390/jcm10040748
APA StyleCalderón-Parra, J., Kestler, M., Ramos-Martínez, A., Bouza, E., Valerio, M., de Alarcón, A., Luque, R., Goenaga, M. Á., Echeverría, T., Fariñas, M. C., Pericàs, J. M., Ojeda-Burgos, G., Fernández-Cruz, A., Plata, A., Vinuesa, D., Muñoz, P., & on behalf of the GAMES Investigators. (2021). Clinical Factors Associated with Reinfection versus Relapse in Infective Endocarditis: Prospective Cohort Study. Journal of Clinical Medicine, 10(4), 748. https://doi.org/10.3390/jcm10040748









