Positive Airway Pressure Therapy Adherence with Mask Resupply: A Propensity-Matched Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Endpoints
2.3. Statistical Analysis
3. Results
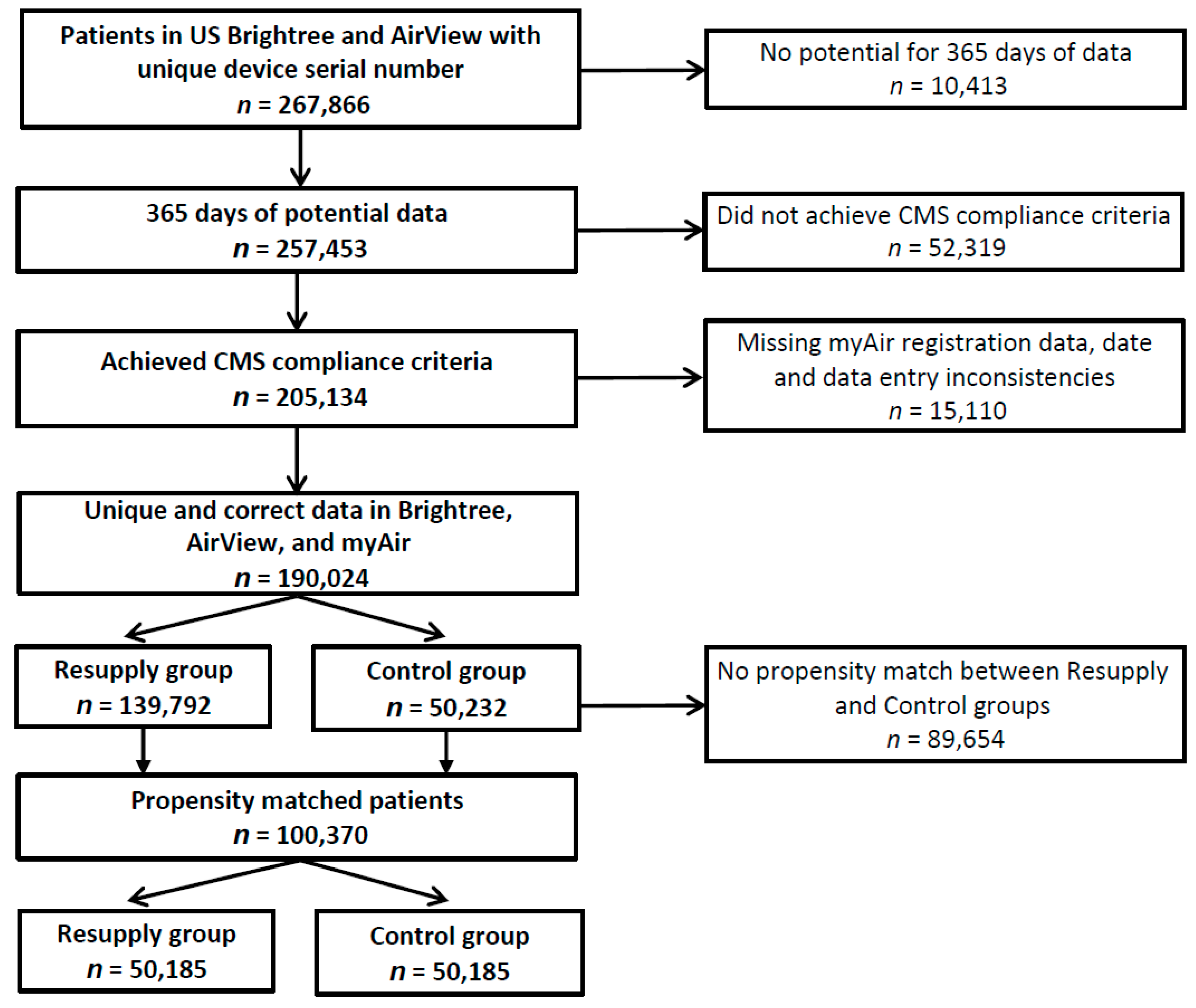
3.1. Study Sample
3.2. Resupply
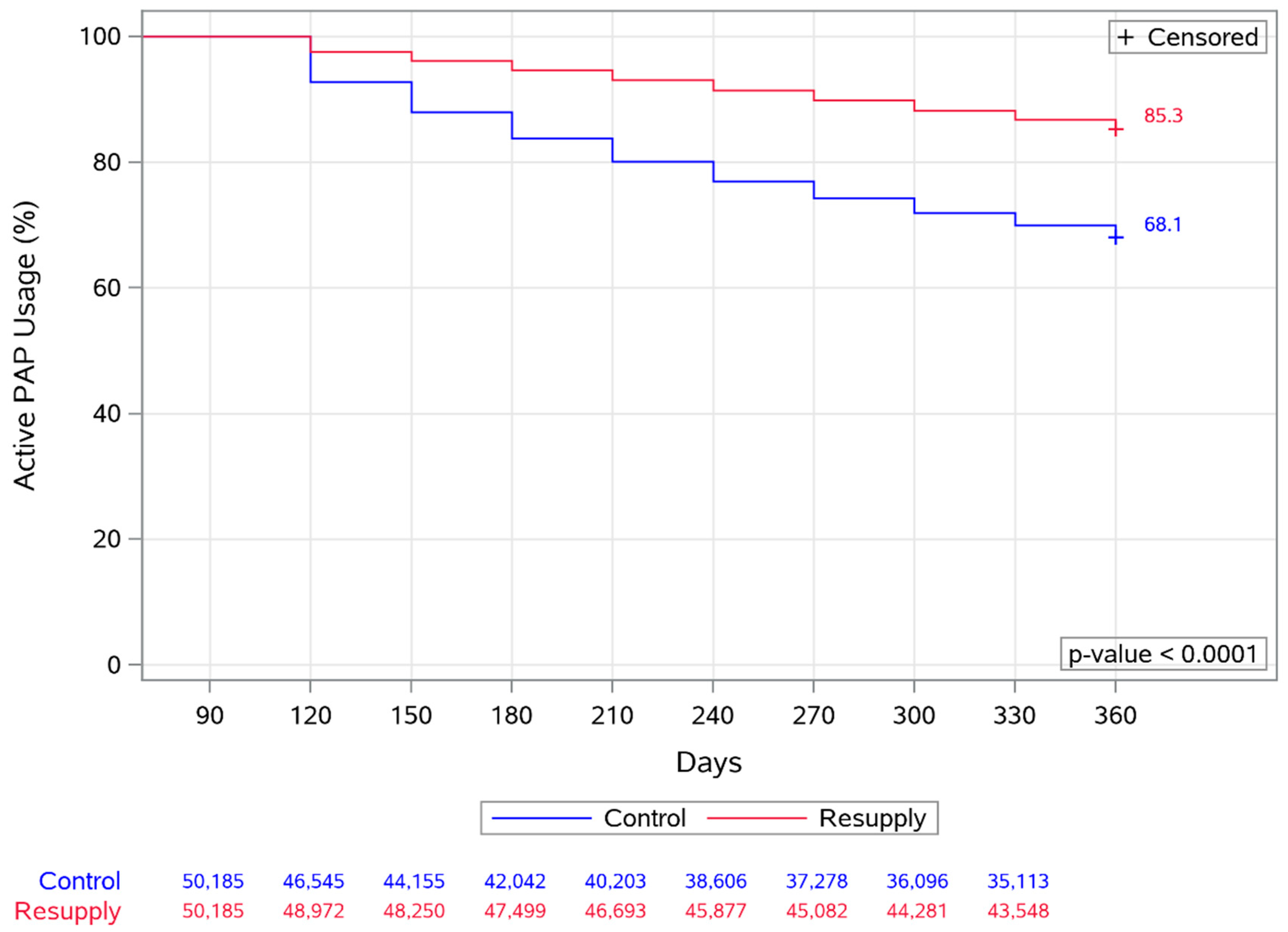
3.3. Adherence
3.4. Respiratory Parameters
3.5. Sensitivity Analysis
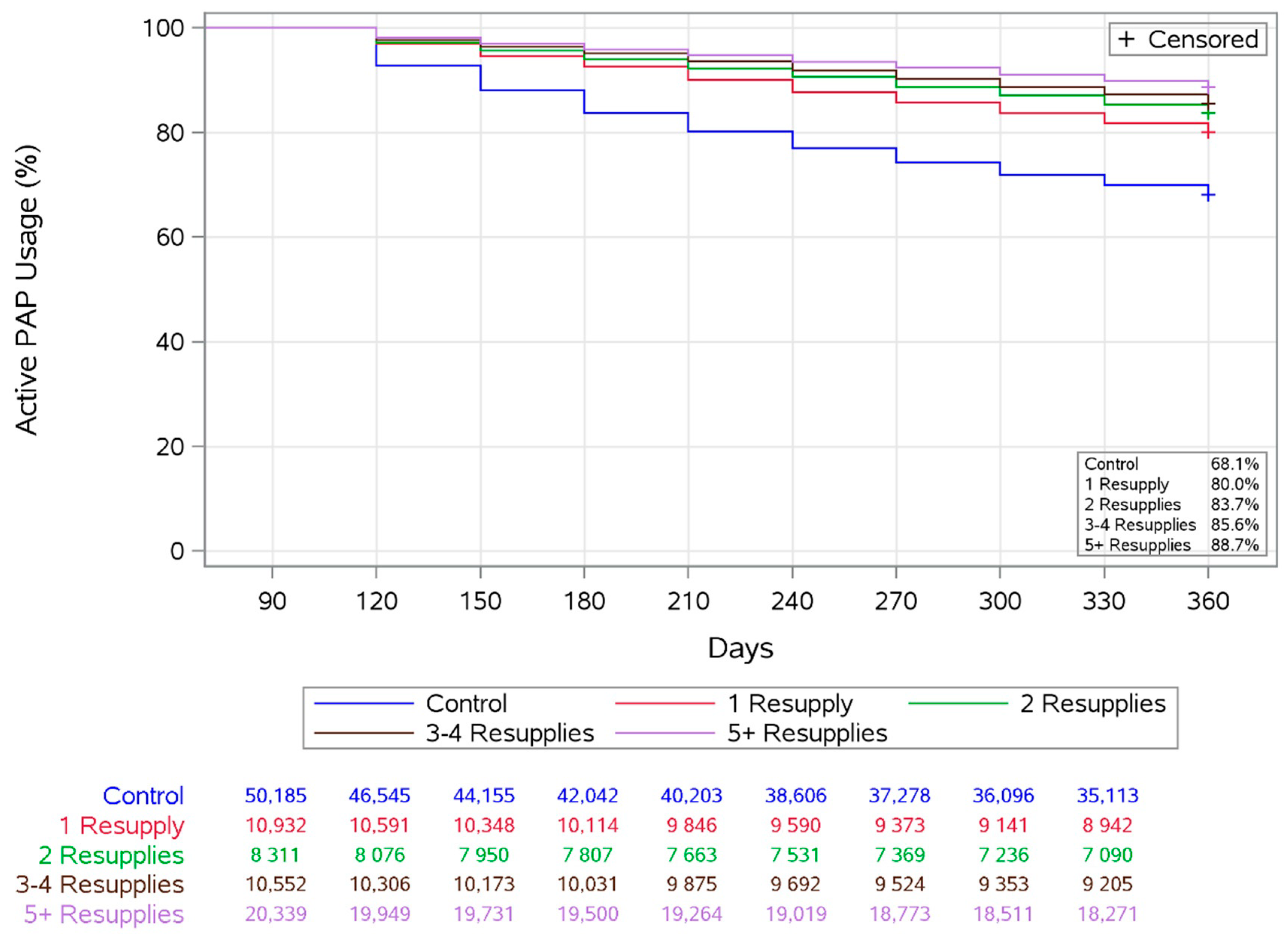
3.6. Longitudinal Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mesarwi, O.A.; Loomba, R.; Malhotra, A. Obstructive sleep apnea, hypoxia, and nonalcoholic fatty liver disease. Am. J. Respir. Crit. Care Med. 2019, 199, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pepin, J.D.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019. [Google Scholar] [CrossRef]
- Phillips, C.L.; Grunstein, R.R.; Darendeliler, M.A.; Mihailidou, A.S.; Srinivasan, V.K.; Yee, B.J.; Marks, G.B.; Cistulli, P.A. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: A randomized controlled trial. Am. J. Respir. Crit. Care Med. 2013, 187, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Kezirian, E.J.; Malhotra, A.; Goldberg, A.N.; White, D.P. Changes in obstructive sleep apnea severity, biomarkers, and quality of life after multilevel surgery. Laryngoscope 2010, 120, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Weaver, E.M.; Maynard, C.; Yueh, B. Survival of veterans with sleep apnea: Continuous positive airway pressure versus surgery. Otolaryngol. Head Neck Surg. 2004, 130, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Strollo, P.J., Jr.; Soose, R.J.; Maurer, J.T.; de Vries, N.; Cornelius, J.; Froymovich, O.; Hanson, R.D.; Padhya, T.A.; Steward, D.L.; Gillespie, M.B.; et al. Upper-airway stimulation for obstructive sleep apnea. N. Engl. J. Med. 2014, 370, 139–149. [Google Scholar] [CrossRef]
- Pepperell, J.; Ramdassingh-Dow, S.; Crosthwaite, N.; Mullins, R.; Jenkinson, C.; Stradling, J.; Davies, R. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: A randomised parallel trial. Lancet 2002, 359, 204–210. [Google Scholar] [CrossRef]
- Jenkinson, C.; Davies, R.J.; Mullins, R.; Stradling, J.R. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: A randomised prospective parallel trial. Lancet 1999, 353, 2100–2105. [Google Scholar] [CrossRef]
- Weaver, T.E.; Mancini, C.; Maislin, G.; Cater, J.; Staley, B.; Landis, J.R.; Ferguson, K.A.; George, C.F.; Schulman, D.A.; Greenberg, H.; et al. CPAP treatment of sleepy patients with milder OSA: Results of the CATNAP randomized clinical trial. Am. J. Respir. Crit. Care Med. 2012. [Google Scholar] [CrossRef]
- Wimms, A.J.; Kelly, J.L.; Turnbull, C.D.; McMillan, A.; Craig, S.E.; O’Reilly, J.F.; Nickol, A.H.; Hedley, E.L.; Decker, M.D.; Willes, L.A.; et al. Continuous positive airway pressure versus standard care for the treatment of people with mild obstructive sleep apnoea (MERGE): A multicentre, randomised controlled trial. Lancet Respir. Med. 2019, 349–358. [Google Scholar] [CrossRef]
- Weaver, T.E.; Maislin, G.; Dinges, D.F.; Bloxham, T.; George, C.F.; Greenberg, H.; Kader, G.; Mahowald, M.; Younger, J.; Pack, A.I. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 2007, 30, 711–719. [Google Scholar] [CrossRef]
- Redline, S.; Adams, N.; Strauss, M.E.; Roebuck, T.; Winters, M.; Rosenberg, C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am. J. Respir. Crit. Care Med. 1998, 157, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Crocker, M.E.; Willes, L.; Kelly, C.; Lynch, S.; Benjafield, A.V. Patient engagement using new technology to improve adherence to positive airway pressure therapy: A retrospective analysis. Chest 2018, 153, 843–850. [Google Scholar] [CrossRef]
- Deacon, N.L.; Jen, R.; Li, Y.; Malhotra, A. Treatment of obstructive sleep apnea. Prospects for personalized combined modality therapy. Ann. Am. Thorac. Soc. 2016, 13, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Kline, L.; Carlson, P. Humidification improves NCPAP acceptance and use. Am. J. Respir. Crit. Care Med. 1999, 159, A427. [Google Scholar]
- Centers for Medicare & Medicaid Services. Local Coverage Determination: Positive Airway Pressure (PAP) Devices for the Treatment of Obstructive Sleep Apnea (L33178). Available online: https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=33718&ver=16&CoverageSelection=Local&ArticleType=All&PolicyType=Final&s=All&CptHcpcsCode=e0601&bc=gAAAACAAAAAA& (accessed on 27 August 2019).
- Patel, N.; Sam, A.; Valentin, A.; Quan, S.; Parthasarathy, S. Refill rates of accessories for positive airway pressure therapy as a surrogate measure of long-term adherence. J. Clin. Sleep Med. 2012, 8, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Armitstead, J.; Benjafield, A.; Shao, S.; Malhotra, A.; Cistulli, P.A.; Pepin, J.L.; Woehrle, H. Trajectories of emergent central sleep apnea during CPAP therapy. Chest 2017, 152, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Woehrle, H.; Arzt, M.; Graml, A.; Fietze, I.; Young, P.; Teschler, H.; Ficker, J.H. Predictors of positive airway pressure therapy termination in the first year: Analysis of big data from a German homecare provider. BMC Pulm. Med. 2018, 18, 186. [Google Scholar] [CrossRef]
- Woehrle, H.; Ficker, J.H.; Graml, A.; Fietze, I.; Young, P.; Teschler, H.; Arzt, M. Telemedicine-based proactive patient management during positive airway pressure therapy: Impact on therapy termination rate. Somnologie (Berl.) 2017, 21, 121–127. [Google Scholar] [CrossRef]
- Rubin, D.B. Using propensity scores to help design observational studies: Application to the tobacco litigation. Health Serv. Outcomes Res. Methodol. 2001, 2, 169–188. [Google Scholar] [CrossRef]
- Bakker, J.P.; Edwards, B.A.; Gautam, S.P.; Montesi, S.B.; Duran-Cantolla, J.; Aizpuru, F.; Barbe, F.; Sanchez-de-la-Torre, M.; Malhotra, A. Blood pressure improvement with continuous positive airway pressure is independent of obstructive sleep apnea severity. J. Clin. Sleep Med. 2014, 10, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Cistulli, P.A.; Armitstead, J.; Pepin, J.L.; Woehrle, H.; Nunez, C.M.; Benjafield, A.; Malhotra, A. Short-term CPAP adherence in obstructive sleep apnea: A big data analysis using real world data. Sleep Med. 2019, 59, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Hoy, C.J.; Vennelle, M.; Kingshott, R.N.; Engleman, H.M.; Douglas, N.J. Can intensive support improve continuous positive airway pressure use in patients with the sleep apnea/hypopnea syndrome? Am. J. Respir. Crit. Care Med. 1999, 159, 1096–1100. [Google Scholar] [CrossRef]
- Kuna, S.T.; Shuttleworth, D.; Chi, L.; Schutte-Rodin, S.; Friedman, E.; Guo, H.; Dhand, S.; Yang, L.; Zhu, J.; Bellamy, S.L.; et al. Web-based access to positive airway pressure usage with or without an initial financial incentive improves treatment use in patients with obstructive sleep apnea. Sleep 2015, 38, 1229–1236. [Google Scholar] [CrossRef]
- Tarasiuk, A.; Reznor, G.; Greenberg-Dotan, S.; Reuveni, H. Financial incentive increases CPAP acceptance in patients from low socioeconomic background. PLoS ONE 2012, 7, e33178. [Google Scholar] [CrossRef]
- Ye, L.; Antonelli, M.T.; Willis, D.G.; Kayser, K.; Malhotra, A.; Patel, S.R. Couples’ experiences with continuous positive airway pressure treatment: A dyadic perspective. Sleep Health 2017, 3, 362–367. [Google Scholar] [CrossRef]
- Aloia, M.S.; Stanchina, M.; Arnedt, J.T.; Malhotra, A.; Millman, R.P. Treatment adherence and outcomes in flexible vs standard continuous positive airway pressure therapy. Chest 2005, 127, 2085–2093. [Google Scholar] [CrossRef][Green Version]
- Nilius, G.; Happel, A.; Domanski, U.; Ruhle, K.H. Pressure-relief continuous positive airway pressure vs constant continuous positive airway pressure: A comparison of efficacy and compliance. Chest 2006, 130, 1018–1024. [Google Scholar] [CrossRef]
- Ballard, R.D.; Gay, P.C.; Strollo, P.J. Interventions to improve compliance in sleep apnea patients previously non-compliant with continuous positive airway pressure. J. Clin. Sleep Med. 2007, 3, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Benjafield, A.V.; Pepin, J.D.; Valentine, K.; Cistulli, P.A.; Woehrle, H.; Nunez, C.M.; Armitstead, J.; Malhotra, A. Compliance after switching from CPAP to bilevel for patients with non-compliant OSA: Big data analysis. BMJ Open Respir. Res. 2019, 6, e000380. [Google Scholar] [CrossRef]
- Horowitz, A.; Horowitz, S.; Chun, C. CPAP masks are sources of microbial contamination [abstract]. In Proceedings of the 23rd Annual Meeting of the Associated Professional Sleep Societies 2009, Boston, MA, USA, 6–11 June 2009. [Google Scholar]
- Mercieca, L.; Pullicino, R.; Camilleri, K.; Abela, R.; Mangion, S.A.; Cassar, J.; Zammit, M.; Gatt, C.; Deguara, C.; Barbara, C.; et al. Continuous positive airway pressure: Is it a route for infection in those with obstructive sleep apnoea? Sleep Sci. 2017, 10, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.J.; George, C.; Lannigan, R.; Rotenberg, B.W. Association of CPAP bacterial colonization with chronic rhinosinusitis. J. Clin. Sleep Med. 2013, 9, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Montesi, S.B.; Bakker, J.P.; Macdonald, M.; Hueser, L.; Pittman, S.; White, D.P.; Malhotra, A. Air leak during CPAP titration as a risk factor for central apnea. J. Clin. Sleep Med. 2013, 9, 1187–1191. [Google Scholar] [CrossRef]
- Platt, A.B.; Kuna, S.T.; Field, S.H.; Chen, Z.; Gupta, R.; Roche, D.F.; Christie, J.D.; Asch, D.A. Adherence to sleep apnea therapy and use of lipid-lowering drugs: A study of the healthy-user effect. Chest 2010, 137, 102–108. [Google Scholar] [CrossRef] [PubMed]
| Heading | Control (n = 50,185) | Resupply (n = 50,185) |
|---|---|---|
| Age, years | ||
| Mean ± SD | 56.9 ± 13.6 | 57.0 ± 13.5 |
| Median | 57.0 | 57.0 |
| Sex, n (%) | ||
| Female | 17,847 (35.6) | 17,858 (35.6) |
| Male | 32,312 (64.4) | 32,301 (64.4) |
| Missing | 26 (<0.1) | 26 (<0.1) |
| Device/mode, n (%) | ||
| APAP | 21,437 (42.7) | 21,449 (42.7) |
| CPAP | 23,949 (47.7) | 24,022 (47.9) |
| Bilevel | 4272 (8.5) | 4187 (8.3) |
| Missing | 527 (1.1) | 527 (1.1) |
| myAirTM use, n (%) | 8807 (17.5) | 8795 (17.5) |
| AHI on the first day of therapy, /h | ||
| Mean ± SD | 3.9 ± 6.0 | 3.8 ± 5.7 |
| Median | 2.0 | 2.0 |
| 95th percentile leak on the first day of therapy, L/min | ||
| Mean ± SD | 23.5 ± 26.3 | 23.2 ± 25.7 |
| Median | 15.6 | 15.6 |
| Device Usage | Control | Resupply | p-Value |
|---|---|---|---|
| (n = 50,185) | (n = 50,185) | ||
| Mean usage, h | |||
| Mean (SE) | 4.5 (0.1) | 5.6 (0.01) | |
| Median | 4.9 | 6.0 | |
| Mean difference vs. control (95% CI) | 1.1 (1.06, 1.13) | <0.0001 | |
| Mean usage ≥4 h, % | |||
| Mean (95% CI) | 59.2 (58.8, 59.6) | 77.0 (76.6, 77.4) | |
| Mean difference vs. control (95% CI) | 17.8 (17.2, 18.3) | <0.0001 | |
| Daily usage ≥4 h for 70% of nights, % | |||
| Mean (95% CI) | 50.9 (50.5, 51.3) | 67.7 (67.3, 68.1) | |
| Mean difference vs. control (95% CI) | 16.8 (16.2, 17.4) | <0.0001 | |
| Respiratory Parameters | (n = 47,373) | (n = 49,359) | |
| Mean AHI, /h | |||
| Mean (SE) | 2.6 (0.02) | 2.5 (0.01) | |
| Median | 1.5 | 1.5 | |
| Mean difference vs. control (95% CI) | −0.2 (−0.21, −0.13) | <0.0001 | |
| Median 95% percentile mask leak, L/min | |||
| Mean (SE) | 20.4 (0.10) | 19.0 (0.09) | |
| Median | 15.0 | 14.4 | |
| Mean difference vs. control (95% CI) | −1.5 (−1.68, −1.24) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benjafield, A.V.; Oldstone, L.M.; Willes, L.A.; Kelly, C.; Nunez, C.M.; Malhotra, A.; on behalf of the medXcloud Group. Positive Airway Pressure Therapy Adherence with Mask Resupply: A Propensity-Matched Analysis. J. Clin. Med. 2021, 10, 720. https://doi.org/10.3390/jcm10040720
Benjafield AV, Oldstone LM, Willes LA, Kelly C, Nunez CM, Malhotra A, on behalf of the medXcloud Group. Positive Airway Pressure Therapy Adherence with Mask Resupply: A Propensity-Matched Analysis. Journal of Clinical Medicine. 2021; 10(4):720. https://doi.org/10.3390/jcm10040720
Chicago/Turabian StyleBenjafield, Adam V., Liesl M. Oldstone, Leslee A. Willes, Colleen Kelly, Carlos M. Nunez, Atul Malhotra, and on behalf of the medXcloud Group. 2021. "Positive Airway Pressure Therapy Adherence with Mask Resupply: A Propensity-Matched Analysis" Journal of Clinical Medicine 10, no. 4: 720. https://doi.org/10.3390/jcm10040720
APA StyleBenjafield, A. V., Oldstone, L. M., Willes, L. A., Kelly, C., Nunez, C. M., Malhotra, A., & on behalf of the medXcloud Group. (2021). Positive Airway Pressure Therapy Adherence with Mask Resupply: A Propensity-Matched Analysis. Journal of Clinical Medicine, 10(4), 720. https://doi.org/10.3390/jcm10040720





