The Use of Antiviral Agents against SARS-CoV-2: Ineffective or Time and Age Dependent Result? A Retrospective, Observational Study among COVID-19 Older Adults †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Participants, and Data Collection
2.2. Lopinavir/Ritonavir (LPV/r), Darunavir/Cobicistat (DVR/c), Hydroxychloroquine (HCQ), and Need for Hospitalization
2.3. Statistical Analysis
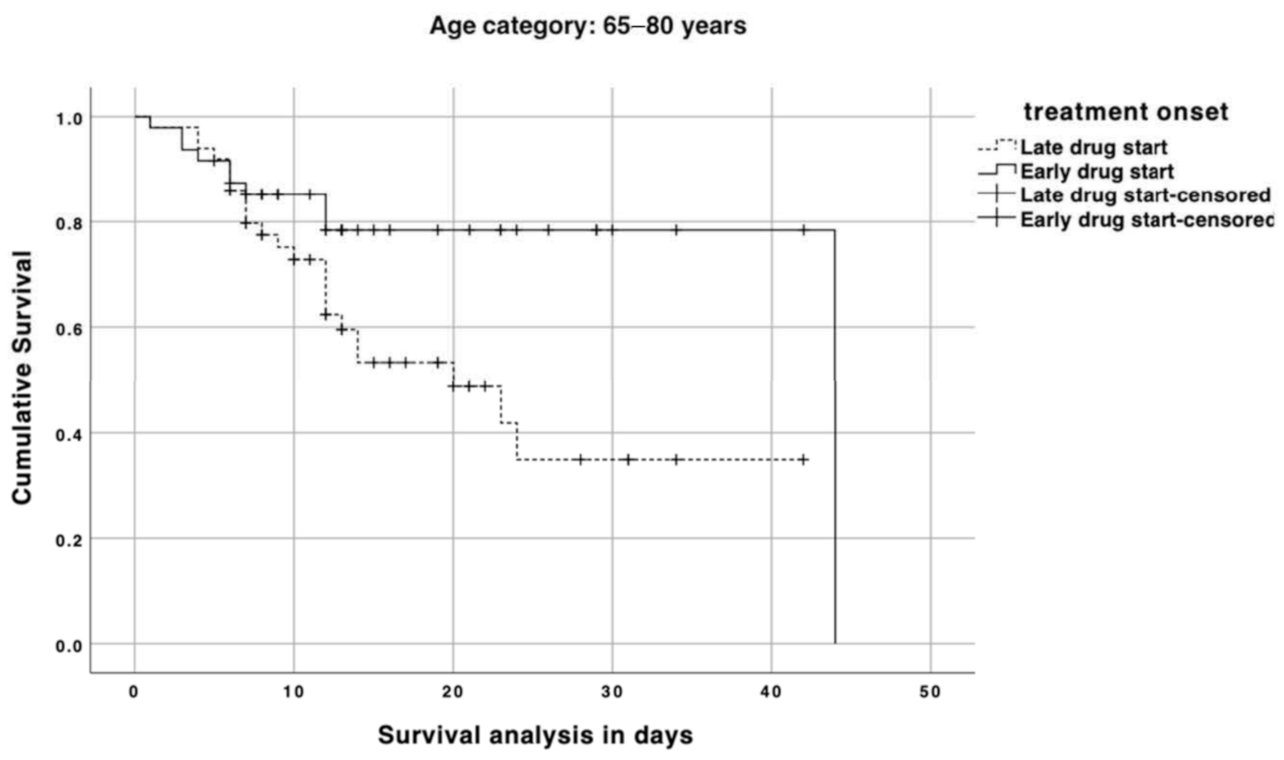
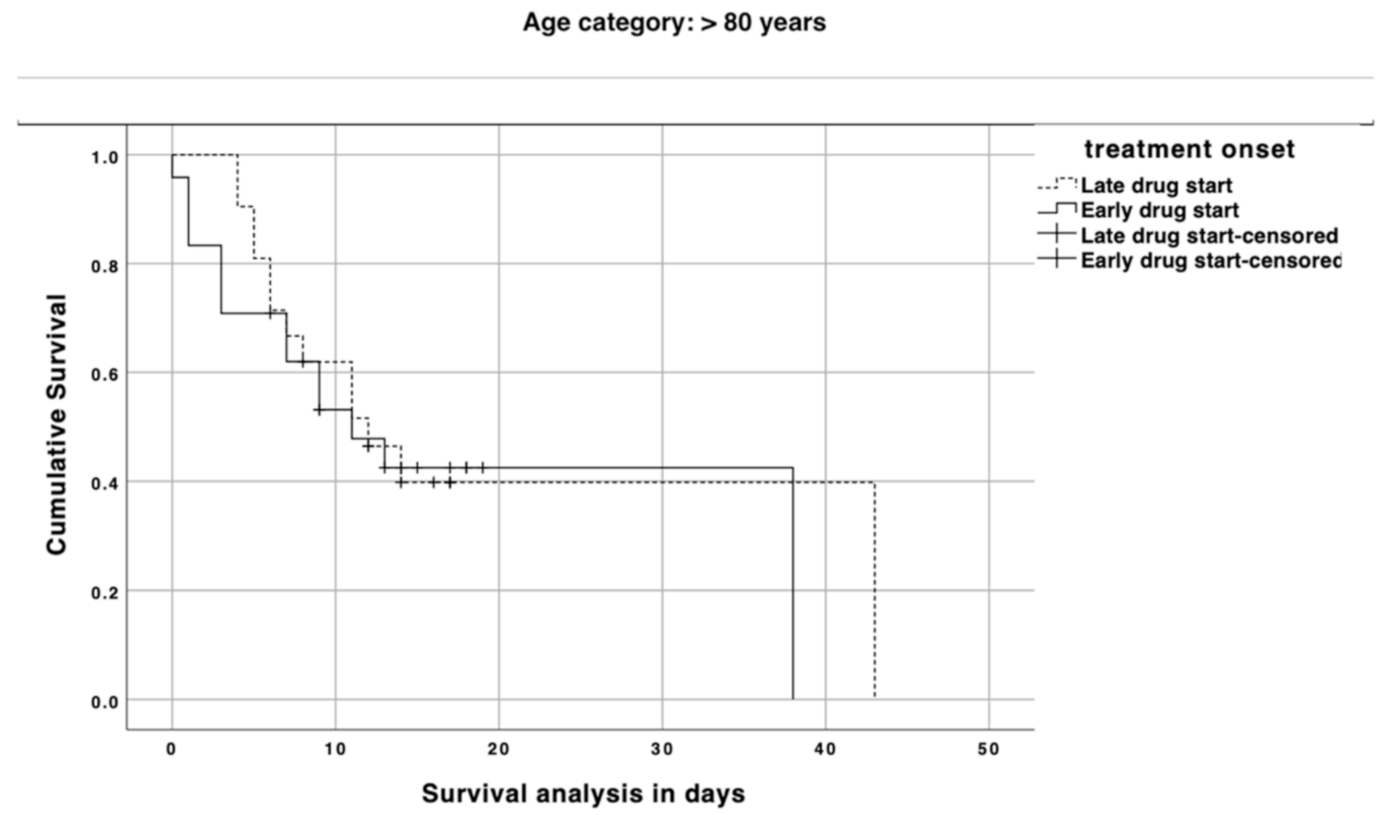
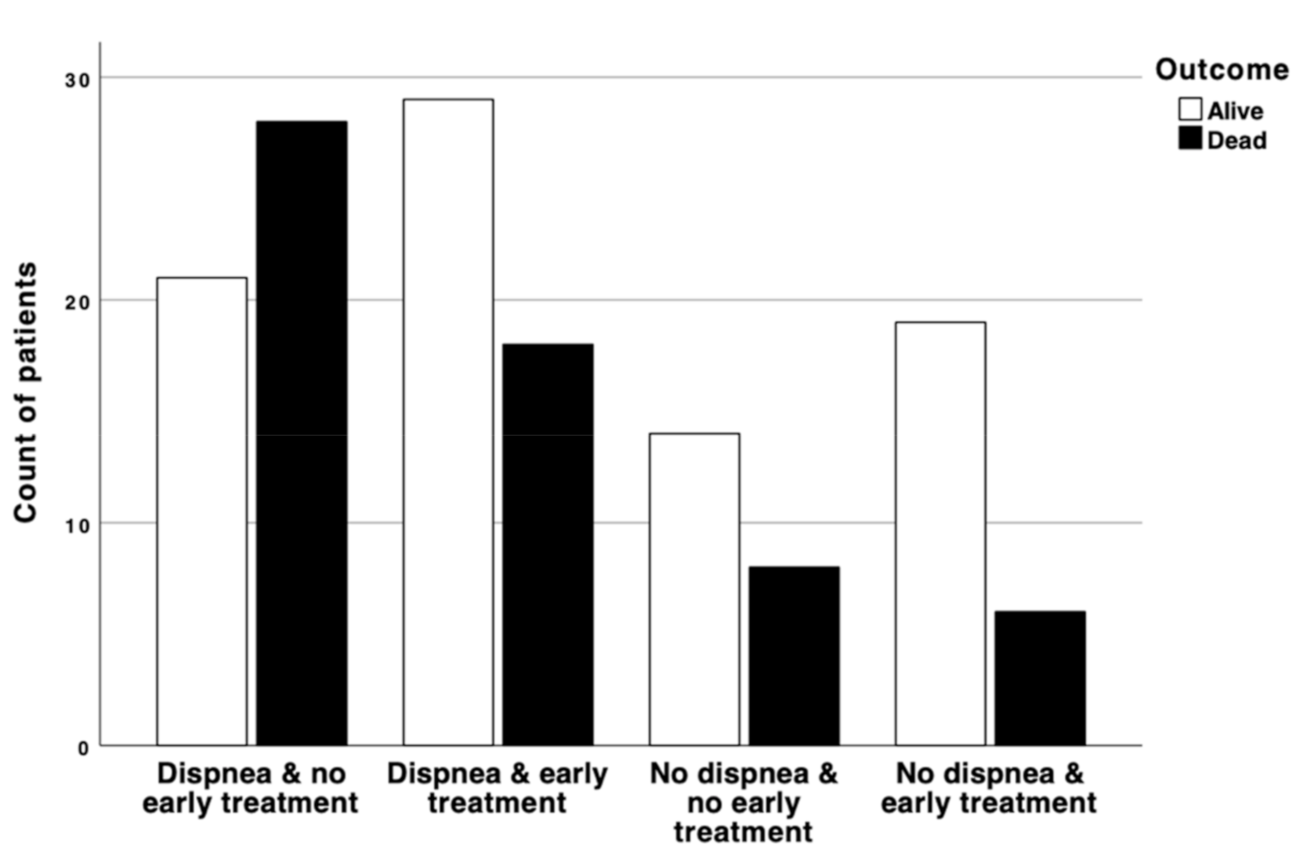
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FDA. Coronavirus (COVID-19) Drugs. Available online: https://www.fda.gov/drugs/emergency-preparedness-drugs/coronavirus-covid-19-drugs (accessed on 28 January 2021).
- Baden, L.R.; Rubin, E.J. Covid-19—The Search for Effective Therapy. N. Engl. J. Med. 2020, 382, 1851–1852. [Google Scholar] [CrossRef]
- Chen, F.; Chan, K.H.; Jiang, Y.; Kao, R.Y.; Lu, H.T.; Fan, K.W.; Cheng, V.C.; Tsui, W.H.; Hung, I.F.; Lee, T.S.; et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 2004, 31, 69–75. [Google Scholar] [CrossRef]
- Kim, E.J.; Choi, S.H.; Park, J.S.; Kwon, Y.S.; Lee, J.; Kim, Y.; Lee, S.Y.; Choi, E.Y. Use of Darunavir-Cobicistat as a Treatment Option for Critically Ill Patients with SARS-CoV-2 Infection. Yonsei Med. J. 2020, 61, 826–830. [Google Scholar] [CrossRef]
- Takahashi, T.; Luzum, J.A.; Nicol, M.R.; Jacobson, P.A. Pharmacogenomics of COVID-19 therapies. NPJ Genom. Med. 2020, 5, 35. [Google Scholar] [CrossRef]
- Pal, A.; Pawar, A.; Goswami, K.; Sharma, P.; Prasad, R. Hydroxychloroquine and Covid-19: A Cellular and Molecular Biology Based Update. Indian J. Clin. Biochem. 2020, 35, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Arshad, S.; Kilgore, P.; Chaudhry, Z.S.; Jacobsen, G.; Wang, D.D.; Huitsing, K.; Brar, I.; Alangaden, G.J.; Ramesh, M.S.; McKinnon, J.E.; et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Dis. 2020, 97, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.; Balayla, G. Hydroxychloroquine and COVID-19. Postgrad. Med. J. 2020, 96, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, M.; Yin, L.; Wang, K.; Zhou, Y.; Zhou, M.; Lu, Y. COVID-19 treatment: Close to a cure? A rapid review of pharmacotherapies for the novel coronavirus (SARS-CoV-2). Int. J. Antimicrob. Agents 2020, 56, 106080. [Google Scholar] [CrossRef] [PubMed]
- Chary, M.A.; Barbuto, A.F.; Izadmehr, S.; Hayes, B.D.; Burns, M.M. COVID-19: Therapeutics and Their Toxicities. J. Med. Toxicol. 2020, 16, 284–294. [Google Scholar] [CrossRef]
- Iyer, M.; Jayaramayya, K.; Subramaniam, M.D.; Lee, S.B.; Dayem, A.A.; Cho, S.G.; Vellingiri, B. COVID-19: An update on diagnostic and therapeutic approaches. BMB Rep. 2020, 53, 191–205. [Google Scholar] [CrossRef]
- Desai, A.; Voza, G.; Paiardi, S.; Teofilo, F.I.; Caltagirone, G.; Pons, M.R.; Aloise, M.; Kogan, M.; Tommasini, T.; Savevski, V.; et al. The role of anti-hypertensive treatment, comorbidities and early introduction of LMWH in the setting of COVID-19: A retrospective, observational study in Northern Italy. Int. J. Cardiol. 2020. [Google Scholar] [CrossRef]
- Bein, B.; Bachmann, M.; Huggett, S.; Wegermann, P. SARS CoV-2/COVID-19: Evidence-Based Recommendation on Diagnosis and Therapy. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 2020, 55, 257–265. [Google Scholar] [CrossRef]
- CDC. COVID-19 Increased Risk of Hospotalization or Death. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html (accessed on 28 January 2021).
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- WHO. Clinical Management of COVID-19. Available online: https://www.who.int/publications/i/item/clinical-management-of-covid-19 (accessed on 28 January 2021).
- Desai, A.; Santonocito, O.G.; Caltagirone, G.; Kogan, M.; Ghetti, F.; Donadoni, I.; Porro, F.; Savevski, V.; Poretti, D.; Ciccarelli, M.; et al. Effectiveness of Streptococcus Pneumoniae Urinary Antigen Testing in Decreasing Mortality of COVID-19 Co-Infected Patients: A Clinical Investigation. Medicina (Kaunas) 2020, 56, 572. [Google Scholar] [CrossRef]
- Fine, M.J.; Auble, T.E.; Yealy, D.M.; Hanusa, B.H.; Weissfeld, L.A.; Singer, D.E.; Coley, C.M.; Marrie, T.J.; Kapoor, W.N. A Prediction Rule to Identify Low-Risk Patients with Community-Acquired Pneumonia. N. Engl. J. Med. 1997, 336, 243–250. [Google Scholar] [CrossRef]
- Lim, W.S.; van der Eerden, M.M.; Laing, R.; Boersma, W.G.; Karalus, N.; Town, G.I.; Lewis, S.A.; Macfarlane, J.T. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax 2003, 58, 377–382. [Google Scholar] [CrossRef]
- Geriatric Medicine Research Collaborative. Age and frailty are independently associated with increased COVID-19 mortality and increased care needs in survivors: Results of an international multi-centre study. Age Ageing 2021. [Google Scholar] [CrossRef]
- Wang, X.; Ji, X. Sample Size Estimation in Clinical Research: From Randomized Controlled Trials to Observational Studies. Chest 2020, 158, S12–S20. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J. Heart Lung Transpl. 2020, 39, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Cocco, A.; Amami, P.; Desai, A.; Voza, A.; Ferreli, F.; Albanese, A. Neurological features in SARS-CoV-2-infected patients with smell and taste disorder. J. Neurol. 2020, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Giotta Lucifero, A.; Marasco, S.; Del Maestro, M.; Bellantoni, G.; Gragnaniello, C. Targeting of renin-angiotensin system in COVID-19 patients affected by stroke: Emerging concerns about detrimental vs. benefit effect. Interdiscip. Neurosurg. 2020, 22, 100822. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Mohiddin, S.A.; Dimarco, A.; Patel, V.; Savvatis, K.; Marelli-Berg, F.M.; Madhur, M.S.; Tomaszewski, M.; Maffia, P.; D’Acquisto, F.; et al. COVID-19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020, 116, 1666–1687. [Google Scholar] [CrossRef]
- McElvaney, O.J.; McEvoy, N.L.; McElvaney, O.F.; Carroll, T.P.; Murphy, M.P.; Dunlea, D.M.; O, N.C.; Clarke, J.; O’Connor, E.; Hogan, G.; et al. Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am. J. Respir. Crit. Care Med. 2020, 202, 812–821. [Google Scholar] [CrossRef]
- Ellinghaus, D.; Degenhardt, F.; Bujanda, L.; Buti, M.; Albillos, A.; Invernizzi, P.; Fernández, J.; Prati, D.; Baselli, G.; Asselta, R.; et al. Genomewide Association Study of Severe Covid-19 with Respiratory Failure. N. Engl. J. Med. 2020, 383, 1522–1534. [Google Scholar] [CrossRef]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; van de Veen, W.; Bruggen, M.C.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Pan, H.; Peto, R.; Karim, Q.A.; Alejandria, M.; Henao-Restrepo, A.M.; García, C.H.; Kieny, M.-P.; Malekzadeh, R.; Murthy, S.; Preziosi, M.-P.; et al. Repurposed antiviral drugs for COVID-19—Interim WHO SOLIDARITY trial results. medRxiv 2020. [Google Scholar] [CrossRef]
- CDC. Assessing Risk Factors for Severe COVID-19 Illness. Available online: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/assessing-risk-factors.html (accessed on 28 January 2021).
- Lithander, F.E.; Neumann, S.; Tenison, E.; Lloyd, K.; Welsh, T.J.; Rodrigues, J.C.L.; Higgins, J.P.T.; Scourfield, L.; Christensen, H.; Haunton, V.J.; et al. COVID-19 in older people: A rapid clinical review. Age Ageing 2020, 49, 501–515. [Google Scholar] [CrossRef]
- Turcotte, J.J.; Meisenberg, B.R.; MacDonald, J.H.; Menon, N.; Fowler, M.B.; West, M.; Rhule, J.; Qureshi, S.S.; MacDonald, E.B. Risk factors for severe illness in hospitalized Covid-19 patients at a regional hospital. PLoS ONE 2020, 15, e0237558. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Ferrucci, L.; Darer, J.; Williamson, J.D.; Anderson, G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Legramante, J.M.; Morciano, L.; Lucaroni, F.; Gilardi, F.; Caredda, E.; Pesaresi, A.; Coscia, M.; Orlando, S.; Brandi, A.; Giovagnoli, G.; et al. Frequent Use of Emergency Departments by the Elderly Population When Continuing Care Is Not Well Established. PLoS ONE 2016, 11, e0165939. [Google Scholar] [CrossRef]
- Valiathan, R.; Ashman, M.; Asthana, D. Effects of Ageing on the Immune System: Infants to Elderly. Scand. J. Immunol. 2016, 83, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Aw, D.; Silva, A.B.; Palmer, D.B. Immunosenescence: Emerging challenges for an ageing population. Immunology 2007, 120, 435–446. [Google Scholar] [CrossRef]
- Vaira, L.A.; Salzano, G.; Fois, A.G.; Piombino, P.; De Riu, G. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int. Forum. Allergy Rhinol. 2020, 10, 1103–1104. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Salzano, G.; Deiana, G.; De Riu, G. Anosmia and Ageusia: Common Findings in COVID-19 Patients. Laryngoscope 2020, 130, 1787. [Google Scholar] [CrossRef]
- Russell, B.; Moss, C.; Rigg, A.; Hopkins, C.; Papa, S.; Van Hemelrijck, M. Anosmia and ageusia are emerging as symptoms in patients with COVID-19: What does the current evidence say? Ecancermedicalscience 2020, 14, ed98. [Google Scholar] [CrossRef] [PubMed]
- Bohn, M.K.; Hall, A.; Sepiashvili, L.; Jung, B.; Steele, S.; Adeli, K. Pathophysiology of COVID-19: Mechanisms Underlying Disease Severity and Progression. Physiology 2020, 35, 288–301. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Ho, Y.C. SARS-CoV-2: A storm is raging. J. Clin. Investig. 2020, 130, 2202–2205. [Google Scholar] [CrossRef]
- Yao, Z.; Zheng, Z.; Wu, K.; Junhua, Z. Immune environment modulation in pneumonia patients caused by coronavirus: SARS-CoV, MERS-CoV and SARS-CoV-2. Aging 2020, 12, 7639–7651. [Google Scholar] [CrossRef]
- Giotta Lucifero, A.; Luzzi, S.; Brambilla, I.; Guarracino, C.; Mosconi, M.; Foiadelli, T.; Savasta, S. Gene therapies for high-grade gliomas: From the bench to the bedside. Acta Bio-Med. 2020, 91, 32–50. [Google Scholar] [CrossRef]
- Giotta Lucifero, A.; Luzzi, S.; Brambilla, I.; Schena, L.; Mosconi, M.; Foiadelli, T.; Savasta, S. Potential roads for reaching the summit: An overview on target therapies for high-grade gliomas. Acta Bio-Med. 2020, 91, 61–78. [Google Scholar] [CrossRef]
- Giotta Lucifero, A.; Luzzi, S.; Brambilla, I.; Trabatti, C.; Mosconi, M.; Savasta, S.; Foiadelli, T. Innovative therapies for malignant brain tumors: The road to a tailored cure. Acta Bio-Med. 2020, 91, 5–17. [Google Scholar] [CrossRef]
- Luzzi, S.; Giotta Lucifero, A.; Brambilla, I.; Magistrali, M.; Mosconi, M.; Savasta, S.; Foiadelli, T. Adoptive immunotherapies in neuro-oncology: Classification, recent advances, and translational challenges. Acta Bio-Med. 2020, 91, 18–31. [Google Scholar] [CrossRef]
| Variables | Age Categories | |||
|---|---|---|---|---|
| Total (n = 143) | 65–80 Years (n = 98) | >80 Years (n = 45) | p-Value | |
| Age, mean (SD), min-max | 76.2 (7.4) 66–93 | 71.9 (3.8) 66–79 | 85.5 (4.2) 80–93 | p < 0.0001 |
| Male, n (%) | 102 (71.3%) | 72 (73.5%) | 30 (66.7%) | p = 0.40 |
| Antivirals, LPV/r:DVR/c (% of LPV/r) | 97:46 (67.8%) | 68:30 (69.4%) | 29:16 (64.4%) | p = 0.55 |
| LMWH, n (%) | 126 (88.1%) | 81 (82.7%) | 45 (100%) | p = 0.003 |
| Fever, n (%) | 126 (88.1%) | 88 (89.8%) | 38 (84.4%) | p = 0.36 |
| Cough, n (%) | 77 (53.8%) | 61 (62.2%) | 16 (35.6%) | p = 0.003 |
| Dyspnea, n (%) | 96 (67.1%) | 61 (62.2%) | 35 (77.8%) | p = 0.06 |
| Gastrointestinal problems, n (%) | 27 (18.9%) | 20 (20.4%) | 7 (15.6%) | p = 0.49 |
| Hyposmia/hypogeusia, n (%) | 8 (5.6%) | 5 (5.1%) | 3 (6.7%) | p = 0.71 |
| >1 symptoms, n (%) | 117 (81.8%) | 83 (84.7%) | 34 (75.6%) | p = 0.19 |
| Time to drug start (<6 days), n (%) | 72 (50.3%) | 48 (49%) | 24 (53.3%) | p = 0.63 |
| In-hospital death, n (%) | 60 (42%) | 33 (33.7%) | 27 (60%) | p = 0.003 |
| ICU transfer, n (%) | 10 (7%) | 10 (10.2%) | 0 (0%) | p = 0.03 |
| Predictors of Mortality | ||||
|---|---|---|---|---|
| Univariate (n = 143) | Multivariate (n = 143) | |||
| Predictors | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Age: >80 vs. 65–80 years | 2.95 (1.4–6.1) | 0.003 | 2.54 (1.2–5.6) | 0.03 |
| Cough, n (%) | 0.48 (0.24–0.94) | 0.03 | 0.53 (0.2–1.1) | 0.09 |
| Dyspnea, n (%) | 2.17 (1.03–4.55) | 0.04 | 2.01 (0.9–4.4) | 0.08 |
| Time to drug start (< 6 days), n (%) | 0.49 (0.2–0.9) | 0.03 | 0.44 (0.2–0.9) | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desai, A.; Caltagirone, G.; Sari, S.; Pocaterra, D.; Kogan, M.; Azzolini, E.; Savevski, V.; Martinelli-Boneschi, F.; Voza, A. The Use of Antiviral Agents against SARS-CoV-2: Ineffective or Time and Age Dependent Result? A Retrospective, Observational Study among COVID-19 Older Adults . J. Clin. Med. 2021, 10, 686. https://doi.org/10.3390/jcm10040686
Desai A, Caltagirone G, Sari S, Pocaterra D, Kogan M, Azzolini E, Savevski V, Martinelli-Boneschi F, Voza A. The Use of Antiviral Agents against SARS-CoV-2: Ineffective or Time and Age Dependent Result? A Retrospective, Observational Study among COVID-19 Older Adults . Journal of Clinical Medicine. 2021; 10(4):686. https://doi.org/10.3390/jcm10040686
Chicago/Turabian StyleDesai, Antonio, Giuseppe Caltagirone, Sharon Sari, Daria Pocaterra, Maria Kogan, Elena Azzolini, Victor Savevski, Filippo Martinelli-Boneschi, and Antonio Voza. 2021. "The Use of Antiviral Agents against SARS-CoV-2: Ineffective or Time and Age Dependent Result? A Retrospective, Observational Study among COVID-19 Older Adults " Journal of Clinical Medicine 10, no. 4: 686. https://doi.org/10.3390/jcm10040686
APA StyleDesai, A., Caltagirone, G., Sari, S., Pocaterra, D., Kogan, M., Azzolini, E., Savevski, V., Martinelli-Boneschi, F., & Voza, A. (2021). The Use of Antiviral Agents against SARS-CoV-2: Ineffective or Time and Age Dependent Result? A Retrospective, Observational Study among COVID-19 Older Adults . Journal of Clinical Medicine, 10(4), 686. https://doi.org/10.3390/jcm10040686







