Same Day Discharge versus Inpatient Surgery for Robot-Assisted Radical Prostatectomy: A Comparative Study
Abstract
1. Introduction
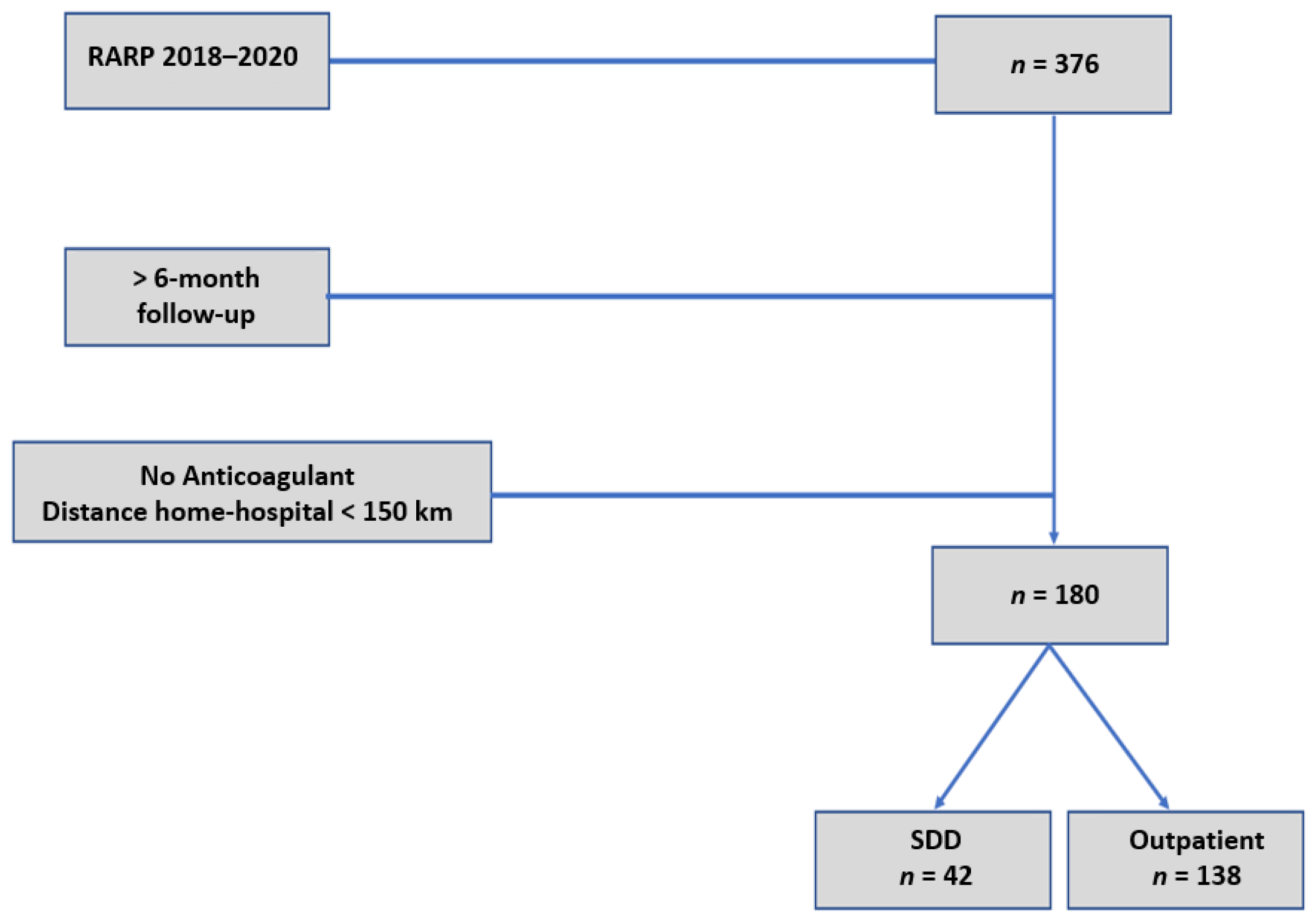
2. Materials and Methods
3. Results
3.1. Overall Cohort (n = 180)
3.2. SDD versus Inpatient RARP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ploussard, G. Robotic surgery in urology: Facts and reality. What are the real advantages of robotic approaches for prostate cancer patients? Curr. Opin. Urol. 2018, 28, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Bergh, R.C.V.D.; Briers, E.; Broeck, T.V.D.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer—2020 update. part 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, G.D.; Yaxley, J.W.; Chambers, S.K.; Occhipinti, S.; Samaratunga, H.; Zajdlewicz, L.; Teloken, P.; Dunglison, N.; Williams, S.; Lavin, M.F.; et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol. 2018, 19, 1051–1060. [Google Scholar] [CrossRef]
- Kehlet, H.; Wilmore, D.W. Multimodal strategies to improve surgical outcome. Am. J. Surg. 2002, 183, 630–641. [Google Scholar] [CrossRef]
- Patel, H.R.; Cerantola, Y.; Valerio, M.; Persson, B.; Jichlinski, P.; Ljungqvist, O.; Hubner, M.; Kassouf, W.; Müller, S.; Baldini, G.; et al. Enhanced recovery after surgery: Are we ready, and can we afford not to imple-ment these pathways for patients undergoing radical cystectomy? Eur. Urol. 2014, 65, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Azhar, R.A.; Bochner, B.; Catto, J.; Goh, A.C.; Kelly, J.; Patel, H.D.; Pruthi, R.S.; Thalmann, G.N.; Desai, M. Enhanced recovery after urological surgery: A contemporary systematic review of outcomes, key elements, and research needs. Eur. Urol. 2016, 70, 176–187. [Google Scholar] [CrossRef]
- Sugi, M.; Matsuda, T.; Yoshida, T.; Taniguchi, H.; Mishima, T.; Yanishi, M.; Komai, Y.; Yasuda, K.; Kinoshita, H.; Yoshida, K.; et al. Introduction of an enhanced recovery after surgery protocol for robot-assisted lap-aroscopic radical prostatectomy. Urol. Int. 2017, 99, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wan, F.; Lu, Y.; Li, G.; Yu, L.; Wang, M. Enhanced recovery after surgery protocol for prostate cancer patients undergoing laparoscopic radical prostatectomy. J. Int. Med. Res. 2018, 47, 114–121. [Google Scholar] [CrossRef]
- Ploussard, G.; Almeras, C.; Beauval, J.; Gautier, J.; Garnault, V.; Frémont, N.; Dallemagne, S.; Loison, G.; Salin, A.; Tollon, C. A combination of enhanced recovery after surgery and prehabilitation pathways improves perioperative outcomes and costs for robotic radical prostatectomy. Cancer 2020, 126, 4148–4155. [Google Scholar] [CrossRef]
- Berger, A.K.; Chopra, S.; Desai, M.M.; Aron, M.; Gill, I.S. Outpatient robotic radical prostatectomy: Matched-pair comparison with inpatient surgery. J. Endourol. 2016, 30 (Suppl. 1), S52–S56. [Google Scholar] [CrossRef]
- Martin, A.D.; Nunez, R.N.; Andrews, J.R.; Martin, G.L.; Andrews, P.E.; Castle, E.P. Outpatient prostatectomy: Too much too soon or just what the patient ordered. Urology 2010, 75, 421–424. [Google Scholar] [CrossRef]
- Banapour, P.; Elliott, P.; Jabaji, R.; Parekh, A.; Pathak, A.; Merchant, M.; Tamaddon, K. Safety and feasibility of outpatient patient robot-assisted radical prostatectomy. J. Robot. Surg. 2019, 13, 261–265. [Google Scholar] [CrossRef]
- Ploussard, G.; Almeras, C.; Beauval, J.-B.; Gautier, J.-R.; Loison, G.; Salin, A.; Tollon, C. Same-day discharge surgery for robot-assisted radical prostatectomy in the era of ERAS and prehabilitation pathways: A contemporary, comparative, feasibility study. World J. Urol. 2020, 1–7. [Google Scholar] [CrossRef]
- Abaza, R.; Martinez, O.; Ferroni, M.C.; Bsatee, A.; Gerhard, R.S. Same day discharge after robotic radical prostatectomy. J. Urol. 2019, 202, 959–963. [Google Scholar] [CrossRef]
- Ploussard, G.; Dumonceau, O.; Thomas, L.; Benamran, D.; Parra, J.; Vaessen, C.; Skowron, O.; Rouprêt, M.; Leclers, F. Multi-institutional assessment of routine same day discharge surgery for robot-assisted radical prostatectomy. J. Urol. 2020, 204, 956–961. [Google Scholar] [CrossRef]
- Khalil, M.I.; Bhandari, N.R.; Payakachat, N.; Davis, R.; Raheem, O.A.; Kamel, M.H. Perioperative mortality and morbidity of outpa-tient versus inpatient robot-assisted radical prostatectomy: A propensity matched analysis. Urol. Oncol. 2020, 38, 3. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, M.C.; Abaza, R. Feasibility of robot-assisted prostatectomy performed at ultra-low pneumoperitoneum pressure of 6 mmHg and comparison of clinical outcomes vs standard pressure of 15 mmHg. BJU Int. 2019, 124, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Shahait, M.; Yezdani, M.; Katz, B.; Lee, A.; Yu, S.-J.; Lee, D.I. Robot-assisted transversus abdominis plane block: Description of the technique and comparative analysis. J. Endourol. 2019, 33, 207–210. [Google Scholar] [CrossRef]
- Dobbs, R.W.; Nguyen, T.T.; Shahait, M.; Lee, D.J.; Kim, J.L.; El-Fahmawi, A.; Lee, D.I. Outpatient robot-assisted radical prostatecto-my: Are patients ready for same-day discharge? J. Endourol. 2020, 34, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Congnard, D.; Vincendeau, S.; Lahjaouzi, A.; Neau, A.-C.; Chaize, C.; Estèbe, J.-P.; Mathieu, R.; Beloeil, H. Outpatient robot-assisted radical prostatectomy: A feasibility study. Urology 2019, 128, 16–22. [Google Scholar] [CrossRef]
- Bajpai, R.R.; Razdan, S.; Barack, J.; Sanchez, M.A.; Razdan, S. Ambulatory robot-assisted laparoscopic prostatectomy: Is it ready for prime time? A quality of life analysis. J. Endourol. 2019, 33, 814–822. [Google Scholar] [CrossRef] [PubMed]
| Overall Cohort n = 180 | |
|---|---|
| Mean age, years | 65.7 |
| (median, IQR) | 66.7 (61.5–70.5) |
| Mean BMI, Kg/m2 | 26.3 |
| (median, IQR) | 26.0 (24.1–28.4) |
| ASA score: | |
| 2 | 85 (47.2%) |
| 3 | 1 (0.6%) |
| Mean Charlson comorbidity index | 4.2 |
| median, IQR) | 4.0 (4–5) |
| Year of RARP | |
| 2018–2019 | n = 87 |
| 2019–2020 | n = 93 |
| Mean PSA, ng/mL | 8.4 |
| (median, IQR) | 7.0 (5.3–9.4) |
| Mean prostate volume, mL | 51.4 |
| (median, IQR) | 47.0 (35–61) |
| Mean operative time, min | 137 |
| (median, IQR) | 134 (118–152) |
| Mean blood loss, mL | 233 |
| (median, IQR) | 200 (100–300) |
| Pelvic lymph node dissection | 138 (76.7%) |
| Nerve-sparing surgery | 148 (82.2%) |
| Mean length of stay, days | 1.2 |
| (median, IQR) | 1.0 (0–2) |
| ERAS pathway | 180 (100%) |
| Transfusion | 1 (0.6%) |
| Unplanned visits | 24 (13.3%) |
| Readmission | 17 (9.4%) |
| Clavien–Dindo grade: | |
| 1 | 17 (9.4%) |
| 2 | 22 (12.2%) |
| 3 | 8 (4.4%) |
| Pathological Grade: | |
| 1 | 7 (3.9%) |
| 2–3 | 150 (83.3%) |
| 4–5 | 23 (12.8%) |
| pT stage: | |
| pT2 | 92 (51.1%) |
| pT3a | 75 (41.7%) |
| pT3b | 13 (7.2%) |
| Positive surgical margins | 57 (31.7%) |
| pN1 status: | 7 (5.0%) |
| No safety pad at 1 month | 92 (51.1%) |
| No safety pad at 6 month | 146 (81.1%) |
| Mean follow-up, months | 19.2 |
| (median, IQR) | 19.5 (10.6–27.2) |
| SDD n = 42 | Inpatient n = 138 | p Value | |
|---|---|---|---|
| Mean age, years | 65.5 | 65.8 | 0.726 |
| (median, IQR) | 66.1 (60.5–69.6) | 64.3 (62.7–71.9) | |
| Mean BMI, Kg/m2 | 26.1 | 26.4 | 0.521 |
| (median, IQR) | 24 (19.3–28.5) | 25.2 (23.4–29.1) | |
| ASA score: | 0.291 | ||
| 2 | 25 (59.5%) | 60 (43.5%) | |
| 3 | 0 | 1 (0.7%) | |
| Mean Charlson comorbidity index | 4.1 | 4.2 | 0.122 |
| median, IQR) | 3.3 (2.9–6.2) | 3.4 (3.1–6.5) | |
| Year of RARP | 0.916 | ||
| 2018–2019 | 23.0% | - | |
| 2019–2020 | 23.7% | - | |
| Mean PSA, ng/mL | 10.0 | 8.0 | 0.081 |
| (median, IQR) | 8.4 (6.7–15.2) | 7.1 (5.8–12.9) | |
| Mean prostate volume, mL | 49.3 | 52.0 | 0.477 |
| (median, IQR) | 50.1 (42.6–53.7) | 50.3 (47.3–58.6) | |
| Mean operative time, min | 135 | 137 | 0.521 |
| (median, IQR) | 129.6 (125.5–148.3) | 131.2 (128.7–150.6) | |
| Mean blood loss, mL | 230 | 234 | 0.886 |
| (median, IQR) | 227.5 (210.4–250.6) | 230.7 (225.7–264.1) | |
| Pelvic lymph node dissection | 32 (76.2%) | 106 (76.8%) | 0.934 |
| Nerve-sparing surgery | 33 (78.6%) | 115 (83.3%) | 0.480 |
| Mean length of stay, days | 1.6 | <0.001 | |
| (median, IQR) | 1 (1.2–2.3) | ||
| ERAS pathway | 42 (100%) | 138 (100%) | 1.00 |
| Transfusion | 0 | 1 (0.7%) | 0.580 |
| Unplanned visits | 4 (9.5%) | 20 (14.5%) | 0.407 |
| Readmission | 3 (7.1%) | 14 (10.1%) | 0.560 |
| Complication | 8 (19.0%) | 39 (28.3%) | 0.234 |
| Clavien–Dindo grade: | 0.538 | ||
| 1 | 2 (4.8%) | 15 (10.9%) | |
| 2 | 5 (11.9%) | 17 (12.3%) | |
| 3 | 1 (2.4%) | 7 (5.1%) | |
| Pathological Grade: | 0.332 | ||
| 1 | 2 (4.8%) | 5 (3.6%) | |
| 2–3 | 34 (81.0%) | 116 (84.1%) | |
| 4–5 | 6 (14.3%) | 17 (12.3%) | |
| pT stage: | 0.603 | ||
| pT2 | 23 (54.8%) | 69 (50.0%) | |
| pT3a | 15 (35.7%) | 60 (43.5%) | |
| pT3b | 4 (9.5%) | 9 (6.5%) | |
| Positive surgical margins | 31 (26.2%) | 46 (33.3%) | 0.384 |
| pN1 status: | 1 (3.1%) | 6 (5.6%) | 0.573 |
| No safety pad at 1 month | 23 (54.8%) | 69 (50.0%) | 0.589 |
| No safety pad at 6 month | 35 (83.3%) | 111 (80.4%) | 0.674 |
| Follow-up, months | 18.4 | 19.4 | 0.492 |
| (median, IQR) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahota, R.G.; Salin, A.; Gautier, J.R.; Almeras, C.; Loison, G.; Tollon, C.; Beauval, J.B.; Ploussard, G. Same Day Discharge versus Inpatient Surgery for Robot-Assisted Radical Prostatectomy: A Comparative Study. J. Clin. Med. 2021, 10, 661. https://doi.org/10.3390/jcm10040661
Rahota RG, Salin A, Gautier JR, Almeras C, Loison G, Tollon C, Beauval JB, Ploussard G. Same Day Discharge versus Inpatient Surgery for Robot-Assisted Radical Prostatectomy: A Comparative Study. Journal of Clinical Medicine. 2021; 10(4):661. https://doi.org/10.3390/jcm10040661
Chicago/Turabian StyleRahota, Razvan George, Ambroise Salin, Jean Romain Gautier, Christophe Almeras, Guillaume Loison, Christophe Tollon, Jean Baptiste Beauval, and Guillaume Ploussard. 2021. "Same Day Discharge versus Inpatient Surgery for Robot-Assisted Radical Prostatectomy: A Comparative Study" Journal of Clinical Medicine 10, no. 4: 661. https://doi.org/10.3390/jcm10040661
APA StyleRahota, R. G., Salin, A., Gautier, J. R., Almeras, C., Loison, G., Tollon, C., Beauval, J. B., & Ploussard, G. (2021). Same Day Discharge versus Inpatient Surgery for Robot-Assisted Radical Prostatectomy: A Comparative Study. Journal of Clinical Medicine, 10(4), 661. https://doi.org/10.3390/jcm10040661







