Metabolic Imaging in Non-Alcoholic Fatty Liver Disease: Applications of Magnetic Resonance Spectroscopy
Abstract
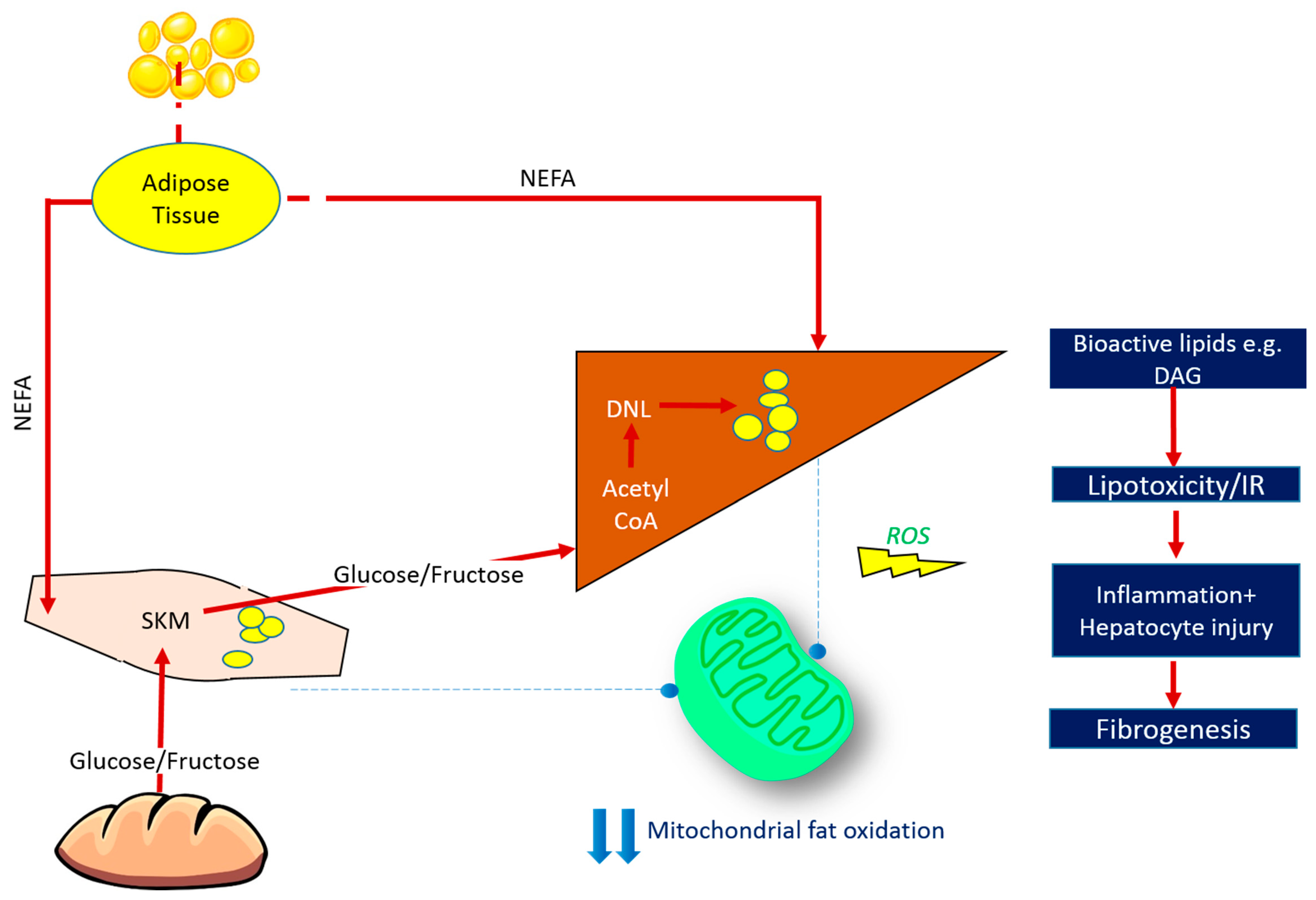
:1. Introduction
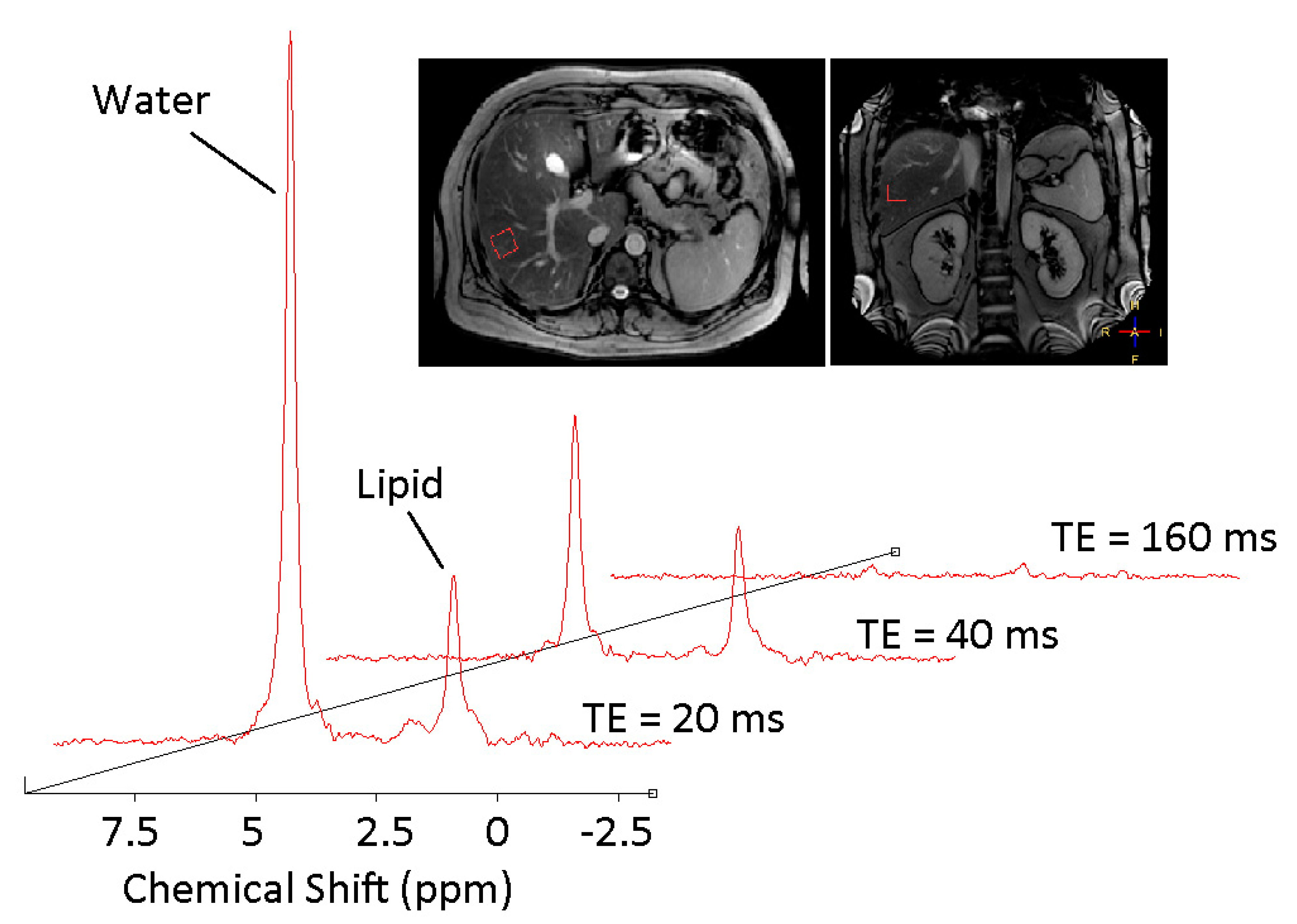
2. Principles of MR Spectroscopy
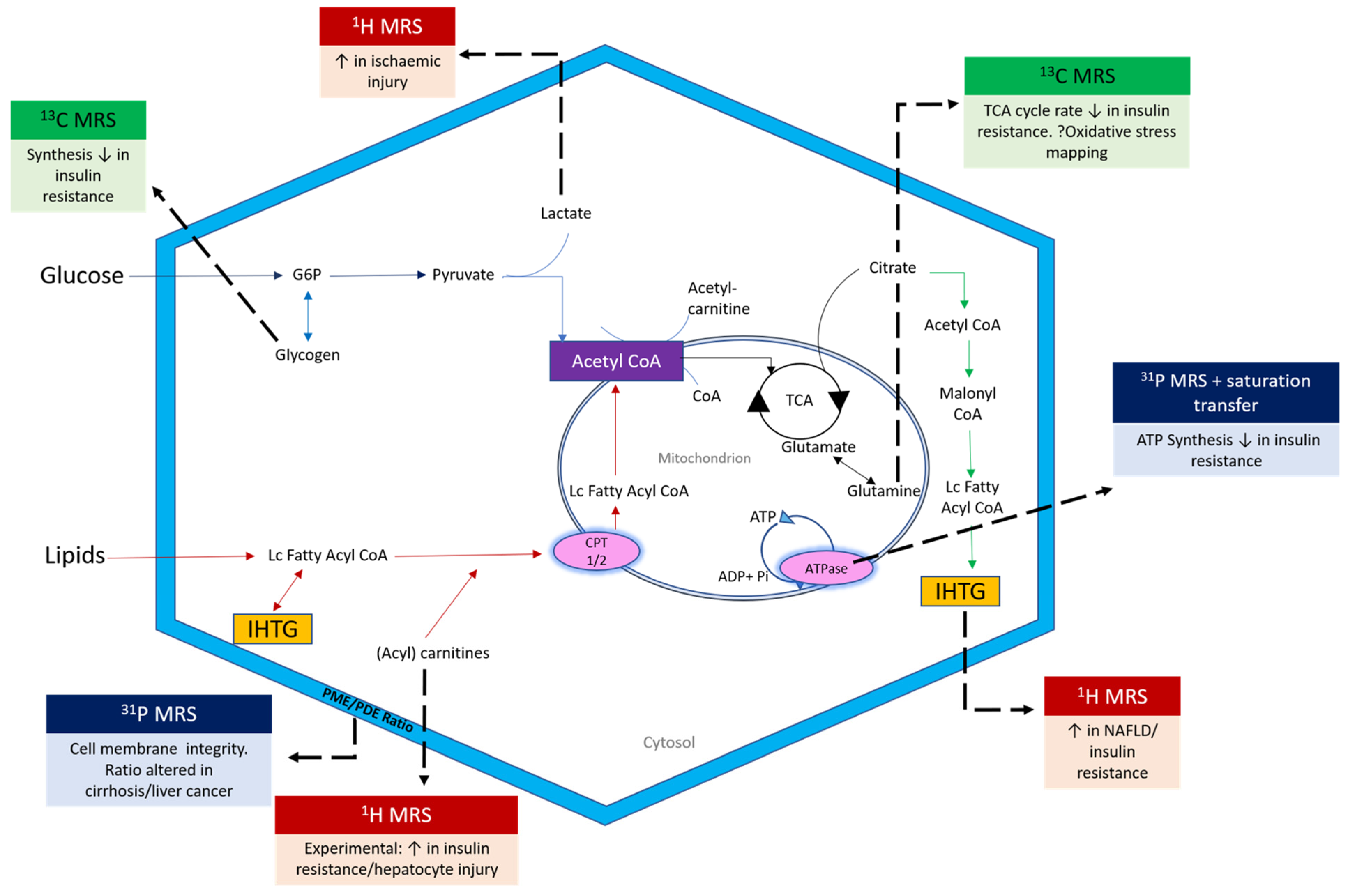
3. Applications of MRS in Metabolic Liver Disease
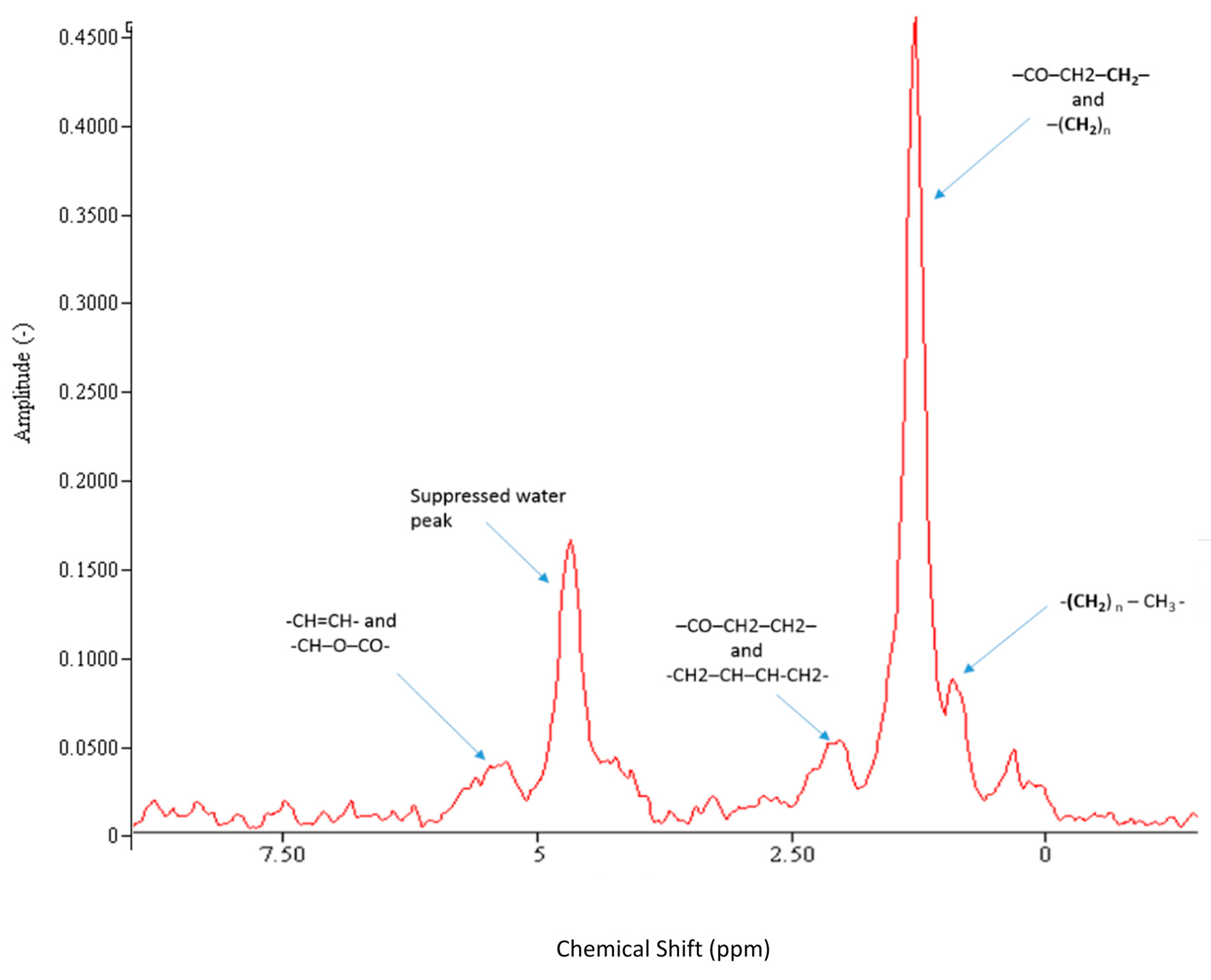
3.1. 1H MRS
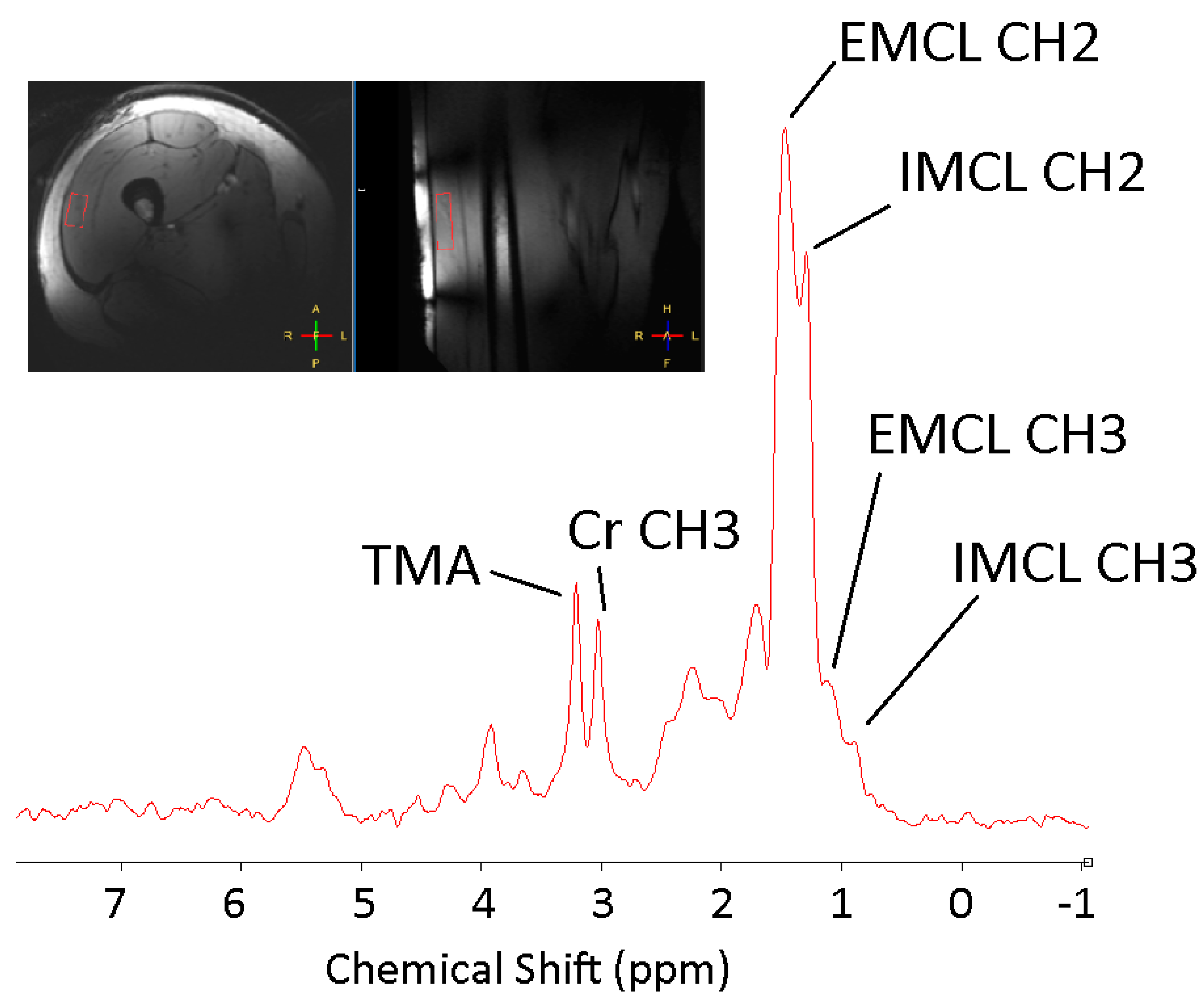
3.1.1. Probing the Liver-Muscle Axis
3.1.2. Evaluating Response to Intervention
3.2. 13C MR Spectroscopy
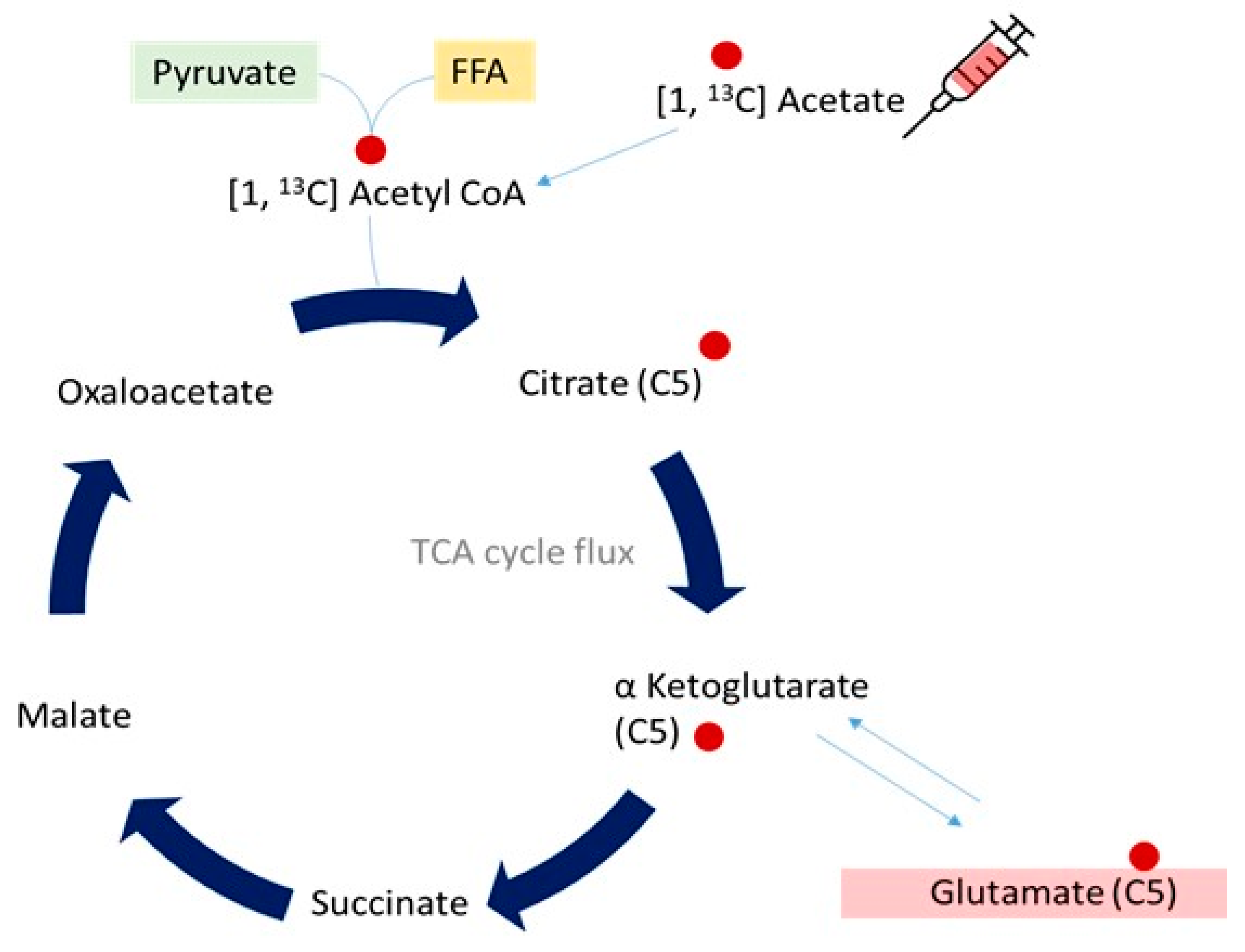
3.2.1. Mitochondrial Oxidative Metabolism
3.2.2. Oxidative Stress
3.3. 31P MRS
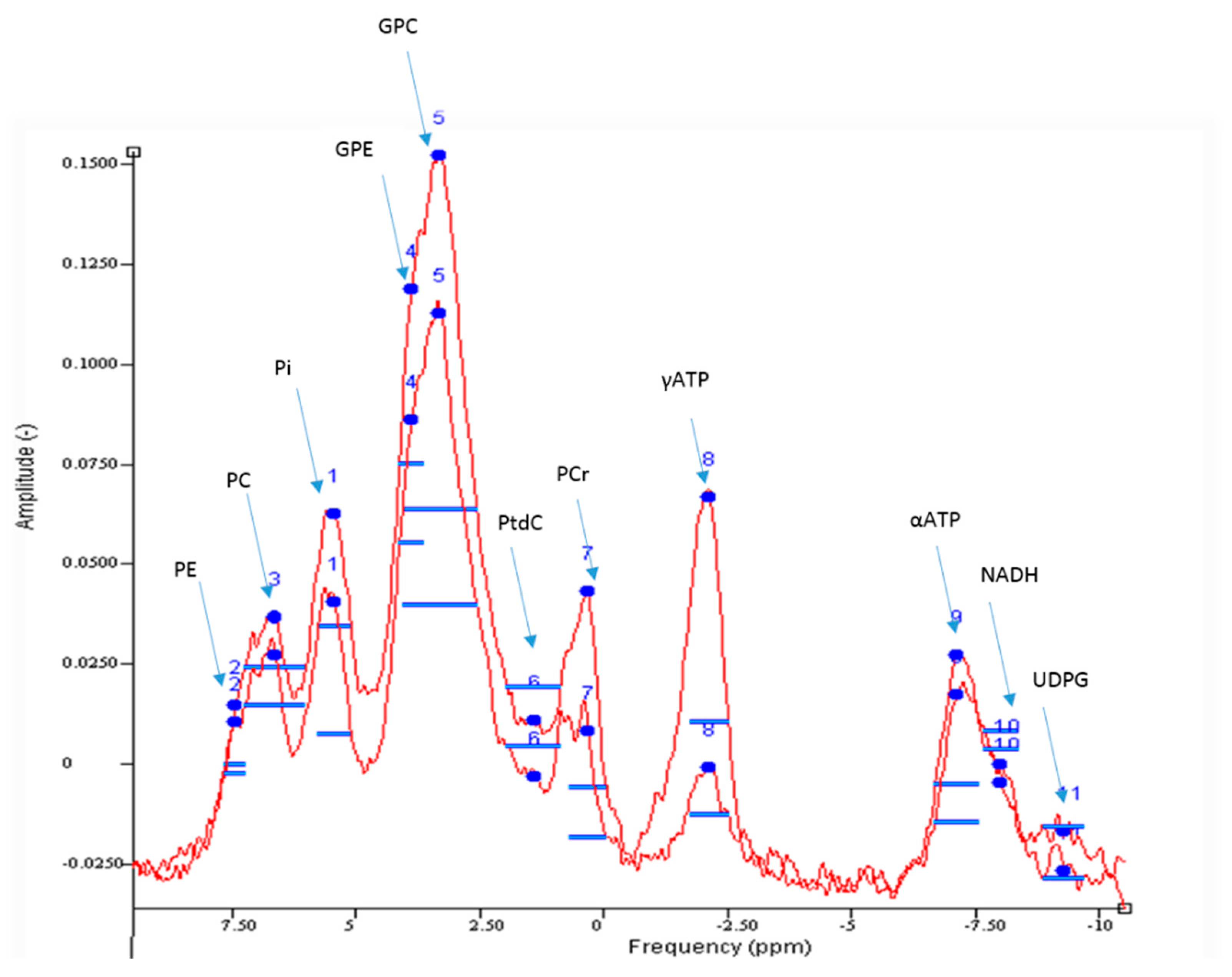
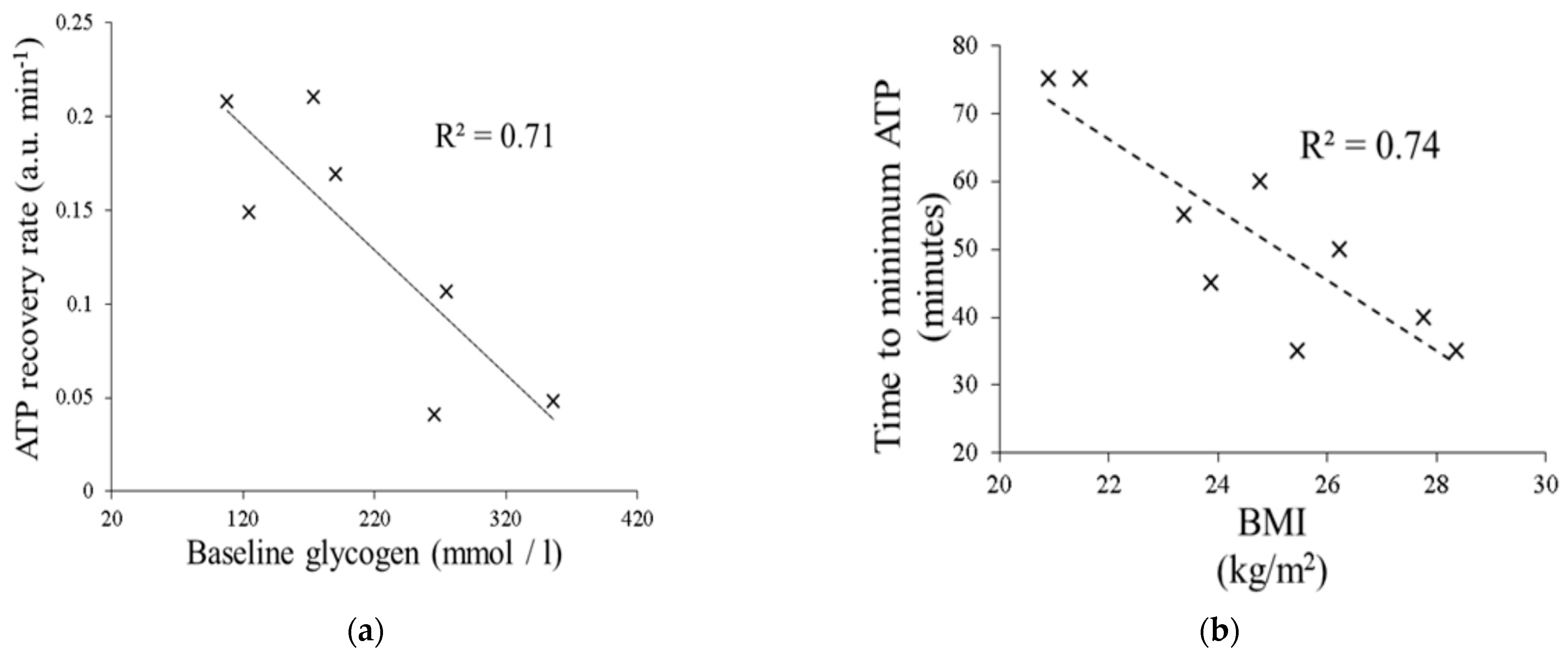
3.3.1. Hepatic ATP Reserves
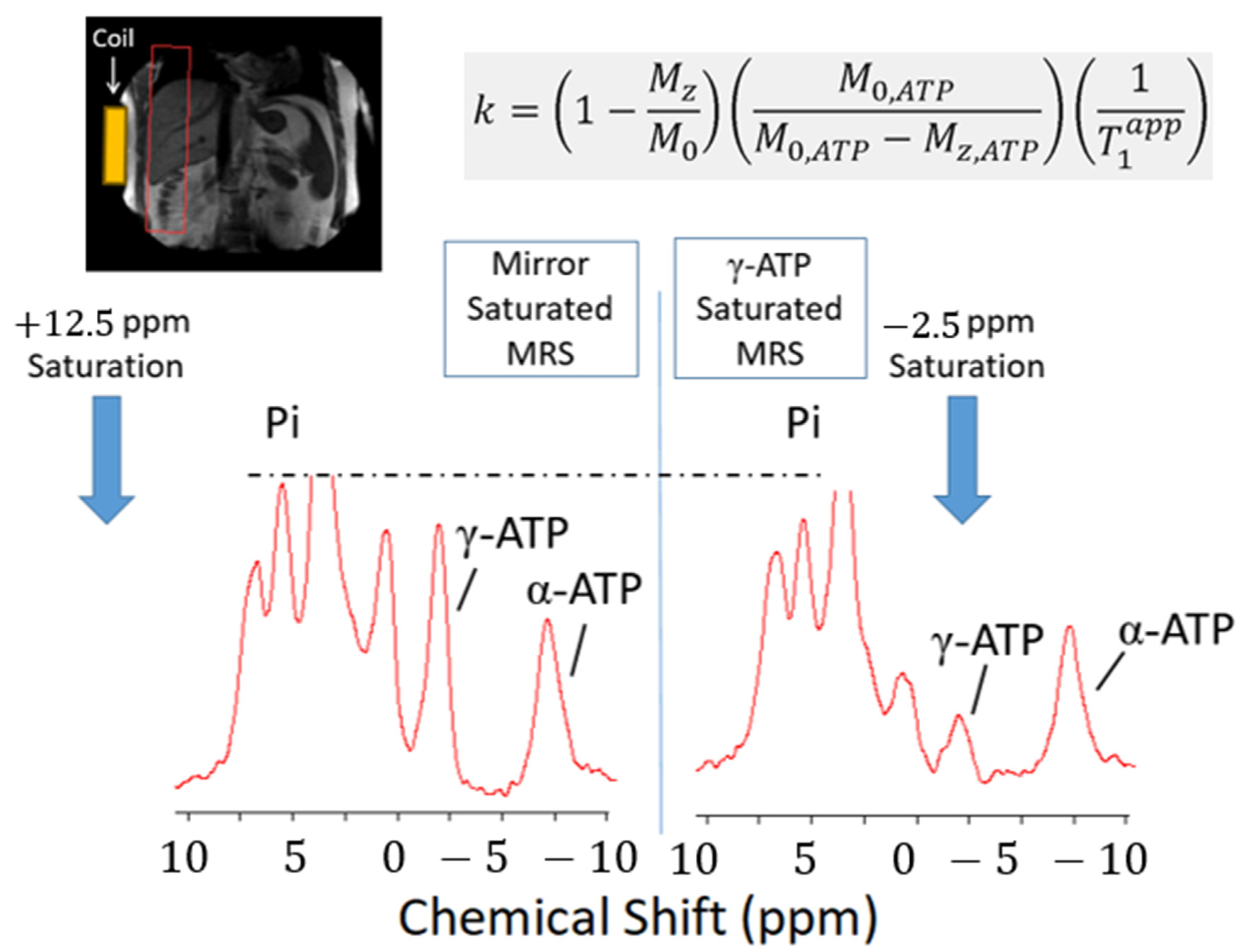
3.3.2. Saturation Transfer Experiment
3.3.3. Other Uses of 31P MRS in the Non-Invasive Stratification of NAFLD
Limitations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [Green Version]
- Gastaldelli, A.; Cusi, K. From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options. JHEP Rep. 2019, 1, 312–328. [Google Scholar] [CrossRef] [Green Version]
- Petersen, M.C.; Shulman, G.I. Roles of Diacylglycerols and Ceramides in Hepatic Insulin Resistance. Trends Pharmacol. Sci. 2017, 38, 649–665. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen-Kirberg, M.; Krausch, M.; Knoefel, W.T.; et al. Adaptation of Hepatic Mitochondrial Function in Humans with Non-Alcoholic Fatty Liver Is Lost in Steatohepatitis. Cell Metab. 2015, 21, 739–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, D.M. Patogenia de la hepatopatía grasa no alcohólica primaria Pathogenesis of primary nonalcoholic fatty liver disease. Med. Clin. 2005, 124, 668–677. [Google Scholar] [CrossRef] [Green Version]
- Samuel, V.T.; Petersen, K.F.; Shulman, G.I. Lipid-induced insulin resistance: Unravelling the mechanism. Lancet 2010, 375, 2267–2277. [Google Scholar] [CrossRef] [Green Version]
- Reeder, S.B. Emerging quantitative magnetic resonance imaging biomarkers of hepatic steatosis. Hepatology 2013, 58, 1877–1880. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, M.; Lam, J.; Peterson, M.R.; Middleton, M.; Hamilton, G.; Le, T.-A.; Bettencourt, R.; Changchien, C.; Brenner, D.A.; Sirlin, C.; et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013, 58, 1930–1940. [Google Scholar] [CrossRef]
- Bawden, S.; Scott, R.A.; Aithal, G.P. Current and Future Magnetic Resonance Technologies for Assessing Liver Disease in Clinical and Experimental Medicine. Dig. Dis. 2017, 35, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, L.S.; Babcock, E.E.; Schick, F.; Dobbins, R.L.; Garg, A.; Burns, D.K.; McGarry, J.D.; Stein, D.T. Measurement of intracellular triglyceride stores by H spectroscopy: Validation in vivo. Am. J. Physiol. Metab. 1999, 276, E977–E989. [Google Scholar] [CrossRef]
- Shulman, G.I. Ectopic Fat in Insulin Resistance, Dyslipidemia, and Cardiometabolic Disease. N. Engl. J. Med. 2014, 371, 1131–1141. [Google Scholar] [CrossRef]
- Jornayvaz, F.R.; Samuel, V.T.; Shulman, G.I. The Role of Muscle Insulin Resistance in the Pathogenesis of Atherogenic Dyslipidemia and Nonalcoholic Fatty Liver Disease Associated with the Metabolic Syndrome. Annu. Rev. Nutr. 2010, 30, 273–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, K.F.; Dufour, S.; Savage, D.B.; Bilz, S.; Solomon, G.; Yonemitsu, S.; Cline, G.W.; Befroy, D.E.; Zemany, L.; Kahn, B.B.; et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 12587–12594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhlmann, J.; Neumann-Haefelin, C.; Belz, U.; Kalisch, J.; Juretschke, H.-P.; Stein, M.; Kleinschmidt, E.; Kramer, W.; Herling, A.W. Intramyocellular lipid and insulin resistance: A longitudinal in vivo 1H-spectroscopic study in Zucker diabetic fatty rats. Diabetes 2003, 52, 138–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krssak, M.; Petersen, K.F.; Dresner, A.; DiPietro, L.; Vogel, S.M.; Rothman, D.L.; Shulman, G.I.; Roden, M. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: A 1H NMR spectroscopy study. Diabetologia 1999, 42, 113–116. [Google Scholar] [CrossRef] [Green Version]
- Perseghin, G.; Scifo, P.; De Cobelli, F.; Pagliato, E.; Battezzati, A.; Arcelloni, C.; Vanzulli, A.; Testolin, G.; Pozza, G.; Del Maschio, A.; et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: A 1H-13C nuclear magnetic resonance spectroscopy assessment in off spring of type 2 diabetic parents. Diabetes 1999, 48, 1600–1606. [Google Scholar] [CrossRef]
- Markova, M.; Pivovarova, O.; Hornemann, S.; Sucher, S.; Frahnow, T.; Wegner, K.; Machann, J.; Petzke, K.J.; Hierholzer, J.; Lichtinghagen, R.; et al. Isocaloric Diets High in Animal or Plant Protein Reduce Liver Fat and Inflammation in Individuals With Type 2 Diabetes. Gastroenterology 2017, 152, 571–585.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Errazuriz, I.; Dube, S.; Slama, M.; Visentin, R.; Nayar, S.; O’Connor, H.; Cobelli, C.; Das, S.K.; Basu, A.; Kremers, W.K.; et al. Randomized Controlled Trial of a MUFA or Fiber-Rich Diet on Hepatic Fat in Prediabetes. J. Clin. Endocrinol. Metab. 2017, 102, 1765–1774. [Google Scholar] [CrossRef] [Green Version]
- Browning, J.D.; Baker, J.A.; Rogers, T.; Davis, J.; Satapati, S.; Burgess, S.C. Short-term weight loss and hepatic triglyceride reduction: Evidence of a metabolic advantage with dietary carbohydrate restriction. Am. J. Clin. Nutr. 2011, 93, 1048–1052. [Google Scholar] [CrossRef]
- Lindeboom, L.; Nabuurs, C.I.; Hesselink, M.K.C.; Wildberger, J.E.; Schrauwen, P.; Schrauwen-Hinderling, V.B. Proton magnetic resonance spectroscopy reveals increased hepatic lipid content after a single high-fat meal with no additional modulation by added protein. Am. J. Clin. Nutr. 2014, 101, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Kotronen, A.; Seppänen-Laakso, T.; Westerbacka, J.; Kiviluoto, T.; Arola, J.; Ruskeepää, A.-L.; Oresic, M.; Yki-Järvinen, H. Hepatic Stearoyl-CoA Desaturase (SCD)-1 Activity and Diacylglycerol but Not Ceramide Concentrations Are Increased in the Nonalcoholic Human Fatty Liver. Diabetes 2008, 58, 203–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haus, J.M.; Solomon, T.P.; Kelly, K.R.; Fealy, C.E.; Kullman, E.L.; Scelsi, A.R.; Lu, L.; Pagadala, M.R.; McCullough, A.J.; Flask, C.A.; et al. Improved Hepatic Lipid Composition Following Short-Term Exercise in Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2013, 98, E1181–E1188. [Google Scholar] [CrossRef]
- Roumans, K.H.M.; Lindeboom, L.; Veeraiah, P.; Remie, C.M.E.; Phielix, E.; Havekes, B.; Bruls, Y.M.H.; Brouwers, M.C.G.J.; Ståhlman, M.; Alssema, M.; et al. Hepatic saturated fatty acid fraction is associated with de novo lipogenesis and hepatic insulin resistance. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Krssak, M.; Brehm, A.; Bernroider, E.; Anderwald, C.; Nowotny, P.; Man, C.D.; Cobelli, C.; Cline, G.W.; Shulman, G.I.; Waldhäusl, W.; et al. Alterations in Postprandial Hepatic Glycogen Metabolism in Type 2 Diabetes. Diabetes 2004, 53, 3048–3056. [Google Scholar] [CrossRef] [Green Version]
- Krššák, M. Novel labeling approaches for the assessment of human hepatic metabolism byin vivomagnetic resonance spectroscopy. Hepatology 2014, 59, 2077–2079. [Google Scholar] [CrossRef]
- Tyson, R.L.; Gallagher, C.; Sutherland, G.R. 13C-Labeled substrates and the cerebral metabolic compartmentalization of acetate and lactate. Brain Res. 2003, 992, 43–52. [Google Scholar] [CrossRef]
- Befroy, D.E.; Perry, R.J.; Jain, N.; Dufour, S.; Cline, G.W.; Trimmer, J.K.; Brosnan, J.; Rothman, D.L.; Petersen, K.F.; Shulman, G.I. Direct assessment of hepatic mitochondrial oxidative and anaplerotic fluxes in humans using dynamic 13C magnetic resonance spectroscopy. Nat. Med. 2014, 20, 98–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, K.F.; Befroy, D.E.; Dufour, S.; Rothman, D.L.; Shulman, G.I. Assessment of Hepatic Mitochondrial Oxidation and Pyruvate Cycling in NAFLD by 13C Magnetic Resonance Spectroscopy. Cell Metab. 2016, 24, 167–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skamarauskas, J.T.; Oakley, F.; Smith, F.E.; Bawn, C.; Dunn, M.; Vidler, D.S.; Clemence, M.; Blain, P.G.; Taylor, R.; Gamcsik, M.P.; et al. Noninvasive in vivo magnetic resonance measures of glutathione synthesis in human and rat liver as an oxidative stress biomarker. Hepatology 2014, 59, 2321–2330. [Google Scholar] [CrossRef]
- Puustinen, L.; Hakkarainen, A.; Kivisaari, R.; Boyd, S.; Nieminen, U.; Färkkilä, M.; Lundbom, N.; Arkkila, P. 31Phosphorus mag-netic resonance spectroscopy of the liver for evaluating inflammation and fibrosis in autoimmune hepatitis. Scand. J. Gastroenterol. 2017, 52, 886–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, S.; Chacko, V.P.; Arnold, C.; Diehl, A.M. Hepatic ATP reserve and efficiency of replenishing: Comparison between obese and nonobese normal individuals. Am. J. Gastroenterol. 2003, 98, 466–470. [Google Scholar] [CrossRef]
- Abdelmalek, M.F.; Lazo, M.; Horska, A.; Bonekamp, S.; Lipkin, E.W.; Balasubramanyam, A.; Bantle, J.P.; Johnson, R.J.; Diehl, A.M.; Clark, J.M.; et al. Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology 2012, 56, 952–960. [Google Scholar] [CrossRef] [Green Version]
- Bawden, S.; Stephenson, M.; Ciampi, E.; Hunter, K.; Marciani, L.; Macdonald, I.A.; Aithal, G.P.; Morris, P.; Gowland, P.A. Investigating the effects of an oral fructose challenge on hepatic ATP reserves in healthy volunteers: A 31P MRS study. Clin. Nutr. 2016, 35, 645–649. [Google Scholar] [CrossRef] [Green Version]
- Schmid, A.I.; Szendroedi, J.; Chmelik, M.; Krššák, M.; Moser, E.; Roden, M. Liver ATP Synthesis Is Lower and Relates to Insulin Sensitivity in Patients With Type 2 Diabetes. Diabetes Care 2011, 34, 448–453. [Google Scholar] [CrossRef] [Green Version]
- Valkovič, L.; Gajdošík, M.; Traussnigg, S.; Wolf, P.; Chmelík, M.; Kienbacher, C.; Bogner, W.; Krebs, M.; Trauner, M.; Trattnig, S.; et al. Application of localized 31P MRS saturation transfer at 7 T for measurement of ATP metabolism in the liver: Reproducibility and initial clinical application in patients with non-alcoholic fatty liver disease. Eur. Radiol. 2014, 24, 1602–1609. [Google Scholar] [CrossRef]
- Traussnigg, S.; Kienbacher, C.; Gajdošík, M.; Valkovič, L.; Halilbasic, E.; Stift, J.; Rechling, C.; Hofer, H.; Steindl-Munda, P.; Ferenci, P.; et al. Ultra-high-field magnetic resonance spectroscopy in non-alcoholic fatty liver disease: Novel mechanistic and diagnostic insights of energy metabolism in non-alcoholic steatohepatitis and advanced fibrosis. Liver Int. 2017, 37, 1544–1553. [Google Scholar] [CrossRef] [Green Version]
- Menon, D.K.; Harris, M.; Sargentoni, J.; Taylor-Robinson, S.D.; Cox, I.J.; Morgan, M.Y. In Vivo hepatic 31P magnetic resonance spectroscopy in chronic alcohol abusers. Gastroenterology 1995, 108, 776–788. [Google Scholar] [CrossRef]
- Lim, A.K.; Patel, N.; Hamilton, G.; Hajnal, J.V.; Goldin, R.D.; Taylor-Robinson, S.D. The relationship of in vivo31P MR spectroscopy to histology in chronic hepatitis C. Hepatology 2003, 37, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Norén, B.; Dahlqvist, O.; Lundberg, P.; Almer, S.; Kechagias, S.; Ekstedt, M.; Franzén, L.; Wirell, S.; Smedby, Ö. Separation of advanced from mild fibrosis in diffuse liver disease using 31P magnetic resonance spectroscopy. Eur. J. Radiol. 2008, 66, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Allen, A.M.; Wang, Z.; Prokop, L.J.; Murad, M.H.; Loomba, R. Fibrosis Progression in Nonalcoholic Fatty Liver vs. Nonalcoholic Steatohepatitis: A Systematic Review and Meta-analysis of Paired-Biopsy Studies. Clin. Gastroenterol. Hepatol. 2015, 13, 643–654.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facciorusso, A.; Del Prete, V.; Turco, A.; Buccino, R.V.; Nacchiero, M.C.; Muscatiello, N. Long-term liver stiffness assessment in hepatitis C virus patients undergoing antiviral therapy: Results from a 5-year cohort study. J. Gastroenterol. Hepatol. 2018, 33, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Knop, V.; Mauss, S.; Goeser, T.; Geier, A.; Zimmermann, T.; Herzer, K.; Postel, N.; Friedrich-Rust, M.; Hofmann, W.P.; German Hepatitis C-Registry. Dynamics of liver stiffness by transient elastography in patients with chronic hepatitis C virus infec-tion receiving direct-acting antiviral therapy-Results from the German Hepatitis C-Registry. J. Viral Hepat. 2020, 27, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Abrigo, J.M.; Shen, J.; Wong, V.W.-S.; Yeung, D.K.-W.; Wong, G.L.-H.; Chim, A.M.-L.; Chan, A.W.-H.; Choi, P.C.-L.; Chan, F.K.-L.; Chan, H.L.-Y.; et al. Non-alcoholic fatty liver disease: Spectral patterns observed from an in vivo phosphorus magnetic resonance spectroscopy study. J. Hepatol. 2014, 60, 809–815. [Google Scholar] [CrossRef] [PubMed]
| MRI | MRS | |
|---|---|---|
| Principle Output | Anatomic | Metabolic |
| Use of signal | Spatial position of water and fat | Chemical composition of tissue |
| Region Covered | Structure of multiple tissues | Tissue specific region of interest One (Single voxel spectroscopy) or multiple (chemical shift imaging) voxels |
| Nuclei of interest | 1H signals from fat and water | 1H, 13C, 31P, 23Na, 19F |
| Data Acquisition | Images | 1D/2D Spectra |
| Advantages | Standard equipment used Known sequences Easily translatable visualisations Shorter scan times Multi-organ single-shot images Non-invasive | Organ specific Metabolite concentration Lipid composition analysis Measure dynamic processes Increased sensitivity on fat Non-invasive |
| Limitations | Cost (compared to biological sampling) Precision of fat fraction measurement Only images fat and water | Non-standard equipment required Expertise/training necessary Cost (compared to MRI) |
| Pathogenic Pathway | Biological Process | Precision Imaging Endpoints | Other Endpoints |
|---|---|---|---|
| Metabolic Inflexibility | Insulin Resistance | 13C MRS (↓ postprandial net hepatic glycogen synthesis) | Dual Step Euglycaemic Hyperinsulinaemic Clamp to assess hepatic and peripheral insulin sensitivity Oral glucose tolerance test Wet biomarkers: Adiponectin, HbA1c, HOMA-IR |
| Liver fat Quantity (%) | MRI-PDFF, 1H MRS | TE with CAP, USS | |
| Liver fat Quality | 1H MRS | ||
| Inflammation | Oxidative Stress | 13C MRS (glutathione flux) | |
| Impaired Energy Kinetics | 31P MRS (ATP flux) 13C MRS (TCA cycle flux and β-oxidation) | ||
| Steatohepatitis | Risk Prediction Tools: OxNASH, NASH resolution score Wet biomarkers: ALT | ||
| Collagen deposition | Fibrosis burden | Magnetic Resonance Elastography (MRE) | Wet biomarkers: ELF, ProC3 |
| 31P MRS (PME/PDE ratios) | Risk prediction tools: FIB-4, NFS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiagarajan, P.; Bawden, S.J.; Aithal, G.P. Metabolic Imaging in Non-Alcoholic Fatty Liver Disease: Applications of Magnetic Resonance Spectroscopy. J. Clin. Med. 2021, 10, 632. https://doi.org/10.3390/jcm10040632
Thiagarajan P, Bawden SJ, Aithal GP. Metabolic Imaging in Non-Alcoholic Fatty Liver Disease: Applications of Magnetic Resonance Spectroscopy. Journal of Clinical Medicine. 2021; 10(4):632. https://doi.org/10.3390/jcm10040632
Chicago/Turabian StyleThiagarajan, Prarthana, Stephen J. Bawden, and Guruprasad P. Aithal. 2021. "Metabolic Imaging in Non-Alcoholic Fatty Liver Disease: Applications of Magnetic Resonance Spectroscopy" Journal of Clinical Medicine 10, no. 4: 632. https://doi.org/10.3390/jcm10040632
APA StyleThiagarajan, P., Bawden, S. J., & Aithal, G. P. (2021). Metabolic Imaging in Non-Alcoholic Fatty Liver Disease: Applications of Magnetic Resonance Spectroscopy. Journal of Clinical Medicine, 10(4), 632. https://doi.org/10.3390/jcm10040632







