Endothelial Nitric Oxide Synthase Knockdown in Human Stem Cells Impacts Mitochondrial Biogenesis and Adipogenesis: Live-Cell Real-Time Fluorescence Imaging
Abstract
1. Introduction
2. Experimental Section
2.1. Human Mesenchymal Stem Cell (hMSC)
2.2. Genetic Knockdown of eNOS and Generation of eNOS-Deficient hMSCs
2.3. Western Blot Analysis
2.4. Cell-Free Mitochondria Isolation and Purification
2.5. Mitochondrial DNA (mtDNA) Preparation and Analysis
2.6. Cell Culture and hMSC Adipogenic Differentiation
2.7. RNA Isolation and Real-Time PCR
2.8. Cell Labeling and Co-Culture
2.9. Live-Cell Real-Time Imaging
2.10. Replication of Results
3. Results
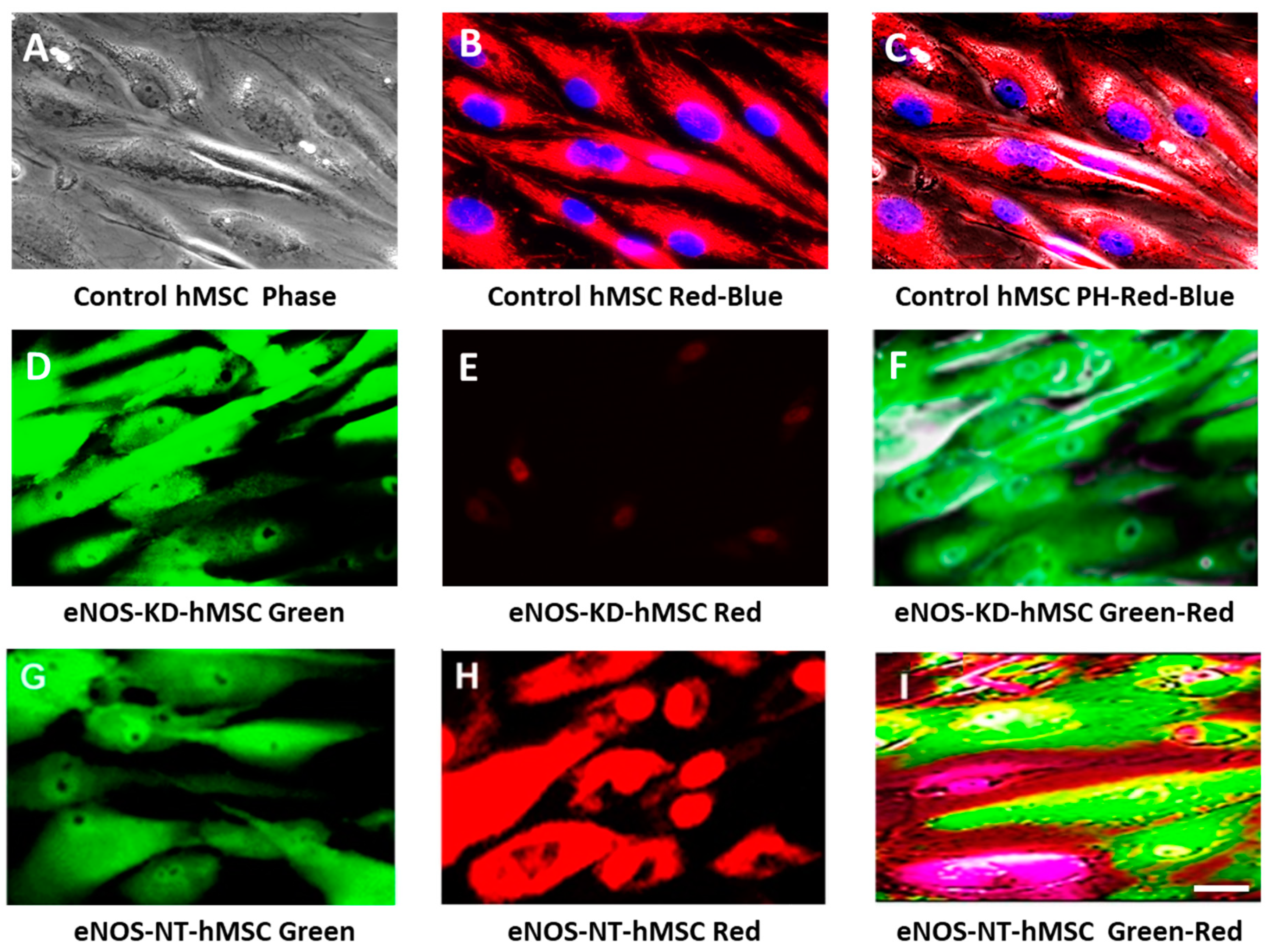
3.1. Confirmation of eNOS Knockdown in hMSCs
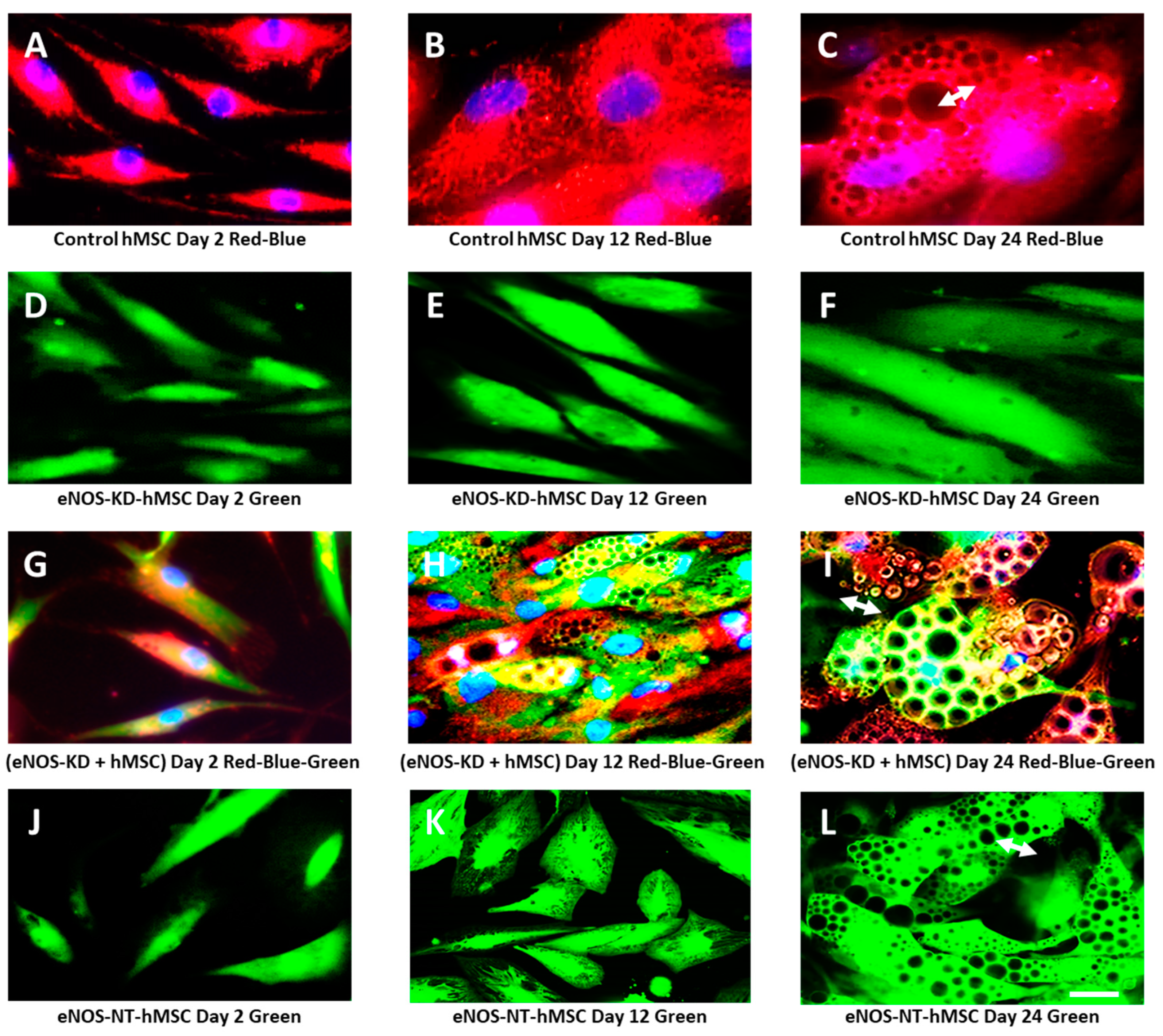
3.2. Live-Cell Real-Time Fluorescence Imaging Showing eNOS KD in hMSCs Impacts Mitochondrial Biogenesis and/or Function
3.3. Confirmation on Impact of eNOS Knockdown on Mitochondrial Biogenesis
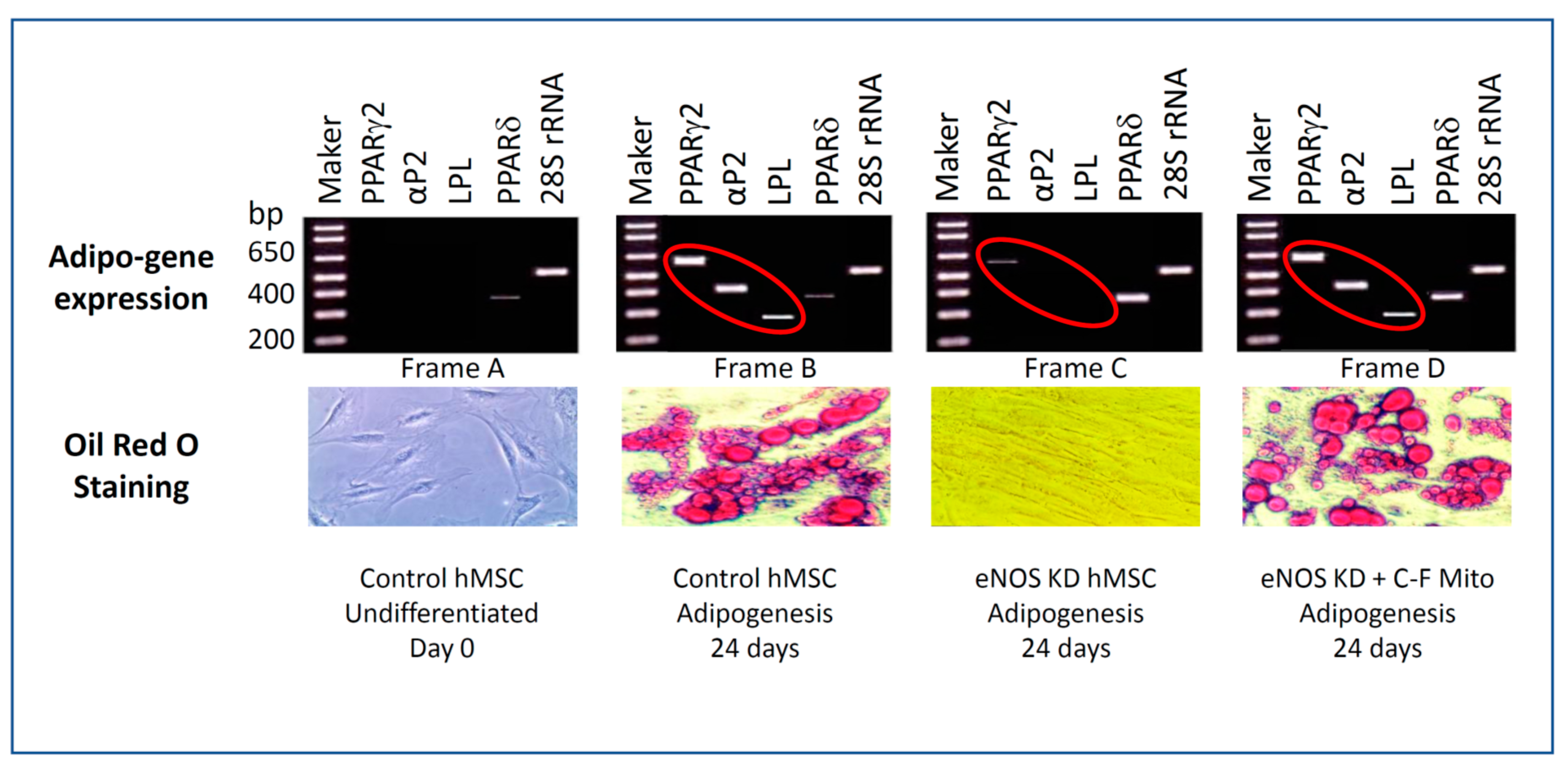
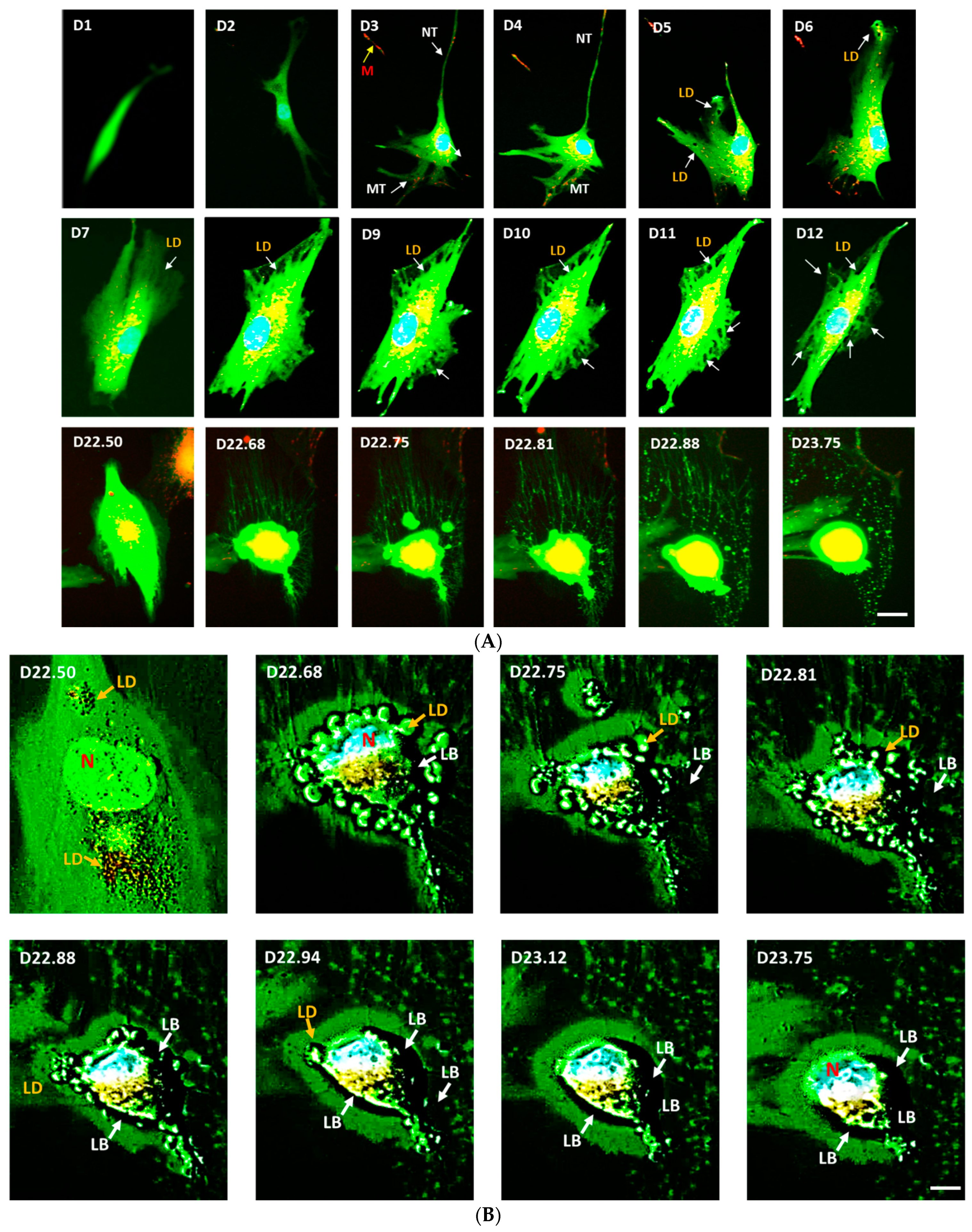
3.4. eNOS KD in hMSCs Impacts Adipogenesis, and Transfer of Healthy Mitochondria from Control hMSCs to eNOS-Deficient hMSCs Restores Adipogenesis
3.5. Confirmation on the Impact of eNOS Knockdown on Adipogenesis
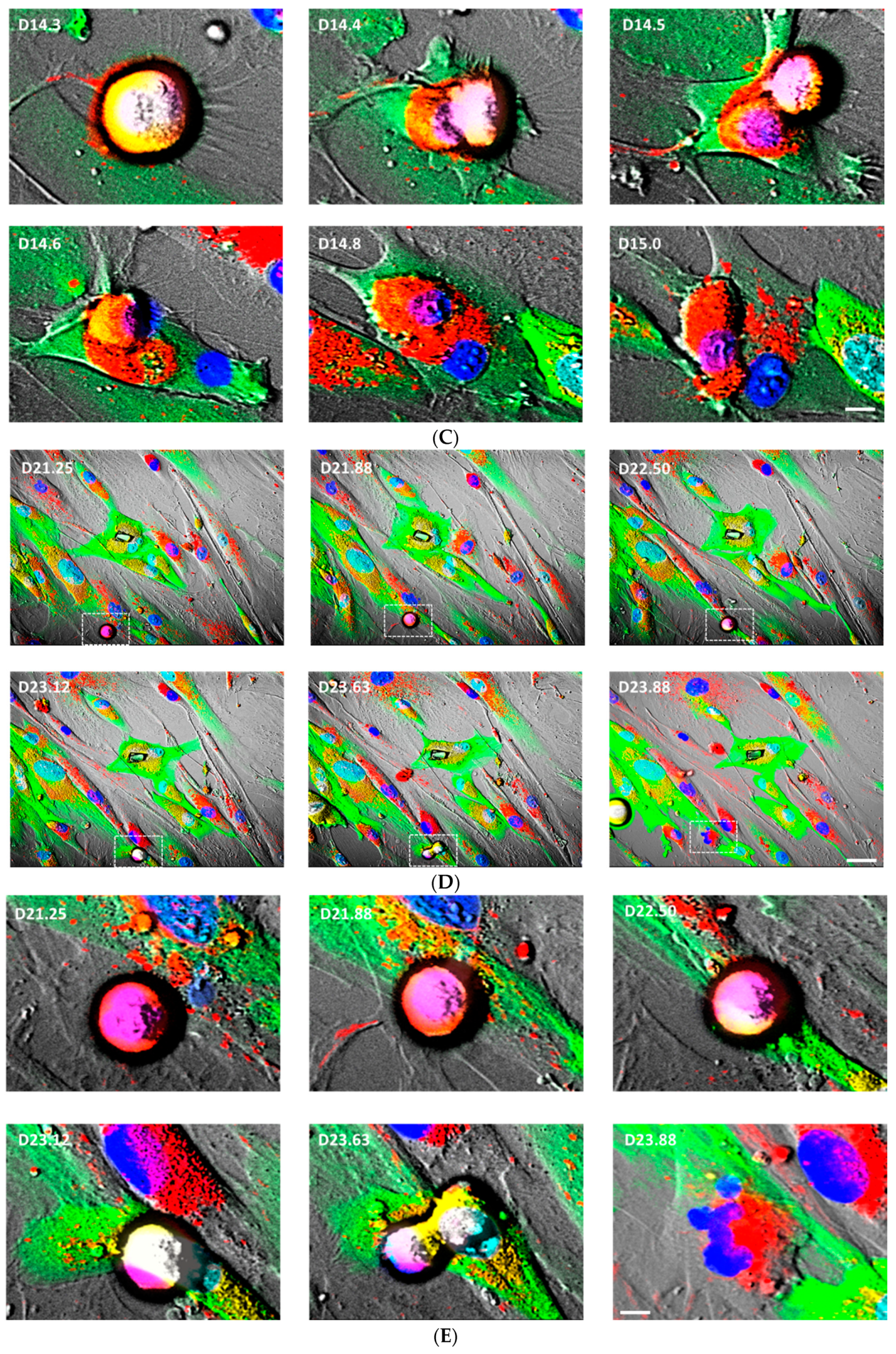
3.6. Mitochondrial Transfer, Adipogenesis Restoration, and Adipocytes Replication
3.7. Cell-Free Mitochondria Purified from Control hMSCs Are Capable of Restoring Adipogenesis in eNOS-Deficient hMSCs
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, P.L. eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol. Metab. 2009, 20, 295–302. [Google Scholar] [CrossRef]
- Atochin, D.N.; Huang, P.L. Endothelial nitric oxide synthase transgenic models of endothelial dysfunction. Pflügers Arch. Eur. J. Physiol. 2010, 460, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, B.; Thibault, H.B.; Raher, M.J.; Popovich, J.R.; Cawley, S.; Atochin, D.N.; Hayton, S.; Shakartzi, H.R.; Huang, P.L.; Bloch, K.D.; et al. Nitric oxide synthase 3 deficiency limits adverse ventricular remodeling after pressure overload in insulin resistance. Am. J. Physiol. Circ. Physiol. 2011, 301, H2093–H2101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- King, A.L.; Polhemus, D.J.; Bhushan, S.; Otsuka, H.; Kondo, K.; Nicholson, C.K.; Bradley, J.M.; Islam, K.N.; Calvert, J.W.; Tao, Y.-X.; et al. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc. Natl. Acad. Sci. USA 2014, 111, 3182–3187. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, A.; Lin, M.I.; Murata, T.; Landskroner-Eiger, S.; Schleicher, M.; Kothiya, M.; Iwakiri, Y.; Yu, J.; Huang, P.L.; Sessa, W.C.; et al. eNOS-derived nitric oxide regulates endothelial barrier function through VE-cadherin and Rho GTPases. J. Cell Sci. 2013, 126, 5541–5552. [Google Scholar] [CrossRef]
- Erusalimsky, J.D.; Moncada, S. Nitric oxide and mitochondrial signaling: From physiology to pathophysiology. Arter. Thromb. Vasc. Biol. 2007, 27, 2524–2531. [Google Scholar] [CrossRef]
- Nisoli, E.; Carruba, M.O. Nitric oxide and mitochondrial biogenesis. J. Cell Sci. 2006, 119, 2855–2862. [Google Scholar] [CrossRef]
- Aquilano, K.; Lettieri-Barbato, D.; Ciriolo, M.R.; Ciriolo, M.R. The multifaceted role of nitric oxide synthases in mitochondrial biogenesis and cell differentiation. Commun. Integr. Biol. 2015, 8, e1017158. [Google Scholar] [CrossRef][Green Version]
- Nisoli, E.; Clementi, E.; Paolucci, C.; Cozzi, V.; Tonello, C.; Sciorati, C.; Bracale, R.; Valerio, A.; Francolini, M.; Moncada, S.; et al. Mitochondrial biogenesis in mammals: The role of endogenous nitric oxide. Science 2003, 299, 896–899. [Google Scholar] [CrossRef]
- Sheldon, R.D.; Meers, G.M.; Morris, E.M.; Linden, M.A.; Cunningham, R.P.; Ibdah, J.A.; Thyfault, J.P.; Laughlin, M.H.; Rector, R.S. eNOS deletion impairs mitochondrial quality control and exacerbates Western diet-induced NASH. Am. J. Physiol. Metab. 2019, 317, E605–E616. [Google Scholar] [CrossRef]
- Huang, P.L.; Lee-Huang, S. Nitric oxide differentially regulates white and brown adipogenesis through effects on thermo-genesis and mitochondrial biogenesis. Circulation 2015, 32, A13115. [Google Scholar]
- Galluzzi, L.; Kepp, O.; Trojel-Hansen, C.; Kroemer, G. Mitochondrial control of cellular life, stress, and death. Circ. Res. 2012, 111, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Supinski, G.S.; Schroder, E.A.; Callahan, L.A. Mitochondria and critical illness. Chest 2020, 157, 310–322. [Google Scholar] [CrossRef]
- Nakamura, Y.; Park, J.-H.; Hayakawa, K. Therapeutic use of extracellular mitochondria in CNS injury and disease. Exp. Neurol. 2020, 324, 113114. [Google Scholar] [CrossRef]
- Roth, K.G.; Mambetsariev, I.; Kulkarni, P.; Salgia, R. The Mitochondrion as an emerging therapeutic target in cancer. Trends Mol. Med. 2020, 26, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, H.; Yao, Y.; Zhao, T.; Chen, Y.-Y.; Shen, Y.-L.; Wang, L.; Zhu, Y. Stem cell-derived mitochondria transplantation: A novel strategy and the challenges for the treatment of tissue injury. Stem Cell Res. Ther. 2018, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Bozi, L.H.; Campos, J.C.; Zambelli, V.O.; Ferreira, N.D.; Ferreira, J.C.B. Mitochondrially-targeted treatment strategies. Mol. Asp. Med. 2020, 71, 100836. [Google Scholar] [CrossRef]
- Vallabhaneni, K.C.; Haller, H.; Dumler, I. Vascular Smooth muscle cells initiate proliferation of mesenchymal stem cells by mitochondrial transfer via tunneling nanotubes. Stem Cells Dev. 2012, 21, 3104–3113. [Google Scholar] [CrossRef]
- Burt, R.; Dey, A.; Aref, S.; Aguiar, M.; Akarca, A.; Bailey, K.; Day, W.; Hooper, S.; Kirkwood, A.; Kirschner, K.; et al. Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood 2019, 134, 1415–1429. [Google Scholar] [CrossRef]
- Qian, X.; Li, X.; Shi, Z.; Bai, X.; Xia, Y.; Zheng, Y.; Xu, D.; Chen, F.; You, Y.; Fang, J.; et al. KDM3A senses oxygen availability to regulate PGC-1α-mediated mitochondrial biogenesis. Mol. Cell 2019, 76, 885–895. [Google Scholar] [CrossRef]
- Mammalian Gene Collection (MGC) Program Team. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc. Natl. Acad. Sci. USA. 2002, 99, 16899–16903. [Google Scholar] [CrossRef] [PubMed]
- Pendergrass, W.; Wolf, N.; Poot, M. Efficacy of MitoTracker Green? and CMXrosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytom. Part A J. Int. Soc. Anal. Cytol. 2004, 61, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Jogdand, P.S.; Singh, S.K.; Christiansen, M.; Dziegiel, M.H.; Singh, S.; Theisen, M. Flow cytometric readout based on Mitotracker Red CMXRos staining of live asexual blood stage malarial parasites reliably assesses antibody dependent cellular inhibition. Malar. J. 2012, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Poot, M.; Zhang, Y.Z.; Krämer, J.A.; Wells, K.S.; Jones, L.J.; Hanzel, D.K.; Lugade, A.G.; Singer, V.L.; Haugland, R.P. Analysis of mitochondrial morphology and function with novel fixable fluorescent stains. J. Histochem. Cytochem. 1996, 44, 1363–1372. [Google Scholar] [CrossRef]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Chen, X.-Y.; Zhang, Z.; Kang, Y.; Liao, W.-M.; Yu, W.-H.; Xiang, A.P. Expression patterns of transcription factor PPARγ and C/EBP family members during In Vitro adipogenesis of human bone marrow mesenchymal stem cells. Cell Biol. Int. 2015, 39, 457–465. [Google Scholar] [CrossRef]
- Tang, Q.-Q.; Lane, M.D. Adipogenesis: From stem cell to adipocyte. Annu. Rev. Biochem. 2012, 81, 715–736. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.; Liu, J.; Wang, J.; Wu, Z.; Zhang, X.; Zhai, Y. Mature adipocytes observed to undergo reproliferation and polyploidy. FEBS Open Bio 2017, 7, 652–658. [Google Scholar] [CrossRef]


| Gene | Primer Sequence (Sense/Antisense) | Product (bp) | Gene ID |
|---|---|---|---|
| Marker | 1 kb plus DNA ladder with 200, 300, 400, 500, 650, 850, 1000 bp | 200–1000 | |
| PPARγ Adipogenic | 5′-GGATGTCGTGTCTGTGGAGA-3′ 5′-TGAGGAGAGTTACTTGGTCG-3′ | 630 | BC006811 |
| LPL Adipogenic | 5′-GAGATTTCTCTGTATGGCACC-3′ 5′-CTGCAAATGAGACACTTTCTC-3′ | 276 | BC011353 |
| αP2 Adipogenic | 5′-GTACCTGGAAACTTGTCTCC-3′ 5′-GTTCAATGCGAACTTCAGTC-3′ | 418 | BC007538 |
| PPARδ Anti-adipogenic | 5′-GGTGAATGGCCTGCCTCCCTACAA-3′ 5′-CACAGAATGATGGCCGCAATGAAT-3′ | 380 | BC007578 |
| 28S RNA Internal control | 5′-GTGCAGATCTTGGTGGTAGTAGC-3′ 5′-AGAGCCAATCCTTATCCCGAAGTT-3′ | 589 | BC000380 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee-Huang, S.; Huang, P.L.; Huang, P.L. Endothelial Nitric Oxide Synthase Knockdown in Human Stem Cells Impacts Mitochondrial Biogenesis and Adipogenesis: Live-Cell Real-Time Fluorescence Imaging. J. Clin. Med. 2021, 10, 631. https://doi.org/10.3390/jcm10040631
Lee-Huang S, Huang PL, Huang PL. Endothelial Nitric Oxide Synthase Knockdown in Human Stem Cells Impacts Mitochondrial Biogenesis and Adipogenesis: Live-Cell Real-Time Fluorescence Imaging. Journal of Clinical Medicine. 2021; 10(4):631. https://doi.org/10.3390/jcm10040631
Chicago/Turabian StyleLee-Huang, Sylvia, Philip Lin Huang, and Paul Lee Huang. 2021. "Endothelial Nitric Oxide Synthase Knockdown in Human Stem Cells Impacts Mitochondrial Biogenesis and Adipogenesis: Live-Cell Real-Time Fluorescence Imaging" Journal of Clinical Medicine 10, no. 4: 631. https://doi.org/10.3390/jcm10040631
APA StyleLee-Huang, S., Huang, P. L., & Huang, P. L. (2021). Endothelial Nitric Oxide Synthase Knockdown in Human Stem Cells Impacts Mitochondrial Biogenesis and Adipogenesis: Live-Cell Real-Time Fluorescence Imaging. Journal of Clinical Medicine, 10(4), 631. https://doi.org/10.3390/jcm10040631






