Trough Concentrations of Specific Antibodies in Primary Immunodeficiency Patients Receiving Intravenous Immunoglobulin Replacement Therapy
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Sample Collection and Handling
2.3. Determination of Specific Antibodies Levels
2.4. Statistics
3. Results
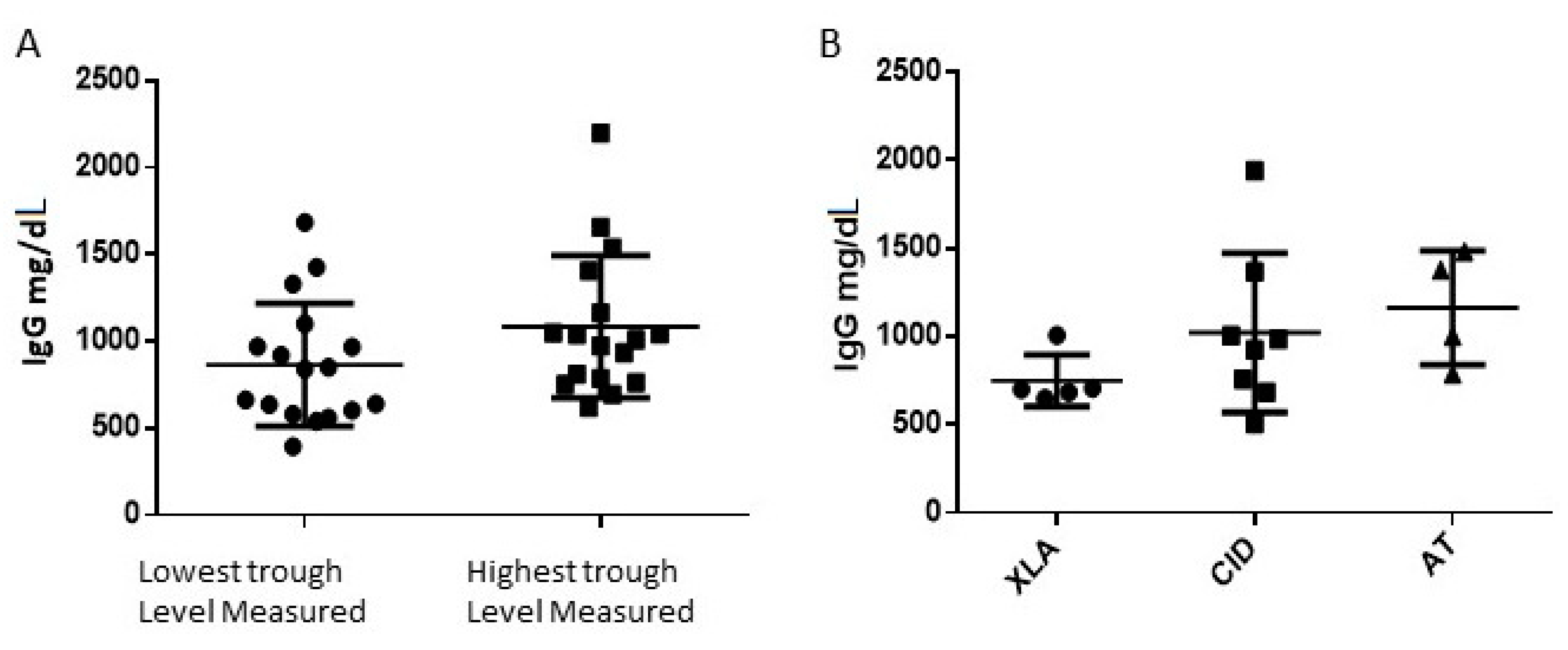
3.1. Patients Epidemiologic Data and Total IgG Levels
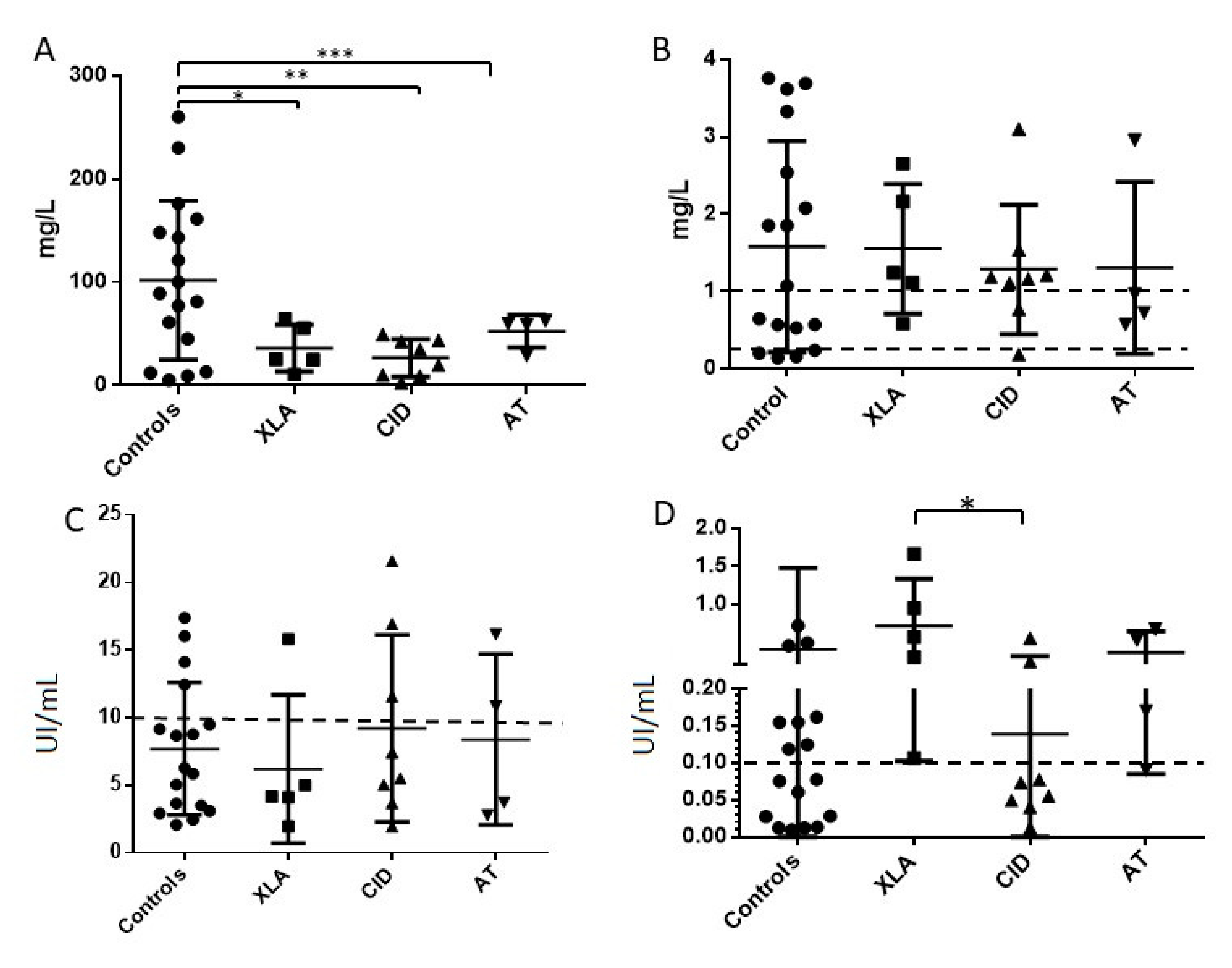
3.2. Specific Antibodies Trough Levels in the Entire Group of Patients
3.3. Specific Antibodies Trough Levels by Diagnosis of Primary Immunodeficiency
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perez, E.E.; Orange, J.S.; Bonilla, F.; Chinen, J.; Chinn, I.K.; Dorsey, M.; El-Gamal, Y.; Harville, T.O.; Hossny, E.; Mazer, B.; et al. Update on the use of immunoglobulin in human disease: A review of evidence. J. Allergy Clin. Immunol. 2017, 139, S1–S46. [Google Scholar] [CrossRef] [PubMed]
- Minegishi, Y.; Saito, M.; Tsuchiya, S.; Tsuge, I.; Takada, H.; Hara, T.; Kawamura, N.; Ariga, T.; Pasic, S.; Stojkovic, O.; et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 2007, 448, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.P.; Woodbine, L.; Gilmour, K.C.; Bibi, S.; Cale, C.M.; Amrolia, P.J.; Veys, P.A.; Davies, E.G.; Jeggo, P.A.; Jones, A. The many faces of Artemis-deficient combined immunodeficiency: Two patients with DCLRE1C mutations and a systematic literature review of genotype-phenotype correlation. Clin. Immunol. 2013, 149, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Nahum, A.; Somech, R.; Shubinsky, G.; Levy, J.; Broides, A. Unusual phenotype in patients with a hypomorphic mutation in the DCLRE1C gene: IgG hypergammaglobulinemia with IgA and IgE deficiency. Clin. Immunol. 2020, 213, 108366. [Google Scholar] [CrossRef] [PubMed]
- Nahum, A.; Sharfe, N.; Broides, A.; Dadi, H.; Naghdi, Z.; Mandola, A.B.; Vong, L.; Arbiv, A.; Dalal, I.; Brami, I.; et al. Defining the biological responses of IL-6 by the study of a novel IL-6 receptor chain immunodeficiency. J. Allergy Clin. Immunol. 2020, 145, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Amirifar, P.; Ranjouri, M.R.; Yazdani, R.; Abolhassani, H.; Aghamohammadi, A. Ataxia-telangiectasia: A review of clinical features and molecular pathology. Pediatr. Allergy Immunol. 2019, 30, 277–288. [Google Scholar] [CrossRef] [PubMed]
- FDA/CBER. Guidance for Industry: Safety, Efficacy, and Pharmacokinetic Studies to Support Marketing of Immune Globulin Intravenous (Human) as Replacement Therapy for Primary Humoral Immunodeficiency. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/safety-efficacy-and-pharmacokinetic-studies-support-marketing-immune-globulin-intravenous-human (accessed on 2 February 2021).
- Mikolajczyk, M.G.; Concepcion, N.F.; Wang, T.; Frazier, D.; Golding, B.; Frasch, C.E.; Scott, D.E. Characterization of antibodies to capsular polysaccharide antigens of Haemophilus influenzae type b and Streptococcus pneumoniae in human immune globulin intravenous preparations. Clin. Diagn. Lab. Immunol. 2004, 11, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, H.W.; Kim, K.H. Antibodies against Hepatitis A and Hepatitis B Virus in Intravenous Immunoglobulin Products. J. Korean Med. Sci. 2016, 31, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Kaplan, M.; Nelson, M. Enterovirus-specific IgG in intravenous immunoglobulin preparations. Ann. Allergy Asthma. Immunol. 2011, 106, 544–545. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, H.W.; Kim, K.H. Functional antibodies to Haemophilus influenzae type b, Neisseria meningitidis, and Streptococcus pneumoniae contained in intravenous immunoglobulin products. Transfusion 2017, 57, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Tuerlinckx, D.; Florkin, B.; Ferster, A.; De Schutter, I.; Chantrain, C.; Haerynck, F.; Philippet, P.; Strengers, P.; Laub, R. Pneumococcal antibody levels in children with PID receiving immunoglobulin. Pediatrics 2014, 133, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, A.P.; Leiva, L.E.; Caruthers, C.; Rodrigues, J.; Sorensen, R.U. Streptococcus pneumoniae antibody titres in patients with primary antibody deficiency receiving intravenous immunoglobulin (IVIG) compared to subcutaneous immunoglobulin (SCIG). Clin. Exp. Immunol. 2015, 182, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Costa-Carvalho, B.T.; Sullivan, K.E.; Fontes, P.M.; Aimé-Nobre, F.; Gonzales, I.G.S.; Lima, E.S.; Granato, C.; de Moraes-Pinto, M.I. Low Rates of Poliovirus Antibodies in Primary Immunodeficiency Patients on Regular Intravenous Immunoglobulin Treatment. J. Clin. Immunol. 2018, 38, 628–634. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Haemophilus influenzae type b (Hib) Vaccination Position Paper—July 2013: Introduction. Wkly. Epidemiol. Rec. Relev. Épidémiol. Hebd. 2013, 88, 413–426. [Google Scholar]
- LaFon, D.C.; Nahm, M.H. Measuring quantity and function of pneumococcal antibodies in immunoglobulin products. Transfusion 2018, 58, 3114–3120. [Google Scholar] [CrossRef] [PubMed]
- Janssen, W.J.; Bloem, A.C.; Vellekoop, P.; Driessen, G.J.; Boes, M.; van Montfrans, J.M. Measurement of pneumococcal polysaccharide vaccine responses for immunodeficiency diagnostics: Combined IgG responses compared to serotype specific IgG responses. J. Clin. Immunol. 2014, 34, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Misin, A.; Antonello, R.M.; Di Bella, S.; Campisciano, G.; Zanotta, N.; Giacobbe, D.R.; Comar, M.; Luzzati, R. Measles: An Overview of a Re-Emerging Disease in Children and Immunocompromised Patients. Microorganisms 2020, 8, 276. [Google Scholar] [CrossRef] [PubMed]
- Davidkin, I.; Jokinen, S.; Broman, M.; Leinikki, P.; Peltola, H. Persistence of Measles, Mumps, and Rubella Antibodies in an MMR-Vaccinated Cohort: A 20-Year Follow-up. J. Infect. Dis. 2008, 197, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Waaijenborg, S.; Hahné, S.J.M.; Mollema, L.; Smits, G.P.; Berbers, G.A.M.; van der Klis, F.R.M.; de Melker, H.E.; Wallinga, J. Waning of Maternal Antibodies Against Measles, Mumps, Rubella, and Varicella in Communities With Contrasting Vaccination Coverage. J. Infect. Dis. 2013, 208, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.G.; Levin, M.J.; Ljungman, P.; Davies, E.G.; Avery, R.; Tomblyn, M.; Bousvaros, A.; Dhanireddy, S.; Sung, L.; Keyserling, H. Executive Summary: 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host. Clin. Infect. Dis. 2014, 58, 309–318. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | PID Patients | Control Group (Non-PID) |
|---|---|---|
| Number | 17 | 17 |
| Median age (range) | 11.9 (1.1–17.9) | 8.6 (1.1–18) |
| Gender No. (%) | Male = 15 (88.2%) Female = 2 (11.8%) | Male = 12 (70.5%) Female = 5 (29.5%) |
| Ethnicity | Jewish = 3 (17.6%) Bedouin = 14 (82.4%) | Jewish = 1 (5.8%) Bedouin = 16 (94.2%) |
| Groups of PID patients No. (%) | CID = 8 (47%) XLA = 5 (29.4%) AT = 4 (23.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassin, O.; Abu Freih, Y.; Hazan, R.; Lev, A.; Zrihen, K.S.; Somech, R.; Broides, A.; Nahum, A. Trough Concentrations of Specific Antibodies in Primary Immunodeficiency Patients Receiving Intravenous Immunoglobulin Replacement Therapy. J. Clin. Med. 2021, 10, 592. https://doi.org/10.3390/jcm10040592
Hassin O, Abu Freih Y, Hazan R, Lev A, Zrihen KS, Somech R, Broides A, Nahum A. Trough Concentrations of Specific Antibodies in Primary Immunodeficiency Patients Receiving Intravenous Immunoglobulin Replacement Therapy. Journal of Clinical Medicine. 2021; 10(4):592. https://doi.org/10.3390/jcm10040592
Chicago/Turabian StyleHassin, Ori, Yahya Abu Freih, Ran Hazan, Atar Lev, Keren S. Zrihen, Raz Somech, Arnon Broides, and Amit Nahum. 2021. "Trough Concentrations of Specific Antibodies in Primary Immunodeficiency Patients Receiving Intravenous Immunoglobulin Replacement Therapy" Journal of Clinical Medicine 10, no. 4: 592. https://doi.org/10.3390/jcm10040592
APA StyleHassin, O., Abu Freih, Y., Hazan, R., Lev, A., Zrihen, K. S., Somech, R., Broides, A., & Nahum, A. (2021). Trough Concentrations of Specific Antibodies in Primary Immunodeficiency Patients Receiving Intravenous Immunoglobulin Replacement Therapy. Journal of Clinical Medicine, 10(4), 592. https://doi.org/10.3390/jcm10040592





