Small Vessel Disease: Another Component of the Hypertrophic Cardiomyopathy Phenotype Not Necessarily Associated with Fibrosis
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
- (a)
- Autopsy hearts of patients with SCD due to HCM (<40 years);
- (b)
- Explanted native hearts of patients who underwent HT due to end-stage HF-HCM;
- (c)
- Autopsy hearts of patients with post-myocardial infarction chronic IHD who died due to HF.
2.2. Gross Examination
- -
- Total heart weight, paying attention to removal of pericardium, postmortem clots, and cutting the great arteries 2 cm above semilunar valves, venae cavae, and pulmonary veins at their junctions with the atria in case of autopsy.
- -
- Wall thickness of the left ventricle (LV) free wall, IVS and right ventricle (RV) free wall, to assess the subtype of hypertrophy (symmetric or asymmetric), in cross-section, excluding papillary muscles and trabeculae [5,8]; normal heart weight and wall thickness for sex and age were calculated according to Schulz and Giordano [9] for children and to Kitzman et al. [10] for adults.
- -
- Coronary artery in terms of origin, course, in particular the presence of myocardial bridge at the level of left anterior descending artery, and patency with serial transverse cuts.
- -
- Presence and site of grossly evident myocardial scars.
2.3. Histological Analysis
2.4. Statistical Analysis
3. Results
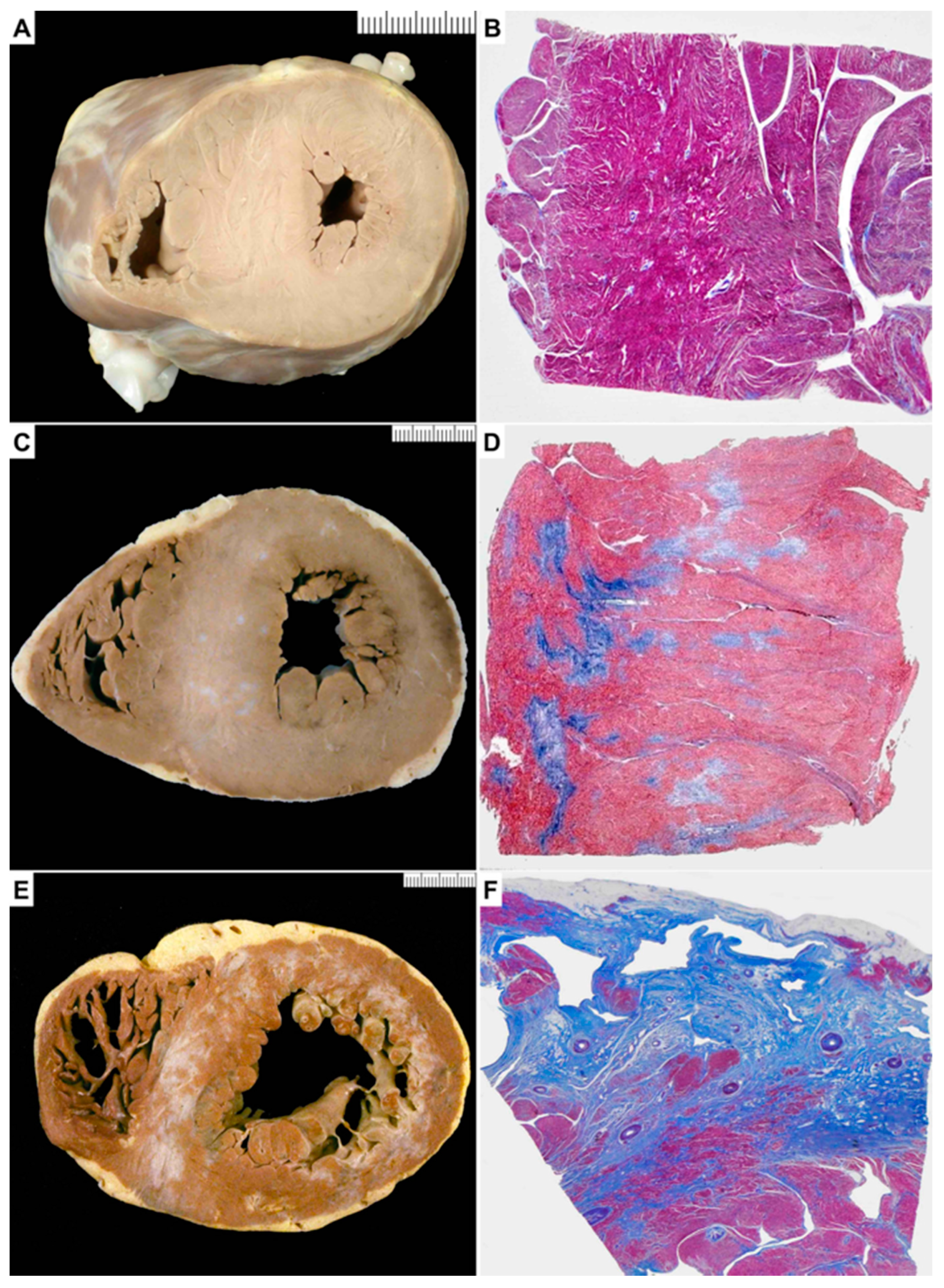
3.1. Gross Examination
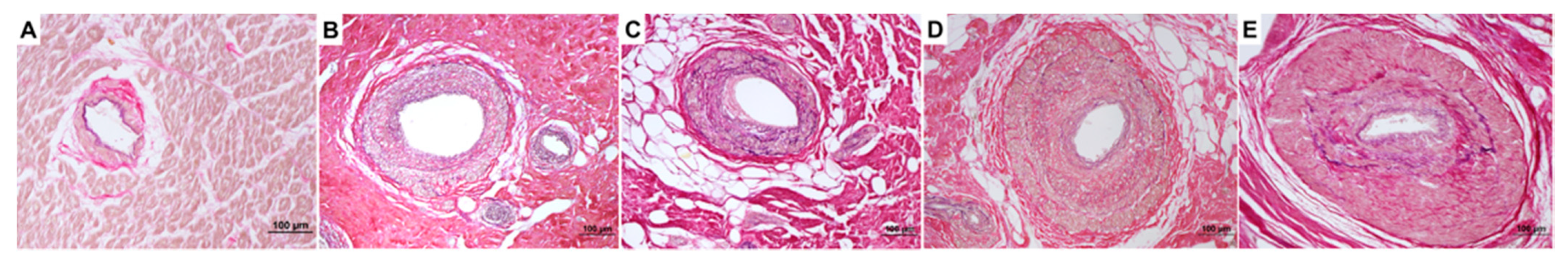
3.2. Histological Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: The task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar]
- Olivotto, I.; d’Amati, G.; Basso, C.; Van Rossum, A.; Patten, M.; Emdin, M.; Pinto, Y.; Tomberli, B.; Camici, P.G.; Michels, M. Defining phenotypes and disease progression in sarcomeric cardiomyopathies: Contemporary role of clinical investigations. Cardiovasc. Res. 2015, 105, 409–423. [Google Scholar] [CrossRef]
- Maron, B.J.; Wolfson, J.K.; Epstein, S.E.; Roberts, W.C. Intramural (“small vessel”) coronary artery disease in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 1986, 8, 545–557. [Google Scholar] [CrossRef]
- Tanaka, M.; Fujiwara, H.; Onodera, T.; Wu, D.J.; Matsuda, M.; Hamashima, Y.; Kawai, C. Quantitative analysis of narrowings of intramyocardial small arteries in normal hearts, hypertensive hearts, and hearts with hypertrophic cardiomyopathy. Circulation 1987, 75, 1130–1139. [Google Scholar] [CrossRef]
- Basso, C.; Thiene, G.; Corrado, D.; Buja, G.; Melacini, P.; Nava, A. Hypertrophic cardiomyopathy and sudden death in the young: Pathologic evidence of myocardial ischemia. Hum. Pathol. 2000, 31, 988–998. [Google Scholar] [CrossRef]
- Foà, A.; Agostini, V.; Rapezzi, C.; Olivotto, I.; Corti, B.; Potena, L.; Biagini, E.; Martin Suarez, S.; Rotellini, M.; Cecchi, F.; et al. Histopathological comparison of intramural coronary artery remodeling and myocardial fibrosis in obstructive versus end-stage hypertrophic cardiomyopathy. Int. J. Cardiol. 2019, 291, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Melacini, P.; Basso, C.; Angelini, A.; Calore, C.; Bobbo, F.; Tokajuk, B.; Bellini, N.; Smaniotto, G.; Zucchetto, M.; Iliceto, S.; et al. Clinicopathological profiles of progressive heart failure in hypertrophic cardiomyopathy. Eur. Heart J. 2010, 31, 2111–2123. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Aguilera, B.; Banner, J.; Cohle, S.; d’Amati, G.; de Gouveia, R.H.; di Gioia, C.; Fabre, A.; Gallagher, P.J.; Leone, O.; et al. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch. 2017, 471, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Schulz, D.M.; Giordano, D.A. Hearts of infants and children: Weights and measurements. Arch. Pathol. 1962, 73, 464–471. [Google Scholar]
- Kitzman, D.W.; Scholtz, D.G.; Hagen, P.T.; Ilstrup, D.M.; Edwards, W.D. Age-related changes in normal human hearts during the first 10 decades of life. Part II (maturity). A qualitative anatomic study of 765 specimens from subjects 20 to 99 years old. Mayo Clin. Proc. 1988, 63, 137–146. [Google Scholar] [CrossRef]
- Maron, M.S. The role of cardiovascular magnetic resonance in sudden death risk stratification in hypertrophic cardiomyopathy. Card. Electrophysiol. Clin. 2015, 7, 187–193. [Google Scholar] [CrossRef]
- Basso, C.; Thiene, G.; Mackey-Bojack, S.; Frigo, A.C.; Corrado, D.; Maron, B.J. Myocardial bridging, a frequent component of the hypertrophic cardiomyopathy phenotype, lacks systematic association with sudden cardiac death. Eur. Heart J. 2009, 30, 1627–1634. [Google Scholar] [CrossRef]
- Cecchi, F.; Olivotto, I.; Gistri, R.; Lorenzoni, R.; Chiriatti, G.; Camici, P.G. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N. Engl. J. Med. 2003, 349, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Krams, R.; Kofflard, M.J.; Duncker, D.J.; Von Birgelen, C.; Carlier, S.; Kliffen, M.; ten Cate, F.J.; Serruys, P.W. Decreased coronary flow reserve in hypertrophic cardiomyopathy is related to remodeling of the coronary microcirculation. Circulation 1998, 97, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Camici, P.G.; Tschöpe, C.; Di Carli, M.F.; Rimoldi, O.; Van Linthout, S. Coronary microvascular dysfunction in hypertrophy and heart failure. Cardiovasc. Res. 2020, 116, 806–816. [Google Scholar] [CrossRef]
- Varnava, A.M.; Elliott, P.M.; Sharma, S.; McKenna, W.J.; Davies, M.J. Hypertrophic cardiomyopathy: The interrelation of disarray, fibrosis, and small vessel disease. Heart 2000, 84, 476–482. [Google Scholar] [CrossRef]
- Galati, G.; Leone, O.; Pasquale, F.; Olivotto, I.; Biagini, E.; Grigioni, F.; Pilato, E.; Lorenzini, M.; Corti, B.; Foà, A.; et al. Histological and Histometric Characterization of Myocardial Fibrosis in End-Stage Hypertrophic Cardiomyopathy: A Clinical-Pathological Study of 30 Explanted Hearts. Circ. Heart Fail. 2016, 9, e003090. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Smedira, N.G.; Rodriguez, E.R.; Tan, C.; Setser, R.; Thamilarasan, M.; Lytle, B.W.; Lever, H.M.; Desai, M.Y. Cardiac magnetic resonance detection of myocardial scarring in hypertrophic cardiomyopathy: Correlation with histopathology and prevalence of ventricular tachycardia. J. Am. Coll. Cardiol. 2009, 54, 242–249. [Google Scholar] [CrossRef]
- Lamkea, G.T.; Allena, R.D.; Edwards, W.; Tazelaar, H.D.; Danielson, G.K. Surgical pathology of subaortic septal myectomy associated with hypertrophic cardiomyopathy: A study of 204 cases (1996–2000). Cardiovasc. Pathol. 2003, 12, 149–158. [Google Scholar] [CrossRef]
- Moravsky, G.; Ofek, E.; Rakowski, H.; Butany, J.; Williams, L.; Ralph- Edwards, A.; Wintersperger, B.J.; Crean, A. Myocardial fibrosis in hypertrophic cardiomyopathy: Accurate reflection of histopathological findings by RMC. JACC Cardiovasc. Imaging 2013, 6, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.; Mörner, S.; Waldenström, A.; Stål, P. Myocardial capillary supply is limited in hypertrophic cardiomyopathy: A morphological analysis. Int. J. Cardiol. 2008, 126, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.R. Hypertrophic cardiomyopathy. Clinical and pathologic correlates. J. Vet. Cardiol. 2005, 5, 39–45. [Google Scholar] [CrossRef]
- Vikstrom, K.L.; Factor, S.M.; Leinwand, L.A. Mice expressing mutant myosin heavy chains are a model for familial hypertrophic cardiomyopathy. Mol. Med. 1996, 2, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Michael, L.H.; Entman, M.L.; Frangogiannis, N.G. Morphological characteristics of the microvasculature in healing myocardial infarcts. J. Histochem. Cytochem. 2002, 50, 71–79. [Google Scholar] [CrossRef]
- Schwartzkopff, B.; Frenzel, H.; Dieckerhoff, J.; Betz, P.; Flasshove, M.; Schulte, H.D.; Mundhenke, M.; Motz, W.; Strauer, B.E. Morphometric investigation of human myocardium in arterial hypertension and valvular aortic stenosis. Eur. Heart J. 1992, 13 (Suppl. D), 17–23. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Cecchi, F.; Poggesi, C.; Yacoub, M.H. Developmental origins of hypertrophic cardiomyopathy phenotypes: A unifying hypothesis. Nat. Rev. Cardiol. 2009, 6, 317–321. [Google Scholar] [CrossRef]


| SCD Group (n = 30) | HF Group (n = 10) | IHD Group (n = 20) | p Value (SCD vs. HF) | |
|---|---|---|---|---|
| Male, n (%) | 25 (83) | 4 (40) | 19 (95) | 0.006 |
| Age, yrs (mean ± SD) | 22.6 ± 7.4 | 51.60 ± 13.21 | 60.6 ± 5.36 | <0.0001 |
| Mean mass, g (mean ± SD) | 538 ± 202.90 | 425.70 ± 96.99 | 446.39 ± 105.43 | 0.10 |
| Thickness of the IVS, mm (mean ± SD) | 20.8 ± 7.14 | 14.7 ± 3.09 | 11.8 ± 2.76 | 0.01 |
| Thickness of the LVFW, mm (mean ± SD) | 16.30 ± 4.04 | 12.11 ± 2.02 | 10.4 ± 2.35 | 0.008 |
| Thickness of the RVFW, mm (mean ± SD) | 4.52 ± 1.45 | 4.77 ± 2.10 | 3.8 ± 1.82 | 0.6 |
| Gross fibrosis, n (%) | 10 (30) | 10 (100) | 20 (100) | <0.0001 |
| Histological replacement-type fibrosis, n (%) | 17 (57) | 10 (100) | 20 (100) | 0.01 |
| SVD presence, n (%) | 22 (73) | 10 (100) | 19 (95) | 0.07 |
| SVD score, mean | 1.18 | 2.4 | 1.95 | <0.0001 |
| Author, Year (Ref) | Source | HCM Cases, n. (Clinical Presentation) | Controls Yes/No (n. and Type) | HCM Mean Age, Years (Range or SD) | SVD Score, Methods | SVD Findings | SVD and Fibrosis |
|---|---|---|---|---|---|---|---|
| Maron et al., 1986 [3] | Autopsy | 48 (26 SCD, 9 HF, 10 at operation, 3 others) | Yes (68, including 14 extracardiac and 54 with heart disease increased LV mass) | 29 (11–60) | Semiquantitative (mild 1+, moderate 2+, severe 3+) | HCM 83% vs. controls 9% | 74% in those with fibrosis vs. 30% in those without |
| Tanaka et al., 1987 [4] | Autopsy | 14 (7 SCD, 1 CVA, 2 others, 4 HF) | Yes (25, hypertensive, cancers and other) | 40 (17–76) no-HF 44 (22–60) HF | Quantitative, mean % lumen in the evaluated section (IVS and LV free wall) | HCM: IVS 30%, LV 31% HCM-HF: IVS 17%, LV 33% Hypertensive: IVS 30%, LV 31% Controls: IVS 40%, LV 38% | % lumen inversely correlates with the extent of IVS fibrosis |
| Basso et al., 2000 [5] | Autopsy | 19 (SCD) | Yes (15, trauma) | 23 (1–35) | Semiquantitative (0, 1+ <20%, 2+ 20–50%, 3+ >50%), reported as the highest observed score in tissue section | HCM: SVD score 1.2 Controls: no SVD (only 3 hearts with a mean score = 1) | SVD score in areas with and without fibrosis: 1.7 ± 0.4 vs. 1.2 ± 0.4, p = 0.04. Positive correlation between %area of fibrosis with SVD mean score (p < 0.01, r = 0.58) |
| Varnava et al., 2000 [16] | Autopsy and HT | 72 (22 with HCM-dilated) | No | 36 (6–81) | Semiquantitative, % of vessels within a given section in which this ratio between external diameter to lumen diameter is ≥3 | % SVD LV: 14.6 (0–50) | No correlation between fibrosis and SVD (r = 0.8, p = 0.5), both in total HCM and in HCM-dilated |
| Kwon et al., 2009 [18] | Myectomy | 60 (LVOTO, preserved EF) | No | 51(18–76) | Semiquantitative, % of arteries affected by SVD: absent, mild (1–25%), moderate 26–50%) and severe (>50%) | SVD absent 25%, mild 55%, moderate 17%, severe 3% | Septal scar by CMR higher in HCM with SVD than in those without (78% vs. 20%, p < 0.001) |
| Moravsky et al., 2013 [20] | Myectomy | 29 (LVOTO, LVEF > 55%) | No | 50 (23–77) | Semiquantitative, % of arteries affected by SVD: absent, mild (1–25%), moderate 26–50%), and severe (>50%) | SVD absent 17%, mild 52%, moderate 24%, severe 7% | Statistically significant association between degree of SVD and % replacement fibrosis (p = 0.01) |
| Foà et al., 2019 [6] | Myectomy and HT | 57 (27 LVOTO, 30 HF) | No | 45.4 (±13.5) LVOTO 46.8 (±12.0) HF | Semiquantitative, lumen stenosis (mild <30%, moderate 30–60%, severe >60%), the highest observed score in tissue section was reported | SVD in 100% LVOTO, severe in 25.9% and multifocal in 55.6% SVD in 93.3% HCM-HF, severe in 30% and multifocal in 70% | No differences between subgroups in terms of SVD, but topographic association between SVD and scar in HCM-HF (70% vs. 40%, p = 0.034) |
| De Gaspari et al., 2021 (current study) | Autopsy and HT | 40 (30 SCD, 10 HF) | Yes (20, IHD with HF) | 22.6 (7–38) SCD 51.6 (22–68) HF | Semiquantitative (0, 1+ <20%, 2+ 20–50%, 3+ >50–90%, 4 >90%), reported as the highest observed score in tissue section | SVD in 73% HCM-SCD, 100% HCM-HF, 95% IHD SVD score HCM-HF 2.4, HCM-SCD 1.18 and IHD 1.95 | SVD score higher in areas with fibrosis vs. those without in both HF-HCM and SCD-HCM hearts (p < 0.05) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Gaspari, M.; Basso, C.; Perazzolo Marra, M.; Elia, S.; Bueno Marinas, M.; Angelini, A.; Thiene, G.; Rizzo, S. Small Vessel Disease: Another Component of the Hypertrophic Cardiomyopathy Phenotype Not Necessarily Associated with Fibrosis. J. Clin. Med. 2021, 10, 575. https://doi.org/10.3390/jcm10040575
De Gaspari M, Basso C, Perazzolo Marra M, Elia S, Bueno Marinas M, Angelini A, Thiene G, Rizzo S. Small Vessel Disease: Another Component of the Hypertrophic Cardiomyopathy Phenotype Not Necessarily Associated with Fibrosis. Journal of Clinical Medicine. 2021; 10(4):575. https://doi.org/10.3390/jcm10040575
Chicago/Turabian StyleDe Gaspari, Monica, Cristina Basso, Martina Perazzolo Marra, Stefania Elia, Maria Bueno Marinas, Annalisa Angelini, Gaetano Thiene, and Stefania Rizzo. 2021. "Small Vessel Disease: Another Component of the Hypertrophic Cardiomyopathy Phenotype Not Necessarily Associated with Fibrosis" Journal of Clinical Medicine 10, no. 4: 575. https://doi.org/10.3390/jcm10040575
APA StyleDe Gaspari, M., Basso, C., Perazzolo Marra, M., Elia, S., Bueno Marinas, M., Angelini, A., Thiene, G., & Rizzo, S. (2021). Small Vessel Disease: Another Component of the Hypertrophic Cardiomyopathy Phenotype Not Necessarily Associated with Fibrosis. Journal of Clinical Medicine, 10(4), 575. https://doi.org/10.3390/jcm10040575







