Testosterone and Bone Health in Men: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
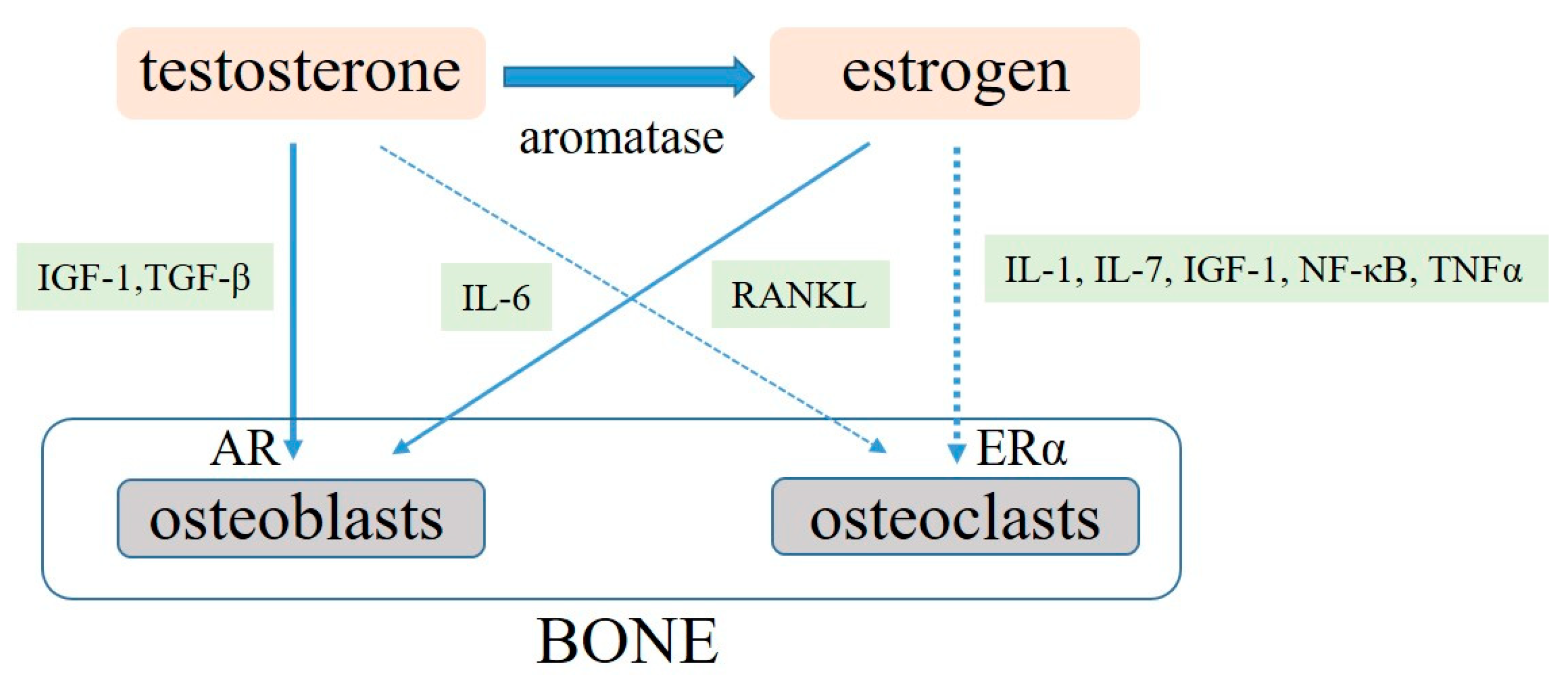
3. Molecular Roles of Sex Hormones on Bone Metabolism
4. The Relationship between Hypogonadism and BMD in Humans
5. The Relationship between Testosterone and Bone Fracture
6. The Effects of TRT on Bone Health among Hypogonadal Men
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feldman, H.A.; Longcope, C.; Derby, C.A.; Johannes, C.B.; Araujo, A.B.; Coviello, A.D.; Bremner, W.J.; McKinlay, J.B. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the Massachusetts male aging study. J. Clin. Endocrinol. Metab. 2002, 87, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Lunenfeld, B.; Mskhalaya, G.; Zitzmann, M.; Arver, S.; Kalinchenko, S.; Tishova, Y.; Morgentaler, A. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male 2015, 18, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Lunenfeld, B.; Arver, S.; Moncada, I.; Rees, D.A.; Schulte, H.M. How to help the aging male? Current approaches to hypogonadism in primary care. Aging Male 2012, 15, 187–197. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.A.; Carson, C.C.; Coward, R.M. Diagnosis and management of testosterone deficiency. Asian. J. Androl. 2015, 17, 177–186. [Google Scholar]
- Bassil, N.; Alkaade, S.; Morley, J.E. The benefits and risks of testosterone replacement therapy: A review. Ther. Clin. Risk Manag. 2009, 5, 427–448. [Google Scholar]
- Yoshimura, N.; Muraki, S.; Oka, H.; Kawaguchi, H.; Nakamura, K.; Akune, T. Cohort profile: Research on osteoarthritis/osteoporosis disability study. Int. J. Epidemiol. 2010, 39, 988–995. [Google Scholar] [CrossRef]
- Melton, L.J.; Atkinson, E.J.; O’Connor, M.K.; O’Fallon, W.M.; Riggs, B.L. Bone density and fracture risk in men. J. Bone Miner. Res. 1998, 13, 1915–1923. [Google Scholar] [CrossRef]
- Yamamoto, L. Guideline for treatment of osteoporosis. Nippon Rinsho. 2002, 60, 280–287. [Google Scholar]
- van Staa, T.P.; Dennison, E.M.; Leufkens, H.G.; Cooper, C. Epidemiology of fractures in England and Wales. Bone 2001, 29, 517–522. [Google Scholar] [CrossRef]
- Almeida, M.; Laurent, M.R.; Dubois, V.; Claessens, F.; O’Brien, C.A.; Bouillon, R.; Vanderschueren, D.; Manolagas, S.C. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol. Rev. 2017, 97, 135–187. [Google Scholar] [CrossRef]
- El Badri, S.A.; Salawu, A.; Brown, J.E. Bone Health in Men with Prostate Cancer: Review Article. Curr. Osteoporos. Rep. 2019, 17, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Mitsuzuka, K.; Arai, Y. Metabolic changes in patients with prostate cancer during androgen deprivation therapy. Int. J. Urol. 2018, 25, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Lassemillante, A.C.; Doi, S.A.; Hooper, J.D.; Prins, J.B.; Wright, O.R. Prevalence of osteoporosis in prostate cancer survivors: A meta-analysis. Endocrine 2014, 45, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Shahinian, V.B.; Kuo, Y.F.; Freeman, J.L.; Goodwin, J.S. Risk of fracture after androgen deprivation for prostate cancer. N. Engl. J. Med. 2005, 352, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Brahamsen, B.; Nielsen, M.F.; Esklldsen, P.; Andersen, J.T.; Walter, S.; Brixen, K. Fracture risk in Danish men with prostate cancer: A nationwide register study. BJU. Int. 2007, 100, 749–754. [Google Scholar] [CrossRef]
- Junjie, W.; Dongsheng, H.; Lei, S.; Hongzhuo, L.; Changying, S. Testosterone Replacement Therapy Has Limited Effect on Increasing Bone Mass Density in Older Men: A Meta-analysis. Curr. Pharm. Des. 2019, 25, 73–84. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Soelaiman, I.N.; Chin, K.Y. A concise review of testosterone and bone health. Clin. Interv. Aging 2016, 11, 1317–1324. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, D.; Li, H. The effects of testosterone on bone health in males with testosterone deficiency: A systematic review and meta-analysis. BMC Endocr. Disord. 2020, 20, 33. [Google Scholar] [CrossRef]
- Drake, M.T.; Murad, M.H.; Mauck, K.F.; Lane, M.A.; Undavalli, C.; Elraiyah, T.; Stuart, L.M.; Prasad, C.; Shahrour, A.; Mullan, R.J.; et al. Clinical review. Risk factors for low bone mass-related fractures in men: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2012, 97, 1861–1870. [Google Scholar] [CrossRef]
- Isidori, A.M.; Giannetta, E.; Greco, E.A.; Gianfrilli, D.; Bonifacio, V.; Isidori, A.; Lenzi, A.; Fabbri, A. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: A meta-analysis. Clin. Endocrinol. 2005, 63, 280–293. [Google Scholar] [CrossRef]
- Tracz, M.J.; Sideras, K.; Boloña, E.R.; Haddad, R.M.; Kennedy, C.C.; Uraga, M.V.; Caples, S.M.; Erwin, P.J.; Montori, V.M. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J. Clin. Endocrinol. Metab. 2006, 91, 2011–2016. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.M.; Vermeulen, A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr. Rev. 2005, 26, 833–876. [Google Scholar] [CrossRef] [PubMed]
- Labrie, F.; Cusan, L.; Gomez, J.L.; Martel, C.; Bérubé, R.; Bélanger, P.; Bélanger, A.; Vandenput, L.; Mellström, D.; Ohlsson, C. Comparable amounts of sex steroids are made outside the gonads in men and women: Strong lesson for hormone therapy of prostate and breast cancer. J. Steroid Biochem. Mol. Biol. 2009, 113, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Kondoh, S.; Kouzmenko, A.; Kato, S. Minireview: Osteoprotective action of estrogens is mediated by osteoclastic estrogen receptor-alpha. Mol. Endocrinol. 2010, 24, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Ima-Nirwana, S. The effects of orchidectomy and supraphysiological testosterone administration on trabecular bone structure and gene expression in rats. Aging Male 2015, 18, 60–66. [Google Scholar] [CrossRef]
- Li, X.; Ominsky, M.S.; Stolina, M.; Warmington, K.S.; Geng, Z.; Niu, Q.T.; Asuncion, F.J.; Tan, H.L.; Grisanti, M.; Dwyer, D.; et al. Increased RANK ligand in bone marrow of orchiectomized ra ts and prevention of their bone loss by the RANK ligand inhibitor osteoprotegerin. Bone 2009, 45, 669–676. [Google Scholar] [CrossRef]
- Gill, R.K.; Turner, R.T.; Wronski, T.J.; Bell, N.H. Orchiectomy markedly reduces the concentration of the three isoforms of transforming growth factor beta in rat bone, and reduction is prevented by testosterone. Endocrinology 1998, 139, 546–550. [Google Scholar] [CrossRef]
- Bellido, T.; Jilka, R.L.; Boyce, B.F.; Girasole, G.; Broxmeyer, H.; Dalrymple, S.A.; Murray, R.; Manolagas, S.C. Regulation of interleukin-6, osteoclastogenesis, and bone mass by androgens. The role of the androgen receptor. J. CIin. Investig. 1995, 95, 2886–2895. [Google Scholar] [CrossRef]
- Chen, Q.; Kaji, H.; Kanatani, M.; Sugimoto, T.; Chihara, K. Testosterone increases osteoprotegerin mRNA expression in mouse osteoblast cells. Horm. Metab. Res. 2004, 36, 674–678. [Google Scholar] [CrossRef]
- Kawano, H.; Sato, T.; Yamada, T.; Matsumoto, T.; Sekine, K.; Watanabe, T.; Nakamura, T.; Fukuda, T.; Yoshimura, K.; Yoshizawa, T.; et al. Suppressive function of androgen receptor in bone resorption. Proc. Natl. Acad. Sci. USA 2003, 100, 9416–9421. [Google Scholar] [CrossRef]
- Smith, E.P.; Boyd, J.; Frank, G.R.; Takahashi, H.; Cohen, R.M.; Specker, B.; Williams, T.C.; Lubahn, D.B.; Korach, K.S. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N. Engl. J. Med. 1994, 331, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Morishima, A.; Grumbach, M.M.; Simpson, E.R.; Fisher, C.; Qin, K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J. Clin. Endocrinol. Metab. 1995, 80, 3689–3698. [Google Scholar] [PubMed]
- Bilezikian, J.P.; Morishima, A.; Bell, J.; Grumbach, M.M. Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. N. Engl. J. Med. 1998, 339, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.E.; Dai, A.; Tiffee, J.C.; Li, H.H.; Mundy, G.R.; Boyce, B.F. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat. Med. 1996, 2, 1132–1136. [Google Scholar] [CrossRef]
- Raisz, L.G. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J. Clin. Investig. 2005, 115, 3318–3325. [Google Scholar] [CrossRef]
- Ding, K.H.; Wang, Z.Z.; Hamrick, W.M.; Deng, Z.B.; Zhou, L.; Kang, B.; Yan, S.L.; She, J.X.; Stern, D.M.; Isales, C.M.; et al. Disordered osteoclast formation in RAGE-deficient mouse establishes an essential role for RAGE in diabetes related bone loss. Biochem. Biophys. Res. Commun. 2006, 340, 1091–1097. [Google Scholar] [CrossRef]
- Xie, J.; Méndez, J.D.; Méndez-Valenzuela, V.; Aguilar-Hernández, M.M. Cellular signaling of the receptor for advanced glycation end products (RAGE). Cell Signal 2013, 25, 2185–2197. [Google Scholar] [CrossRef]
- Kimble, R.B.; Matayoshi, A.B.; Vannice, J.L.; Kung, V.T.; Williams, C.; Pacifici, R. Simultaneous block of interleukin-1 and tumor necrosis factor is required to completely prevent bone loss in the early postovariectomy period. Endocrinology 1995, 136, 3054–3061. [Google Scholar] [CrossRef]
- Lorenzo, J.A.; Naprta, A.; Rao, Y.; Alander, C.; Glaccum, M.; Widmer, M.; Gronowicz, G.; Kalinowski, J.; Pilbeam, C.C. Mice lacking the type I interleukin-1 receptor do not lose bone mass after ovariectomy. Endocrinology 1998, 139, 3022–3025. [Google Scholar] [CrossRef]
- Antonio, L.; Caerels, S.; Jardi, F.; Delaunay, E.; Vanderschueren, D. Testosterone replacement in congenital hypogonadotropic hypogonadism maintains bone density but has only limited osteoanabolic effects. Andrology 2019, 7, 302–306. [Google Scholar] [CrossRef]
- Rochira, V.; Antonio, L.; Vanderschueren, D. EAA clinical guideline on management of bone health in the andrological outpatient clinic. Andrology 2018, 6, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Stanley, H.L.; Schmitt, B.P.; Poses, R.M.; Deiss, W.P. Does hypogonadism contribute to the occurrence of a minimal trauma hip fracture in elderly men? J. Am. Geriatr. Soc. 1991, 39, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Baillie, S.P.; Davison, C.E.; Johnson, F.J.; Francis, R.M. Pathogenesis of vertebral crush fractures in men. Age Ageing 1992, 21, 139–141. [Google Scholar] [CrossRef]
- Kelepouris, N.; Harper, K.D.; Gannon, F.; Kaplan, F.S.; Haddad, J.G. Severe osteoporosis in men. Ann. Intern. Med. 1995, 123, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Fink, H.A.; Ewing, S.K.; Ensrud, K.E.; Barrett-Connor, E.; Taylor, B.C.; Cauley, J.A.; Orwoll, E.S. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J. Clin. Endocrinol. Metab. 2006, 91, 3908–3915. [Google Scholar] [CrossRef]
- Ryan, C.S.; Petkov, V.I.; Adler, R.A. Osteoporosis in men: The value of laboratory testing. Osteoporos. Int. 2011, 22, 1845–1853. [Google Scholar] [CrossRef]
- Kotwal, N.; Upreti, V.; Nachankar, A.; Hari Kumar, K.V.S. A prospective, observational study of osteoporosis in men. Indian J. Endocrinol. Metab. 2018, 22, 62–66. [Google Scholar]
- Liu, Z.Y.; Yang, Y.; Wen, C.Y.; Rong, L.M. Serum Osteocalcin and Testosterone Concentrations in Adult Males with or without Primary Osteoporosis: A Meta-Analysis. Biomed. Res. Int. 2017, 2017, 9892048. [Google Scholar] [CrossRef]
- Greendale, G.A.; Edelstein, S.; Barrett-Connor, E. Endogenous sex steroids and bone mineral density in older women and men: The Rancho Bernardo Study. J. Bone Miner. Res. 1997, 12, 1833–1843. [Google Scholar] [CrossRef]
- Amin, S.; Zhang, Y.; Sawin, C.T.; Evans, S.R.; Hannan, M.T.; Kiel, D.P.; Wilson, P.W.; Felson, D.T. Association of hypogonadism and estradiol levels with bone mineral density in elderly men from the Framingham study. Ann. Intern. Med. 2000, 133, 951–963. [Google Scholar] [CrossRef]
- Mellström, D.; Johnell, O.; Ljunggren, O.; Eriksson, A.L.; Lorentzon, M.; Mallmin, H.; Holmberg, A.; Redlund-Johnell, I.; Orwoll, E.; Ohlsson, C. Free Testosterone is an Independent Predictor of BMD and Prevalent Fractures in Elderly Men: MrOS Sweden. J. Bone Miner. Res. 2006, 21, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Bjørnerem, A.; Emaus, N.; Berntsen, G.K.; Joakimsen, R.M.; Fønnebø, V.; Wilsgaard, T.; Oian, P.; Seeman, E.; Straume, B. Circulating sex steroids, sex hormone-binding globulin, and longitudinal changes in forearm bone mineral density in postmenopausal women and men: The Tromsø study. Calcif. Tissue Int. 2007, 81, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Kuchuk, N.O.; van Schoor, N.M.; Pluijm, S.M.; Smit, J.H.; de Ronde, W.; Lips, P. The association of sex hormone levels with quantitative ultrasound, bone mineral density, bone turnover and osteoporotic fractures in older men and women. Clin. Endocrinol. (Oxford) 2007, 67, 295–303. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, E.S.; Nielson, C.M.; Marshall, L.M.; Lapidus, J.A.; Barrett-Connor, E.; Ensrud, K.E.; Hoffman, A.R.; Laughlin, G.; Ohlsson, C.; Orwoll, E.S.; et al. The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J. Clin. Endocrinol. Metab. 2009, 94, 3337–3346. [Google Scholar] [CrossRef]
- Vanderschueren, D.; Pye, S.R.; Venken, K.; Borghs, H.; Gaytant, J.; Huhtaniemi, I.T.; Adams, J.E.; Ward, K.A.; Bartfai, G.; Casanueva, F.F.; et al. EMAS Study Group. Gonadal sex steroid status and bone health in middle-aged and elderly European men. Osteoporos. Int. 2010, 21, 1331–1339. [Google Scholar] [CrossRef]
- Cauley, J.A.; Ewing, S.K.; Taylor, B.C.; Fink, H.A.; Ensrud, K.E.; Bauer, D.C.; Barrett-Connor, E.; Marshall, L.; Orwoll, E.S.; Osteoporotic Fractures in Men Study (MrOS) Research Group. Sex steroid hormones in older men: Longitudinal associations with 4.5-year change in hip bone mineral density–the osteoporotic fractures in men study. J. Clin. Endocrinol. Metab. 2010, 95, 4314–4323. [Google Scholar] [CrossRef]
- Woo, J.; Kwok, T.; Leung, J.C.; Ohlsson, C.; Vandenput, L.; Leung, P.C. Sex steroids and bone health in older Chinese men. Osteoporos. Int. 2012, 23, 1553–1562. [Google Scholar] [CrossRef]
- Hsu, B.; Seibel, M.J.; Cumming, R.G.; Blyth, F.M.; Naganathan, V.; Bleicher, K.; Le Couteur, D.G.; Waite, L.M.; Handelsman, D.J. Progressive temporal change in serum SHBG, but not in serum testosterone or estradiol, is associated with bone loss and incident fractures in older men: The concord health and ageing in men project. J. Bone Miner. Res. 2016, 31, 2115–2122. [Google Scholar] [CrossRef]
- Gennari, L. Aromatase activity and bone homeostasis in men. J. Clin. Endocrinol. Metab. 2004, 89, 5898–5907. [Google Scholar] [CrossRef]
- Catalano, A.; Gaudio, A.; Agostino, R.M.; Morabito, N.; Bellone, F.; Lasco, A. Trabecular bone score and quantitative ultrasound measurements in the assessment of bone health in breast cancer survivors assuming aromatase inhibitors. J. Endocrinol. Investig. 2019, 42, 1337–1343. [Google Scholar] [CrossRef]
- Zitzmann, M.; Brune, M.; Vieth, V.; Nieschlag, E. Monitoring bone density in hypogonadal men by quantitative phalangeal ultrasound. Bone 2002, 31, 422–429. [Google Scholar] [CrossRef]
- Burnett-Bowie, S.-A.M.; McKay, E.A.; Lee, H.; Leder, B.Z. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. J. Clin. Endocrinol. Metab. 2009, 94, 4785–4792. [Google Scholar] [CrossRef] [PubMed]
- Vandenput, L.; Labrie, F.; Mellström, D.; Swanson, C.; Knutsson, T.; Peeker, R.; Ljunggren, O.; Orwoll, E.; Eriksson, A.L.; Damber, J.E.; et al. Serum Levels of Specific Glucuronidated Androgen Metabolites Predict BMD and Prostate Volume in Elderly Men. J. Bone Miner. Res. 2007, 22, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.M.Y.; Wong, H.; Zhang, N.; Chow, S.K.H.; Chau, W.W.; Wang, J.; Chim, Y.N.; Leung, K.S.; Cheung, W.H. The relationship between sarcopenia and fragility fracture-a systematic review. Osteoporos. Int. 2019, 30, 541–553. [Google Scholar] [CrossRef]
- Tarantino, U.; Piccirilli, E.; Fantini, M.; Baldi, J.; Gasbarra, E.; Bei, R. Sarcopenia and fragility fractures: Molecular and clinical evidence of the bone-muscle interaction. J. Bone Joint. Surg. Am. 2015, 97, 429–437. [Google Scholar] [CrossRef]
- Szulc, P.; Claustrat, B.; Marchand, F.; Delmas, P.D. Increased risk of falls and increased bone resorption in elderly men with partial androgen deficiency: The MINOS study. J. Clin. Endocrinol. Metab. 2003, 88, 5240–5247. [Google Scholar] [CrossRef]
- Kenny, A.M.; Prestwood, K.M.; Marcello, K.M.; Raisz, L.G. Determinants of bone density in healthy older men with low testosterone levels. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M492–M497. [Google Scholar] [CrossRef]
- Tuck, S.P.; Scane, A.C.; Fraser, W.D.; Diver, M.J.; Eastell, R.; Francis, R.M. Sex steroids and bone turnover markers in men with symptomatic vertebral fractures. Bone 2008, 43, 999–1005. [Google Scholar] [CrossRef]
- Mellstrom, D.; Vandenput, L.; Mallmin, H.; Holmberg, A.H.; Lorentzon, M.; Odén, A.; Johansson, H.; Orwoll, E.S.; Labrie, F.; Karlsson, M.K.; et al. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J. Bone Miner. Res. 2008, 23, 1552–1560. [Google Scholar] [CrossRef]
- Meier, C.; Nguyen, T.V.; Handelsman, D.J.; Schindler, C.; Kushnir, M.M.; Rockwood, A.L.; Meikle, A.W.; Center, J.R.; Eisman, J.A.; Seibel, M.J. Endogenous sex hormones and incident fracture risk in older men: The Dubbo Osteoporosis Epidemiology Study. Arch. Intern. Med. 2008, 168, 47–54. [Google Scholar] [CrossRef]
- Roddam, A.W.; Appleby, P.; Neale, R.; Dowsett, M.; Folkerd, E.; Tipper, S.; Allen, N.E.; Key, T.J. Association between endogenous plasma hormone concentrations and fracture risk in men and women: The EPIC-Oxford prospective cohort study. J. Bone Miner. Metab. 2009, 27, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Risto, O.; Hammar, E.; Hammar, K.; Fredrikson, M.; Hammar, M.; Wahlström, O. Elderly men with a history of distal radius fracture have significantly lower calcaneal bone density and free androgen index than age-matched controls. Aging Male 2012, 15, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Torremadé-Barreda, J.; Rodríguez-Tolrà, J.; Román-Romera, I.; Padró-Miquel, A.; Rius-Moreno, J.; Franco-Miranda, E. Testosterone-deficiency as a risk factor for hip fracture in elderly men. Actas. Urol. Esp. 2013, 37, 142–146. [Google Scholar] [CrossRef]
- Tran, T.S.; Center, J.R.; Seibel, M.J.; Eisman, J.A.; Kushnir, M.M.; Rockwood, A.L.; Nguyen, T.V. Relationship between Serum Testosterone and Fracture Risk in Men: A Comparison of RIA and LC-MS/MS. Clin. Chem. 2015, 61, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Vandenput, L.; Ohlsson, C. Estrogens as regulators of bone health in men. Nat. Rev. Endocrinol. 2009, 5, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Kenny, A.M.; Kleppinger, A.; Annis, K.; Rathier, M.; Browner, B.; Judge, J.O.; McGee, D. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J. Am. Geriatr. Soc. 2010, 58, 1134–1143. [Google Scholar] [CrossRef]
- Aversa, A.; Bruzziches, R.; Francomano, D.; Greco, E.A.; Fornari, R.; Di Luigi, L.; Lenzi, A.; Migliaccio, S. Effects of long-acting testosterone undecanoate on bone mineral density in middle-aged men with late-onset hypogonadism and metabolic syndrome: Results from a 36 months controlled study. Aging Male 2012, 15, 96–102. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhan, J.K.; Huang, W.; Wang, Y.; Liu, Y.; Wang, S.; Tan, P.; Tang, Z.Y.; Liu, Y.S. Effects of low-dose testosterone undecanoate treatment on bone mineral density and bone turnover markers in elderly male osteoporosis with low serum testosterone. Int. J. Endocrinol. 2013, 2013, 570413. [Google Scholar] [CrossRef]
- Bouloux, P.M.; Legros, J.J.; Elbers, J.M.; Geurts, T.B.; Kaspers, M.J.; Meehan, A.G.; Meuleman, E.J.; Study 43203 Investigators. Effects of oral testosterone undecanoate therapy on bone mineral density and body composition in 322 aging men with symptomatic testosterone deficiency: A 1 year, a randomized, placebo-controlled, dose-ranging study. Aging Male 2013, 16, 38–47. [Google Scholar] [CrossRef]
- Rodriguez-Tolrà, J.; Torremadé, J.; di Gregorio, S.; Del Rio, L.; Franco, E. Effects of testosterone treatment on bone mineral density in men with testosterone deficiency syndrome. Andrology 2013, 1, 570–575. [Google Scholar] [CrossRef]
- Permpongkosol, S.; Khupulsup, K.; Leelaphiwat, S.; Pavavattananusorn, S.; Thongpradit, S.; Petchthong, T. Effects of 8–year treatment of long-acting testosterone undecanoate on metabolic parameters, urinary symptoms, bone mineral density, and sexual function in men with late-onset hypogonadism. J. Sex. Med. 2016, 13, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Rogol, A.D.; Tkachenko, N.; Bryson, N. Natesto™, a novel testosterone nasal gel, normalizes androgen levels in hypogonadal men. Andrology 2016, 4, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Shigehara, K.; Konaka, H.; Koh, E.; Nakashima, K.; Iijima, M.; Nohara, T.; Izumi, K.; Kitagawa, Y.; Kadono, Y.; Sugimoto, K.; et al. Effects of testosterone replacement therapy on hypogonadal men with osteopenia or osteoporosis: A subanalysis of a prospective randomized controlled study in Japan (EARTH study). Aging Male 2017, 20, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J.; Kopperdahl, D.L.; Stephens-Shields, A.J.; Ellenberg, S.S.; Cauley, J.A.; Ensrud, K.E.; Lewis, C.E.; Barrett-Connor, E.; Schwartz, A.V.; Lee, D.C.; et al. Effect of Testosterone Treatment on Volumetric Bone Density and Strength in Older Men With Low Testosterone: A Controlled Clinical Trial. JAMA Intern. Med. 2017, 177, 471–479. [Google Scholar] [CrossRef]
- Permpongkosol, S.; Tantirangsee, N.; Ratana-olarn, K. Treatment of 161 men with symptomatic late onset hypogonadism with long-acting parenteral testosterone undecanoate: Effects on body composition, lipids, and psychosexual complaints. J. Sex. Med. 2010, 7, 3765–3774. [Google Scholar] [CrossRef]
- Konaka, H.; Sugimoto, K.; Orikasa, H.; Iwamoto, T.; Takamura, T.; Takeda, Y.; Shigehara, K.; Iijima, M.; Koh, E.; Namiki, M.; et al. Effects of long-term androgen replacement therapy on the physical and mental statuses of aging males with late-onset hypogonadism: A multicenter randomized controlled trial in Japan (EARTH Study). Asian. J. Androl. 2016, 18, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Ng Tang Fui, M.; Hoermann, R.; Nolan, B.; Clarke, M.; Zajac, J.D.; Grossmann, M. Effect of testosterone treatment on bone remodelling markers and mineral density in obese dieting men in a randomized clinical trial. Sci. Rep. 2018, 8, 9099. [Google Scholar] [CrossRef]
- Bhasin, S.; Cunningham, G.R.; Hayes, F.J.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Montori, V.M. Testosterone therapy in men with androgen deficiency syndromes: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2010, 95, 2536–2559. [Google Scholar] [CrossRef]
- Watts, N.B.; Adler, R.A.; Bilezikian, J.P.; Drake, M.T.; Eastell, R.; Orwoll, E.S.; Finkelstein, J.S. Osteoporosis in men: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2012, 97, 1802–1822. [Google Scholar] [CrossRef] [PubMed]
- Vescini, F.; Attanasio, R.; Balestrieri, A.; Bandeira, F.; Bonadonna, S.; Camozzi, V.; Cassibba, S.; Cesareo, R.; Chiodini, I.; Francucci, C.M.; et al. Italian association of clinical endocrinologists (AME) position statement: Drug therapy of osteoporosis. J. Endocrinol. Investig. 2016, 39, 807–834. [Google Scholar] [CrossRef] [PubMed]
- Hoppé, E.; Bouvard, B.; Royer, M.; Chappard, D.; Audran, M.; Legrand, E. Is androgen therapy indicated in men with osteoporosis? Joint Bone Spine 2013, 80, 459–465. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gallagher, J.C.; Fowler, S.E.; Detter, J.R.; Sherman, S.S. Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J. Clin. Endocrinol. Metab. 2001, 86, 3618–3628. [Google Scholar] [CrossRef]
- Smith, M.R.; Eastham, J.; Gleason, D.M.; Shasha, D.; Tchekmedyian, S.; Zinner, N. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J. Urol. 2003, 169, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Egerdie, B.; Hernández Toriz, N.; Feldman, R.; Tammela, T.L.; Saad, F.; Heracek, J.; Szwedowski, M.; Ke, C.; Kupic, A.; et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N. Engl. J. Med. 2009, 361, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Morabito, N.; Gaudio, A.; Lasco, A.; Catalano, A.; Atteritano, M.; Trifiletti, A.; Anastasi, G.; Melloni, D.; Frisina, N. Neridronate prevents bone loss in patients receiving androgen deprivation therapy for prostate cancer. J. Bone Miner. Res. 2004, 19, 1766–1770. [Google Scholar] [CrossRef]
| Author | Year | Subjects | Prevalence of HG | Reference |
|---|---|---|---|---|
| Stanley | 1991 | 17 with MTHF | 58% | [42] |
| 61 controls | 18% | |||
| Baillie | 1992 | 70 with vertebral fracture | 16% | [43] |
| Kelepouris | 1995 | 47 with atraumatic fracture | 15% | [44] |
| Fink HA | 2006 | 2447 community-dwelling men including 130 with osteoporosis | 7% | [45] |
| Ryan | 2011 | 234 with osteoporosis | 24% | [46] |
| Kotwal N | 2018 | 200 male attendants of patients attending endocrine outpatient department | 35% | [47] |
| Author | Year | Country | Study Subjects | Hormones | Results | Ref |
|---|---|---|---|---|---|---|
| Greendale (Rancho Bernardo Study) | 1997 | USA | 457 women and 534 men (50–89 years) | TT, DHT, E2, E1, BioT | Higher bioavailable (but not total) testosterone levels were associated with higher BMD. | [49] |
| Amin (Framingham Study) | 2000 | USA | 448 men (68–96 years) | TT, E2, LH | Hypogonadism related to aging has little influence on BMD. | [50] |
| Fink | 2006 | USA | 2447 community-dwelling men (>65 years) | TT, E2 | The incidence rates of hip bone loss in men with deficient and normal total testosterone were 22.5% and 8.6%. | [45] |
| Mellström (MrOS Sweden) | 2006 | Sweden | 2908 men (69–80 years) | TT, E2, FT, FE2, SHBG | FT levels were positively correlated with BMD in the hip, femur, and arm but not in the lumbar spine. | [51] |
| Bjørnerem (Tromsø Study) | 2007 | Norway | 927 women (37–80 years), 894 men (25–80 years) | TT, E2, cFT SHBG | The relationship between all gender steroids and bone loss was weak. | [52] |
| Kuchuk NO (Longitudinal Ageing Study Amsterdam) | 2007 | Netherlands | 623 men and 634 women (65–88 years) | TT, E2 SHBG | TT had no correlations with BMD | [53] |
| LeBlanc (MrOS Research) | 2009 | USA | 5995 community-dwelling men (>65 years) | BioT, BioE2 SHBG | The combination of low BioE2, low BaT, and high SHBG was associated with significantly faster rates of BMD loss. | [54] |
| Vanderschueren (EMAS study) | 2010 | Belgium | 3140 men (40–79 years) | TT, FT, E2 SHBG | TT and FT had no correlations with BMD. | [55] |
| Cauley (MrOS Research) | 2010 | USA | 1238 men (cross-sectional) 969 men (longitudinal) >65 years | BioT, BioE2 SHBG | No association existed between BioT and hip BMD loss. | [56] |
| Woo | 2012 | Hong Kong | 1448 men (>65 years) | TT, FT, E2, BioE2, SHBG | TT and FT were not correlated with bone loss. | [57] |
| Hsu (CHAMP Cohort) | 2016 | Australia | 1705, 1367, and 958 men (>70 men) | TT, DHT, E2 E1, SHBG, LH FSH, cFT | Both TT and cFT had no correlations with bone loss. | [58] |
| Author | Year | Country | Study Subjects | Hormones | Results | Ref |
|---|---|---|---|---|---|---|
| Szulc (MINOS study) | 2003 | France | 1040 elderly men (19–85 years) | TT, FT | Hypogonadal men had increased rates of falls and markers of bone resorption. | [66] |
| Kenny | 2005 | USA | 83 community-dwelling white men (>65 years) | BioT | Fifty-two percent of men with low BioT levels had lower BMD and were likely at an increased risk of fracture. | [67] |
| Fink | 2006 | USA | 2447 community-dwelling men (>65 years) | TT, E2 | Prevalence rates of osteoporosis in men with deficient and normal total testosterone were 12.3% and 6.0%. | [45] |
| Mellström (MrOS Sweden) | 2006 | Sweden | 2908 men (69–80 years) | TT, E2, FT, FE2, SHBG | FT levels below the median were independent predictors of prevalent osteoporosis-related fractures and X-ray-verified vertebral fractures. | [51] |
| Tuck | 2008 | UK | 57 men with symptomatic vertebral fractures 57 age-matched controls | FT, BioT, SHBG | Calculated FT was lower in the fracture group than the controls. | [68] |
| Mellstrom (MrOS Sweden) | 2008 | Sweden | 2639 men (69–80 years) | TT, E2, FT, FE2, SHBG | TT and FT were not significantly associated with fracture risk. | [69] |
| Meier (The Dubbo Study) | 2008 | Switzerland | 609 men (>60 years) | TT, E2, SHBG | Lower testosterone increased the risk of osteoporotic fracture, particularly with hip and nonvertebral fractures. | [70] |
| Roddam (EPIC-Oxford Study) | 2009 | UK | 155 men and 281 women | TT, E2, SHBG | There were no associations between fracture risk and testosterone levels. | [71] |
| Risto | 2012 | Sweden | 39 treated for fracture 45 controls | TT, BioT, BioE2 | BioT was a possible marker for increased fracture risk. | [72] |
| Woo | 2012 | Hong Kong | 1448 men (>65 years) | TT, FT, E2, BioE2, SHBG | TT and FT had no correlations with an increased bone fracture. | [57] |
| Torremadé-Barreda | 2013 | Spain | 54 men with hip fracture 54 age-matched controls | TT, FT, BioT | Men with hip fractures had significantly lower calculated FT and BiaT levels. | [73] |
| Hsu (CHAMP Cohort) | 2016 | Australia | 1705, 1367, and 958 men over 70 men | TT, DHT, E2, E1, SHBG, LH FSH, cFT | Both TT and cFT had no correlations with incident fractures. | [58] |
| Tran | 2017 | Australia | 602 men with incident fractures | TT | TT was significantly correlated with the incidence of fracture risk. | [74] |
| Author | Country | Design | Study Subjects | TRT | Periods | Results | Ref |
|---|---|---|---|---|---|---|---|
| Kenny (2010) | USA | RCT | 131 men with hypogonadism, bone fracture, low BMD, frailty | Transdermal testosterone (5 mg/day) | 12–24 months | TRT could increase axial BMD. | [76] |
| Permpongkosol (2010) | Thailand | Single arm | 161 hypogonadal men | 1000 mg TU (Nebido) | 54–150 weeks | No change in BMD was observed in TRT. | [85] |
| Aversa (2012) | Italy | Case–control | 40 hypogonadal men 20 aged-match control | Intramuscular TU (4 times/year) | 36 months | TRT increased vertebral and femoral BMD. | [77] |
| Wang (2013) | China | RCT | 186 men with osteoporosis and hypogonadism | TU (20 or 40 mg/day) | 24 months | TRT improved the lumbar spine and femoral neck BMD. | [78] |
| Bouloux (2013) | UK | RCT | 322 men with LOH syndrome | Oral TU (80, 160, 240 mg/day) | 1 year | Treatment with oral TU led to BMD improvement. | [79] |
| Rodriguez-Tolrà (2013) | Spain | Single arm | 50 men with LOH syndrome | TG (50 mg/day for 12 months) 1000 mg TU (every 2–3 months from 12–24 months) | 2 years | TRT improved lumbar spine and hip BMD. | [80] |
| Permpongkosol (2016) | Thailand | Single arm | 120 hypogonadal men | 1000 mg TU (Nebido) | 5–8 years | A statistically significant increase was found in vertebral and femoral BMD. | [81] |
| Rogol (2016) | USA | RCT | 306 hypogonadal men | TG (22 or 33 mg) | 90–360 days | BMD improved from baseline by TRT. | [82] |
| Konaka (2016) | Japan | RCT | 334 hypogonadal men | TE (250 mg/4 W) | 12 months | 12-month TRT could not improve BMD. | [86] |
| Shigehara (2017) | Japan | RCT | 74 hypogonadal men with osteopenia | TE (250 mg/4 W) | 12 months | TRT for 12 months could improve BMD. | [83] |
| Snyder (2017) | USA | RCT | 211 hypogonadal men | TG (5 mg/day initially) | 1 year | TRT increased BMD and bone strength, more in trabecula. | [84] |
| Ng Tang Fui (2018) | Australia | RCT | 100 obese men with hypogonadism | TU (1000 mg/0, 6, 26, 36, and 46 weeks) | 56 weeks | No significant changes in the lumbar spine and femoral BMD were observed. | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shigehara, K.; Izumi, K.; Kadono, Y.; Mizokami, A. Testosterone and Bone Health in Men: A Narrative Review. J. Clin. Med. 2021, 10, 530. https://doi.org/10.3390/jcm10030530
Shigehara K, Izumi K, Kadono Y, Mizokami A. Testosterone and Bone Health in Men: A Narrative Review. Journal of Clinical Medicine. 2021; 10(3):530. https://doi.org/10.3390/jcm10030530
Chicago/Turabian StyleShigehara, Kazuyoshi, Kouji Izumi, Yoshifumi Kadono, and Atsushi Mizokami. 2021. "Testosterone and Bone Health in Men: A Narrative Review" Journal of Clinical Medicine 10, no. 3: 530. https://doi.org/10.3390/jcm10030530
APA StyleShigehara, K., Izumi, K., Kadono, Y., & Mizokami, A. (2021). Testosterone and Bone Health in Men: A Narrative Review. Journal of Clinical Medicine, 10(3), 530. https://doi.org/10.3390/jcm10030530






