Accuracy of Guided Implant Surgery in the Edentulous Jaw Using Desktop 3D-Printed Mucosal Supported Guides
Abstract
1. Introduction
2. Experimental Section
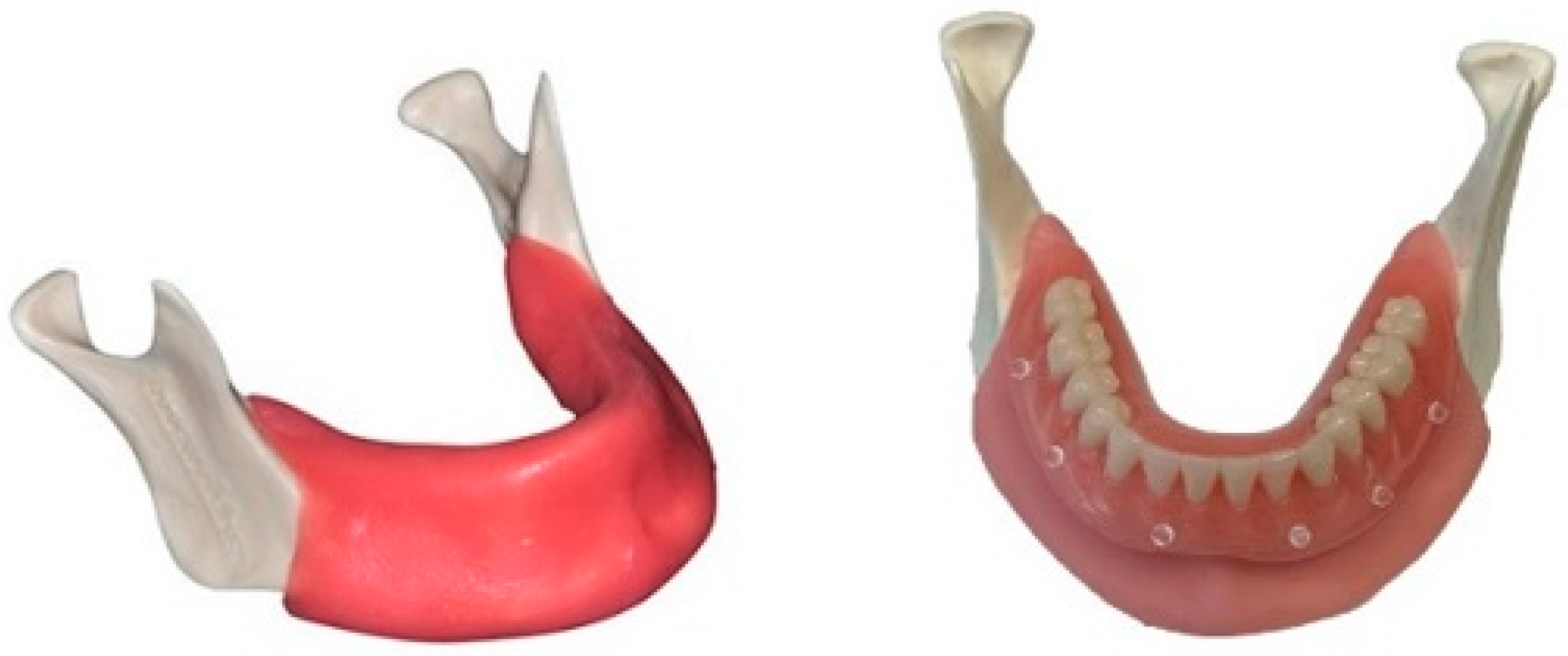
2.1. Cast
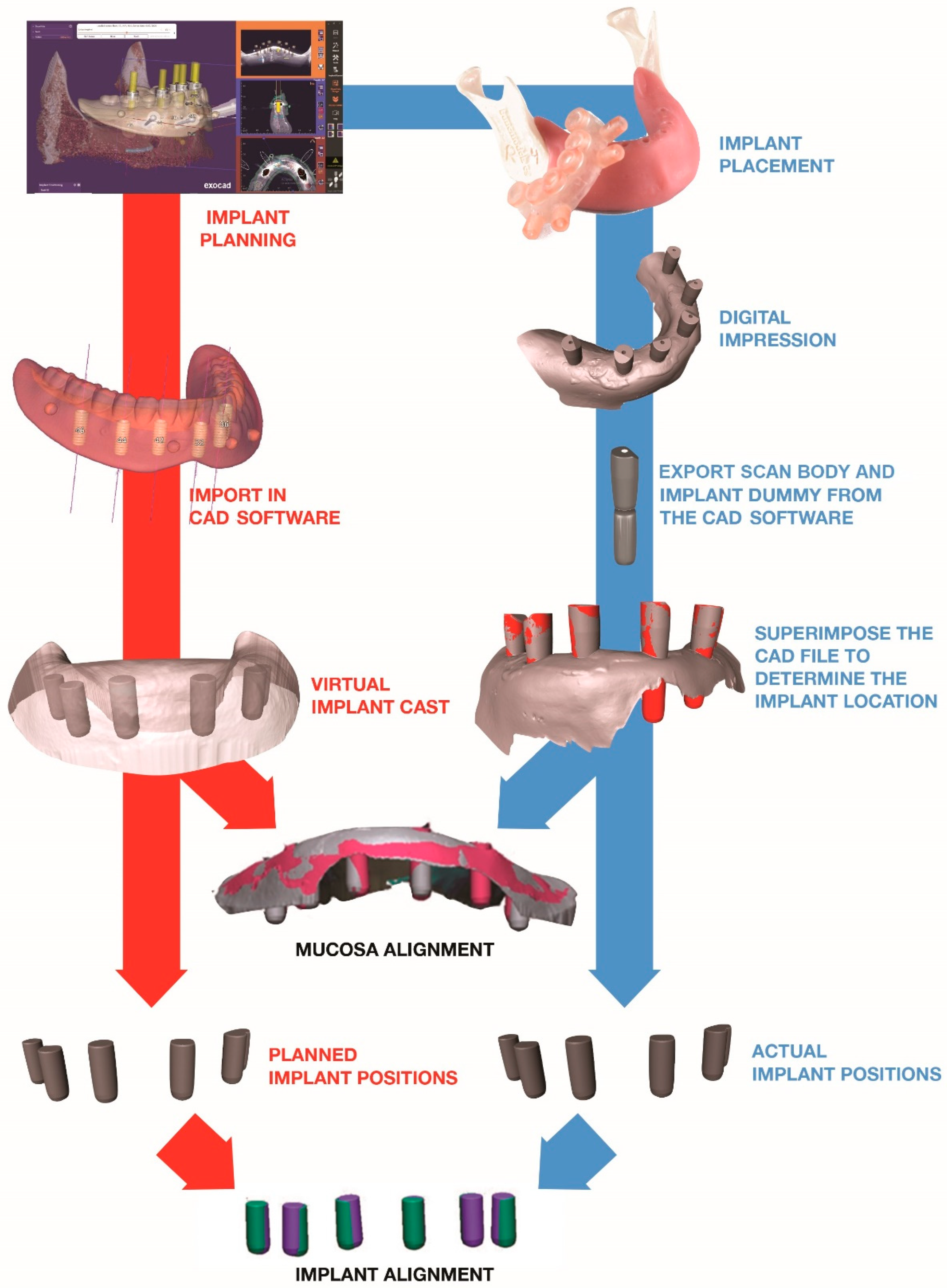
2.2. Implant Planning
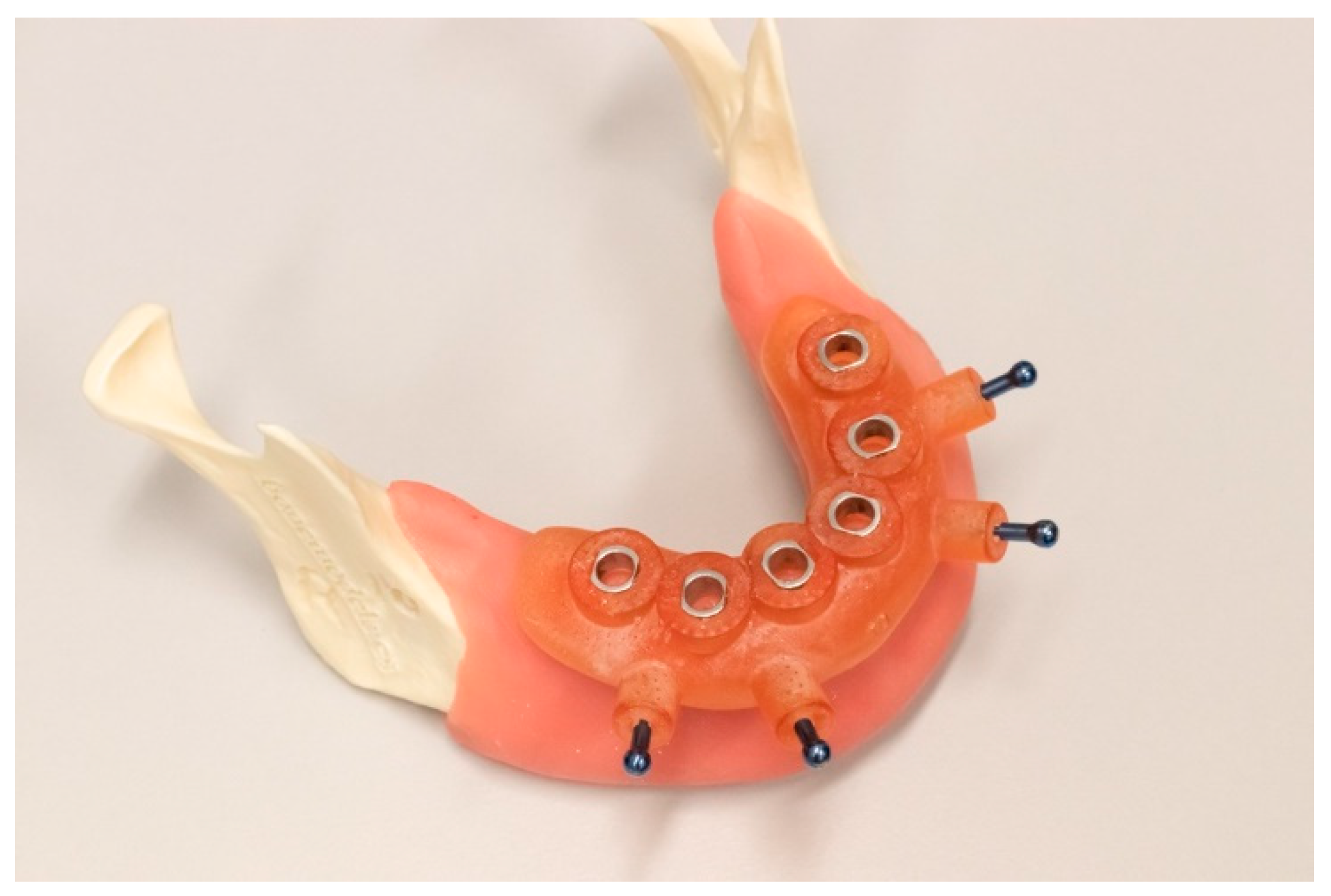
2.3. Guide Fabrication
2.4. Implant Placement
2.5. Scan
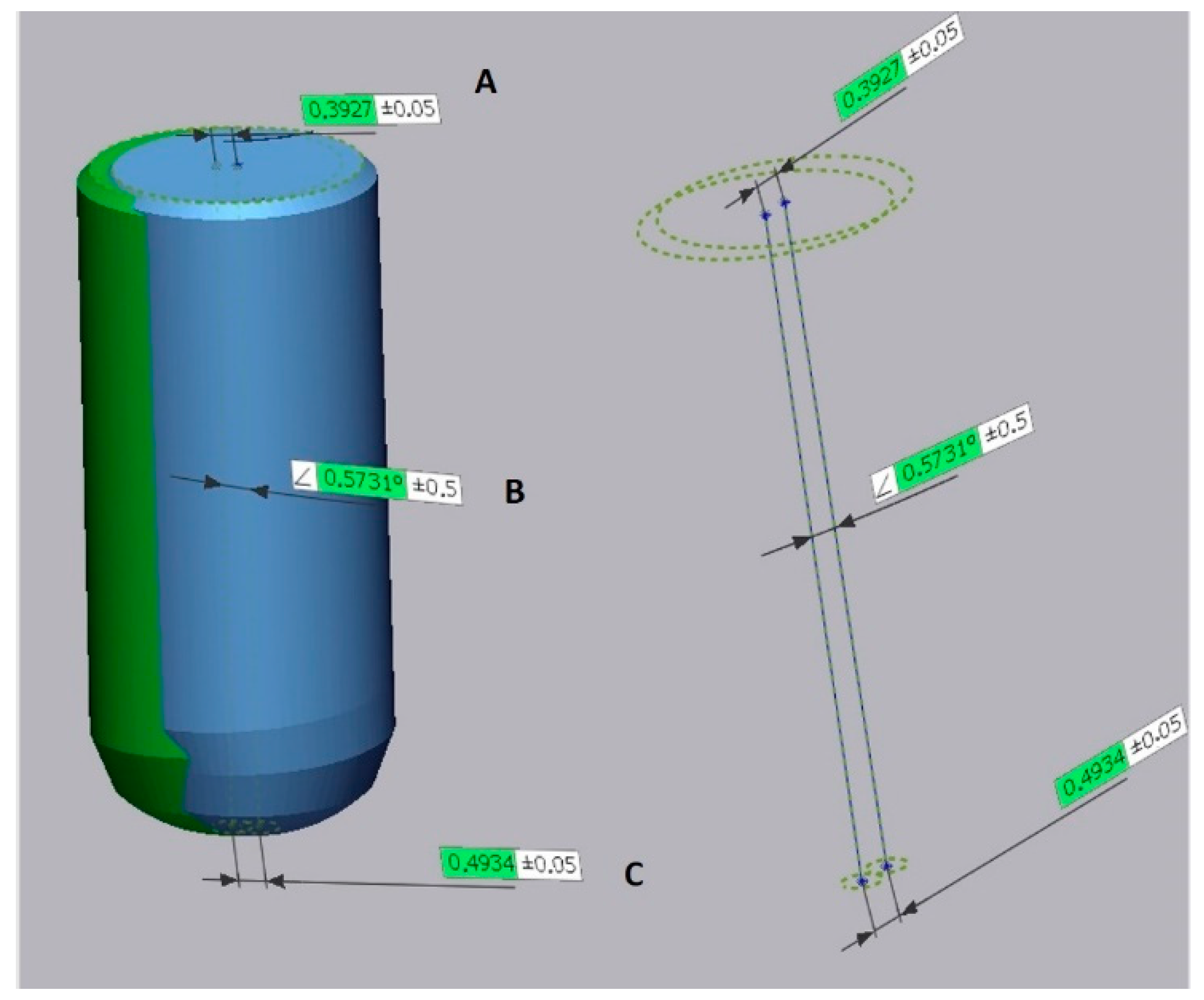
2.6. Comparison
2.6.1. Creating the Reference Model
2.6.2. Creating the Test Model
2.6.3. Implant and Mucosa Alignment
2.7. Statistical Analyses
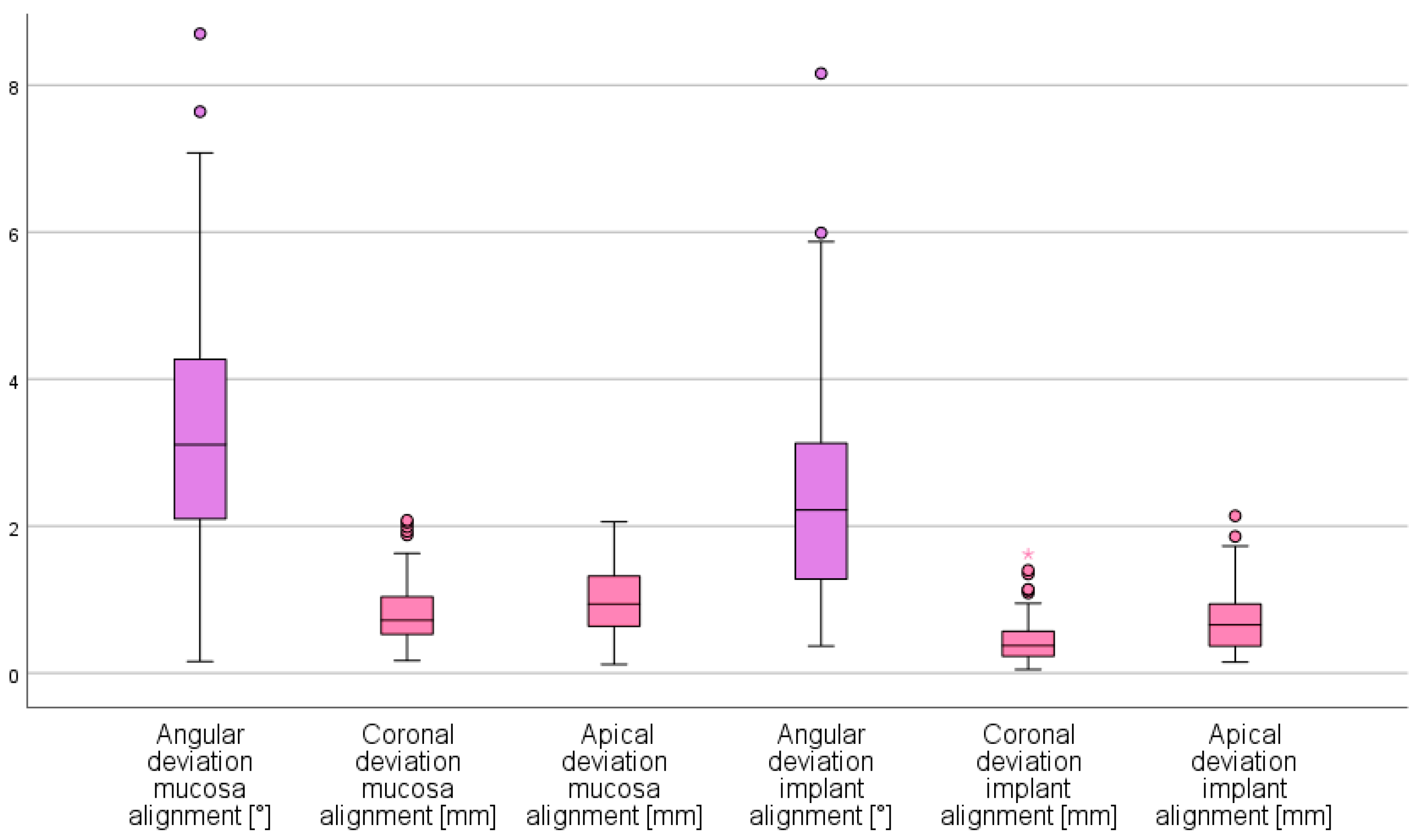
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seo, C.; Juodzbalys, G. Accuracy of Guided Surgery via Stereolithographic Mucosa-Supported Surgical Guide in Implant Surgery for Edentulous Patient: A Systematic Review. J. Oral Maxillofac. Res. 2018, 9, e1. [Google Scholar] [CrossRef] [PubMed]
- Tahmaseb, A.; Wu, V.; Wismeijer, D.; Coucke, W.; Evans, C. The accuracy of static computer-aided implant surgery: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. 16), 416–435. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, Z.; Song, L.; Kuo, C.L.; Shafer, D.M. Clinical Factors Affecting the Accuracy of Guided Implant Surgery-A Systematic Review and Meta-analysis. J. Evid. Based Dent. Pract. 2018, 18, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.; Marquardt, P.; Zwahlen, M.; Jung, R.E. A systematic review on the accuracy and the clinical outcome of computer-guided template-based implant dentistry. Clin. Oral Implant. Res. 2009, 20 (Suppl. 4), 73–86. [Google Scholar] [CrossRef]
- Naeini, E.N.; Atashkadeh, M.; De Bruyn, H.; D’Haese, J. Narrative review regarding the applicability, accuracy, and clinical outcome of flapless implant surgery with or without computer guidance. Clin. Implant Dent. Relat. Res. 2020. [Google Scholar] [CrossRef]
- Marliere, D.A.A.; Demetrio, M.S.; Picinini, L.S.; Oliveira, R.G.; Netto, H. Accuracy of computer-guided surgery for dental implant placement in fully edentulous patients: A systematic review. Eur. J. Dent. 2018, 12, 153–160. [Google Scholar] [CrossRef]
- Vinci, R.; Manacorda, M.; Abundo, R.; Lucchina, A.G.; Scarano, A.; Crocetta, C.; Muzio, L.L.; Gherlone, E.F.; Mastrangelo, F. Accuracy of Edentulous Computer-Aided Implant Surgery as Compared to Virtual Planning: A Retrospective Multicenter Study. J. Clin. Med. 2020, 9, 774. [Google Scholar] [CrossRef]
- Arisan, V.; Karabuda, Z.C.; Ozdemir, T. Accuracy of two stereolithographic guide systems for computer-aided implant placement: A computed tomography-based clinical comparative study. J. Periodontol. 2010, 81, 43–51. [Google Scholar] [CrossRef]
- Abduo, J.; Lau, D. Accuracy of static computer-assisted implant placement in anterior and posterior sites by clinicians new to implant dentistry: In vitro comparison of fully guided, pilot-guided, and freehand protocols. Int. J. Implant Dent. 2020, 6, 10. [Google Scholar] [CrossRef]
- Abduo, J.; Lau, D. Effect of Manufacturing Technique on the Accuracy of Surgical Guides for Static Computer-Aided Implant Surgery. Int. J. Oral Maxillofac. Implant. 2020, 35, 931–938. [Google Scholar] [CrossRef]
- Henprasert, P.; Dawson, D.V.; El-Kerdani, T.; Song, X.; Couso-Queiruga, E.; Holloway, J.A. Comparison of the Accuracy of Implant Position Using Surgical Guides Fabricated by Additive and Subtractive Techniques. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ozan, O.; Turkyilmaz, I.; Ersoy, A.E.; McGlumphy, E.A.; Rosenstiel, S.F. Clinical accuracy of 3 different types of computed tomography-derived stereolithographic surgical guides in implant placement. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2009, 67, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Park, T.; Chun, I.; Yun, K. The accuracy of a 3D printing surgical guide determined by CBCT and model analysis. J. Adv. Prosthodont. 2018, 10, 279–285. [Google Scholar] [CrossRef] [PubMed]
- D’Haese, J.; Van De Velde, T.; Elaut, L.; De Bruyn, H. A prospective study on the accuracy of mucosally supported stereolithographic surgical guides in fully edentulous maxillae. Clin. Implant Dent. Relat. Res. 2012, 14, 293–303. [Google Scholar] [CrossRef]
- Gjelvold, B.; Mahmood, D.J.H.; Wennerberg, A. Accuracy of surgical guides from 2 different desktop 3D printers for computed tomography-guided surgery. J. Prosthet. Dent. 2019, 121, 498–503. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Sinjab, K.; Mendonca, G.; Yu, H.; Wang, H.L. Accuracy of flapless immediate implant placement in anterior maxilla using computer-assisted versus freehand surgery: A cadaver study. Clin. Oral Implant. Res. 2018, 29, 1186–1194. [Google Scholar] [CrossRef]
- Arisan, V.; Karabuda, Z.C.; Piskin, B.; Ozdemir, T. Conventional multi-slice computed tomography (CT) and cone-beam CT (CBCT) for computer-aided implant placement. Part II: Reliability of mucosa-supported stereolithographic guides. Clin. Implant Dent. Relat. Res. 2013, 15, 907–917. [Google Scholar] [CrossRef]
- Ersoy, A.E.; Turkyilmaz, I.; Ozan, O.; McGlumphy, E.A. Reliability of implant placement with stereolithographic surgical guides generated from computed tomography: Clinical data from 94 implants. J. Periodontol. 2008, 79, 1339–1345. [Google Scholar] [CrossRef]
- Lin, Y.K.; Yau, H.T.; Wang, I.C.; Zheng, C.; Chung, K.H. A novel dental implant guided surgery based on integration of surgical template and augmented reality. Clin. Implant Dent. Relat. Res. 2015, 17, 543–553. [Google Scholar] [CrossRef]
- Pettersson, A.; Komiyama, A.; Hultin, M.; Näsström, K.; Klinge, B. Accuracy of virtually planned and template guided implant surgery on edentate patients. Clin. Implant Dent. Relat. Res. 2012, 14, 527–537. [Google Scholar] [CrossRef]
- Vercruyssen, M.; Cox, C.; Coucke, W.; Naert, I.; Jacobs, R.; Quirynen, M. A randomized clinical trial comparing guided implant surgery (bone- or mucosa-supported) with mental navigation or the use of a pilot-drill template. J. Clin. Periodontol. 2014, 41, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Verhamme, L.M.; Meijer, G.J.; Boumans, T.; Schutyser, F.; Berge, S.J.; Maal, T.J. A clinically relevant validation method for implant placement after virtual planning. Clin. Oral Implant. Res. 2013, 24, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, A.; Kero, T.; Gillot, L.; Cannas, B.; Fäldt, J.; Söderberg, R.; Näsström, K. Accuracy of CAD/CAM-guided surgical template implant surgery on human cadavers: Part I. J. Prosthet. Dent. 2010, 103, 334–342. [Google Scholar] [CrossRef]
- Tan, P.L.B.; Layton, D.M.; Wise, S.L. In vitro comparison of guided versus freehand implant placement: Use of a new combined TRIOS surface scanning, Implant Studio, CBCT, and stereolithographic virtually planned and guided technique. Int. J. Comput. Dent. 2018, 21, 87–95. [Google Scholar] [PubMed]
- Cassetta, M.; Giansanti, M.; Di Mambro, A.; Stefanelli, L.V. Accuracy of positioning of implants inserted using a mucosa-supported stereolithographic surgical guide in the edentulous maxilla and mandible. Int. J. Oral Maxillofac. Implant. 2014, 29, 1071–1078. [Google Scholar] [CrossRef]
- Deeb, G.R.; Allen, R.K.; Hall, V.P.; Whitley, D., 3rd; Laskin, D.M.; Bencharit, S. How Accurate Are Implant Surgical Guides Produced with Desktop Stereolithographic 3-Dimentional Printers? J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2017, 75, 2559.e2551–2559.e2558. [Google Scholar] [CrossRef]
- Lin, C.C.; Wu, C.Z.; Huang, M.S.; Huang, C.F.; Cheng, H.C.; Wang, D.P. Fully Digital Workflow for Planning Static Guided Implant Surgery: A Prospective Accuracy Study. J. Clin. Med. 2020, 9, 980. [Google Scholar] [CrossRef]
- Kiatkroekkrai, P.; Takolpuckdee, C.; Subbalekha, K.; Mattheos, N.; Pimkhaokham, A. Accuracy of implant position when placed using static computer-assisted implant surgical guides manufactured with two different optical scanning techniques: A randomized clinical trial. Int. J. Oral Maxillofac. Surg. 2020, 49, 377–383. [Google Scholar] [CrossRef]
- Schulze, R.K.; Berndt, D.; d’Hoedt, B. On cone-beam computed tomography artifacts induced by titanium implants. Clin. Oral Implant. Res. 2010, 21, 100–107. [Google Scholar] [CrossRef]
- de Waard, O.; Rangel, F.A.; Fudalej, P.S.; Bronkhorst, E.M.; Kuijpers-Jagtman, A.M.; Breuning, K.H. Reproducibility and accuracy of linear measurements on dental models derived from cone-beam computed tomography compared with digital dental casts. Am. J. Orthod. Dentofac. Orthop. 2014, 146, 328–336. [Google Scholar] [CrossRef]
- Becker, K.; Schmucker, U.; Schwarz, F.; Drescher, D. Accuracy and eligibility of CBCT to digitize dental plaster casts. Clin. Oral Investig. 2018, 22, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Son, K.; Huang, M.Y.; Lee, K.B. A method to evaluate the accuracy of dental implant placement without postoperative radiography after computer-guided implant surgery: A dental technique. J. Prosthet. Dent. 2020, 123, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Skjerven, H.; Olsen-Bergem, H.; Ronold, H.J.; Riis, U.H.; Ellingsen, J.E. Comparison of postoperative intraoral scan versus cone beam computerised tomography to measure accuracy of guided implant placement-A prospective clinical study. Clin. Oral Implant. Res. 2019, 30, 531–541. [Google Scholar] [CrossRef]
- Brandt, J.; Brenner, M.; Lauer, H.C.; Brandt, S. Accuracy of a Template-Guided Implant Surgery System with a CAD/CAM-Based Measurement Method: An In Vitro Study. Int. J. Oral Maxillofac. Implant. 2018, 33, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Derksen, W.; Wismeijer, D.; Flugge, T.; Hassan, B.; Tahmaseb, A. The accuracy of computer-guided implant surgery with tooth-supported, digitally designed drill guides based on CBCT and intraoral scanning. A prospective cohort study. Clin. Oral Implant. Res. 2019, 30, 1005–1015. [Google Scholar] [CrossRef]
- Ochi, M.; Kanazawa, M.; Sato, D.; Kasugai, S.; Hirano, S.; Minakuchi, S. Factors affecting accuracy of implant placement with mucosa-supported stereolithographic surgical guides in edentulous mandibles. Comput. Biol. Med. 2013, 43, 1653–1660. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, F.Y.; Wu, G.H.; Zhang, W.; Yin, J. Measurement of mucosal thickness in denture-bearing area of edentulous mandible. Chin. Med. J. 2015, 128, 342–347. [Google Scholar] [CrossRef]
- D’Haese, J.; De Bruyn, H. Effect of smoking habits on accuracy of implant placement using mucosally supported stereolithographic surgical guides. Clin. Implant. Dent. Relat. Res. 2013, 15, 402–411. [Google Scholar] [CrossRef]
- Kuhl, S.; Payer, M.; Zitzmann, N.U.; Lambrecht, J.T.; Filippi, A. Technical accuracy of printed surgical templates for guided implant surgery with the coDiagnostiX software. Clin. Implant Dent. Relat. Res. 2015, 17 (Suppl. 1), e177–e182. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’haese, R.; Vrombaut, T.; Hommez, G.; De Bruyn, H.; Vandeweghe, S. Accuracy of Guided Implant Surgery in the Edentulous Jaw Using Desktop 3D-Printed Mucosal Supported Guides. J. Clin. Med. 2021, 10, 391. https://doi.org/10.3390/jcm10030391
D’haese R, Vrombaut T, Hommez G, De Bruyn H, Vandeweghe S. Accuracy of Guided Implant Surgery in the Edentulous Jaw Using Desktop 3D-Printed Mucosal Supported Guides. Journal of Clinical Medicine. 2021; 10(3):391. https://doi.org/10.3390/jcm10030391
Chicago/Turabian StyleD’haese, Rani, Tom Vrombaut, Geert Hommez, Hugo De Bruyn, and Stefan Vandeweghe. 2021. "Accuracy of Guided Implant Surgery in the Edentulous Jaw Using Desktop 3D-Printed Mucosal Supported Guides" Journal of Clinical Medicine 10, no. 3: 391. https://doi.org/10.3390/jcm10030391
APA StyleD’haese, R., Vrombaut, T., Hommez, G., De Bruyn, H., & Vandeweghe, S. (2021). Accuracy of Guided Implant Surgery in the Edentulous Jaw Using Desktop 3D-Printed Mucosal Supported Guides. Journal of Clinical Medicine, 10(3), 391. https://doi.org/10.3390/jcm10030391




