Benchmarking PASADENA Consensus along the Learning Curve of Robotic Radical Cystectomy with Intracorporeal Neobladder: CUSUM Based Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Technique
2.2. Learning Curve Assessment
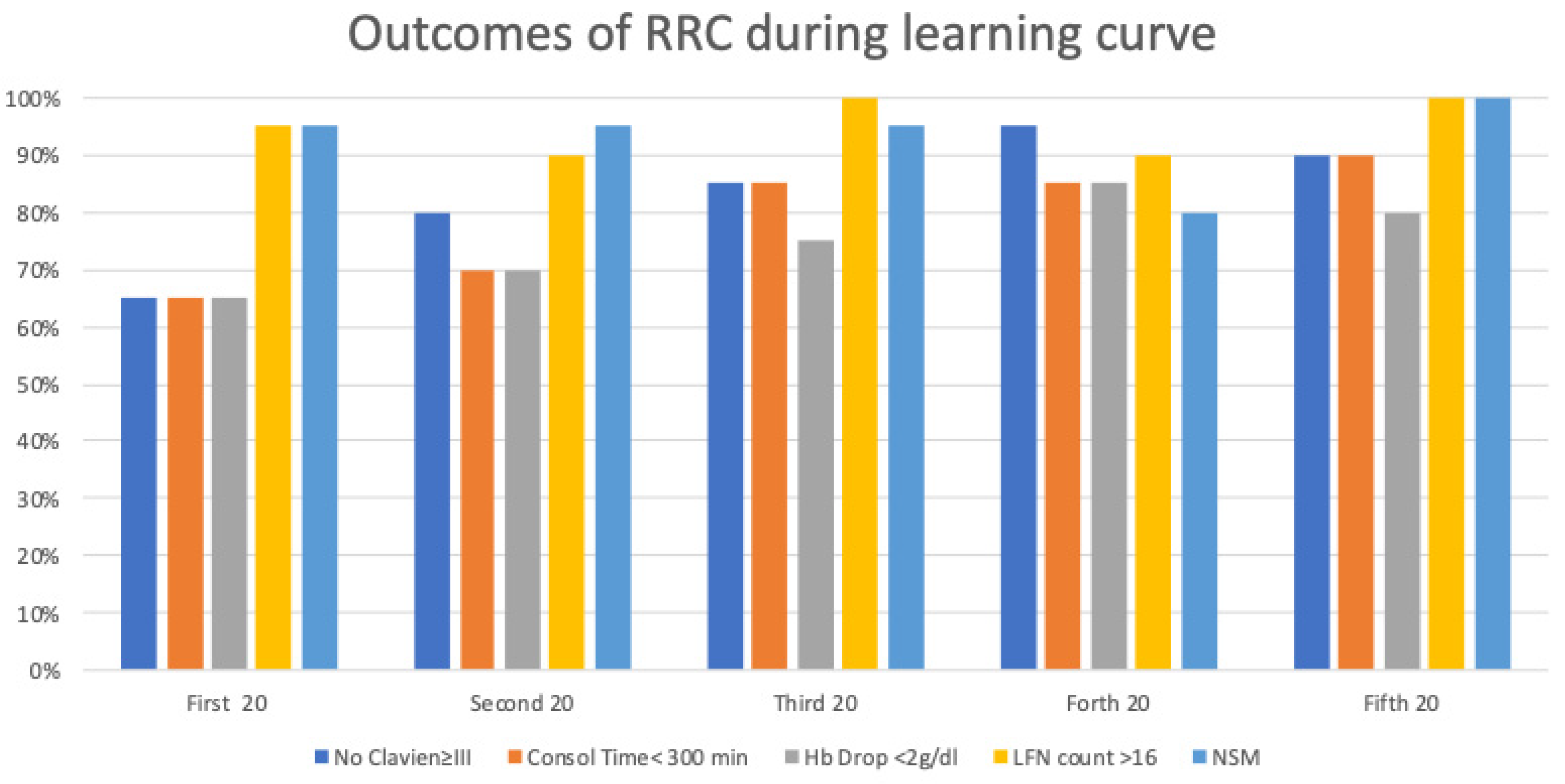
- Operative time (OT) <5 h;
- 24-h Hemoglobin (Hb) drop <2 g/dl;
- Severe complications (according to the Clavien classification system) <30%;
- Positive surgical margins <5%;
- Complete lymph-node dissection (defined as more than 16 nodes).
Graph Interpretation
2.3. Statistical Analysis
3. Results
3.1. Operative Time
3.2. Complications
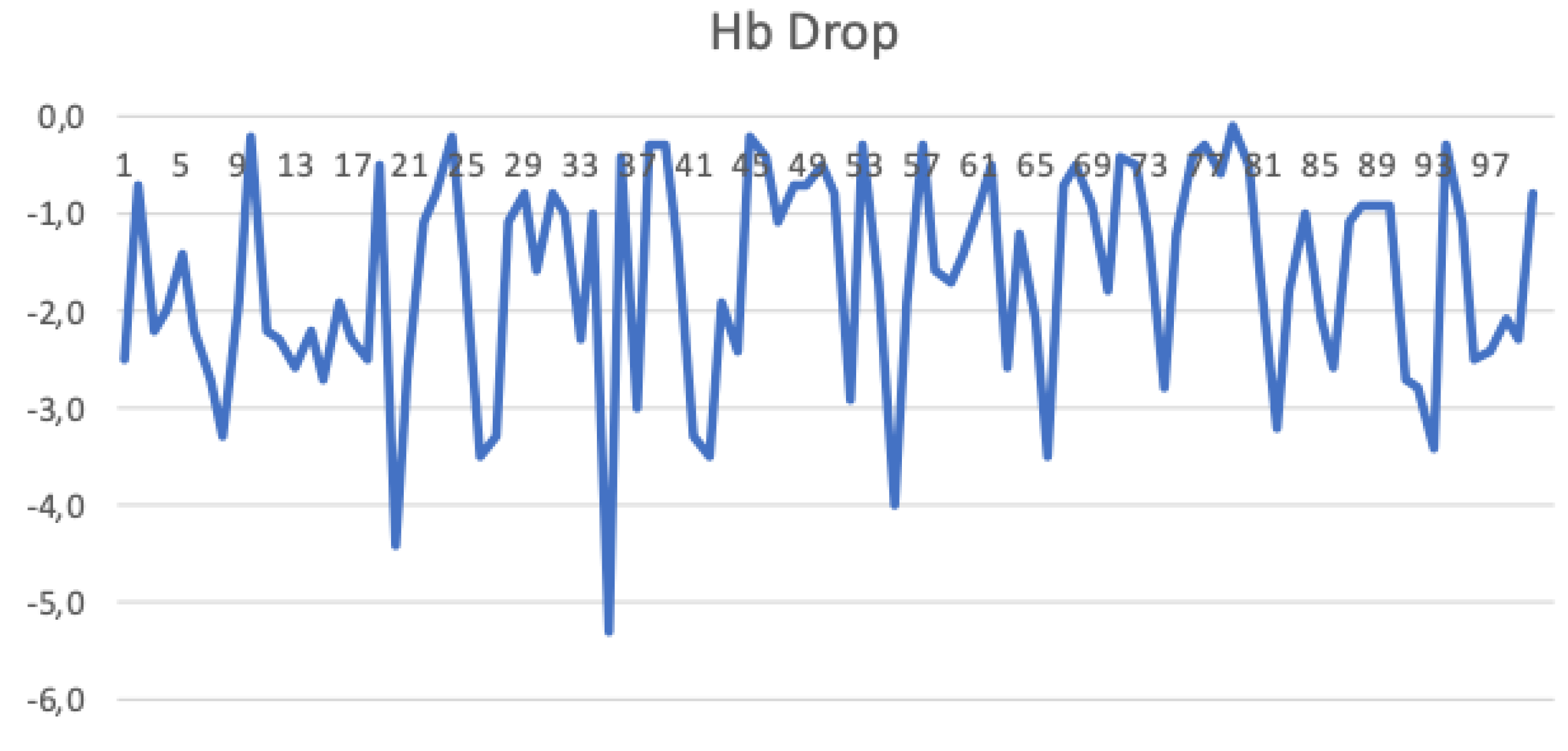
3.3. Haemoglobin Drop
3.4. Positive Surgical Margins
3.5. Lymphnode Count
3.6. Comprehensive Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The American Cancer Society medical and editorial content team What is Bladder Cancer? Am. Cancer Soc. 2014, 17–18. Available online: https://www.cancer.org/cancer/bladder-cancer/about/what-is-bladder-cancer.html (accessed on 18 December 2021).
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, C.; Cicione, A.; Izquierdo, L.; Lombardo, R.; Tema, G.; Lotrecchiano, G.; Minervini, A.; Simone, G.; Cindolo, L.; D’Orta, C.; et al. Multicenter Analysis of Postoperative Complications in Octogenarians After Radical Cystectomy and Ureterocutaneostomy: The Role of the Frailty Index. Clin. Genitourin. Cancer 2019, 17, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Cicione, A.; de Nunzio, C.; Lombardo, R.; Trucchi, A.; Manno, S.; Lima, E.; Tubaro, A. Complications and quality of life of ileal conduit, orthotopic neobladder and ureterocutaneostomy: Systematic review of reports using the Clavien-Dindo Classification. Minerva Urol. Nefrol. 2020, 72, 408–419. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, C.; Franco, G.; Cindolo, L.; Autorino, R.; Cicione, A.; Perdonà, S.; Falsaperla, M.; Gacci, M.; Leonardo, C.; Damiano, R.; et al. Transuretral resection of the bladder (TURB): Analysis of complications using a modified Clavien system in an Italian real life cohort. Eur. J. Surg. Oncol. 2014, 40, 90–95. [Google Scholar] [CrossRef]
- De Nunzio, C.; Cindolo, L.; Leonardo, C.; Antonelli, A.; Ceruti, C.; Franco, G.; Falsaperla, M.; Gallucci, M.; Alvarez-Maestro, M.; Minervini, A.; et al. Analysis of radical cystectomy and urinary diversion complications with the Clavien classification system in an Italian real life cohort. Eur. J. Surg. Oncol. 2013, 39, 792–798. [Google Scholar] [CrossRef]
- Mastroianni, R.; Tuderti, G.; Anceschi, U.; Bove, A.M.; Brassetti, A.; Ferriero, M.; Zampa, A.; Giannarelli, D.; Guaglianone, S.; Gallucci, M.; et al. Comparison of Patient-reported Health-related Quality of Life Between Open Radical Cystectomy and Robot-assisted Radical Cystectomy with Intracorporeal Urinary Diversion: Interim Analysis of a Randomised Controlled Trial. Eur. Urol. Focus 2021, 1–7. [Google Scholar] [CrossRef]
- Simone, G.; Tuderti, G.; Misuraca, L.; Anceschi, U.; Ferriero, M.; Minisola, F.; Guaglianone, S.; Gallucci, M. Perioperative and mid-term oncologic outcomes of robotic assisted radical cystectomy with totally intracorporeal neobladder: Results of a propensity score matched comparison with open cohort from a single-centre series. Eur. J. Surg. Oncol. 2018, 44, 1432–1438. [Google Scholar] [CrossRef]
- Simone, G.; Papalia, R.; Misuraca, L.; Tuderti, G.; Minisola, F.; Ferriero, M.; Vallati, G.; Guaglianone, S.; Gallucci, M. Robotic Intracorporeal Padua Ileal Bladder: Surgical Technique, Perioperative, Oncologic and Functional Outcomes. Eur. Urol. 2018, 73, 934–940. [Google Scholar] [CrossRef]
- Bochner, B.H.; Dalbagni, G.; Sjoberg, D.D.; Silberstein, J.; Keren Paz, G.E.; Donat, S.M.H.; Coleman, J.A.; Mathew, S.; Vickers, A.; Schnorr, G.C.; et al. Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: A randomized clinical trial. Eur. Urol. 2015, 67, 1042–1050. [Google Scholar] [CrossRef]
- Rai, B.P.; Bondad, J.; Vasdev, N.; Adshead, J.; Lane, T.; Ahmed, K.; Khan, M.S.; Dasgupta, P.; Guru, K.; Chlosta, P.L.; et al. Robotic versus open radical cystectomy for bladder cancer in adults. Cochrane Database Syst. Rev. 2019, 2019. [Google Scholar] [CrossRef]
- Porreca, A.; Mineo Bianchi, F.; Romagnoli, D.; D’Agostino, D.; Corsi, P.; Giampaoli, M.; Salvaggio, A.; Bianchi, L.; Schiavina, R.; Brunocilla, E.; et al. Robot-assisted radical cystectomy with totally intracorporeal urinary diversion: Surgical and early functional outcomes through the learning curve in a single high-volume center. J. Robot. Surg. 2020, 14, 261–269. [Google Scholar] [CrossRef]
- MacKenzie, K.R.; Aning, J. Defining competency in flexible cystoscopy: A novel approach using cumulative Sum analysis. BMC Urol. 2016, 16, 31. [Google Scholar] [CrossRef][Green Version]
- Woodall, W.H.; Rakovich, G.; Steiner, S.H. An overview and critique of the use of cumulative sum methods with surgical learning curve data. Stat. Med. 2021, 40, 1400–1413. [Google Scholar] [CrossRef] [PubMed]
- Fortea-Sanchis, C.; Martínez-Ramos, D.; Escrig-Sos, J. CUSUM charts in the quality control of colon cancer lymph node analysis: A population-registry study 11 Medical and Health Sciences 1112 Oncology and Carcinogenesis. World J. Surg. Oncol. 2018, 16, 230. [Google Scholar] [CrossRef] [PubMed]
- Wohl, H. The Cusum Plot: Its Utility in the Analysis of Clinical Data. N. Engl. J. Med. 1977, 296, 1044–1045. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Pautler, S.E. Use of cumulative summation (CUSUM) as a tool for early feedback and monitoring in robot-assisted radical prostatectomy outcomes and performance. Can. Urol. Assoc. J. 2019, 13, 53–58. [Google Scholar] [CrossRef]
- Norisue, Y.; Tokuda, Y.; Juarez, M.; Uchimido, R.; Fujitani, S.; Stoeckel, D.A. Combined cumulative sum (CUSUM) and chronological environmental analysis as a tool to improve the learning environment for linear-probe endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) trainees: A pilot study. BMC Pulm. Med. 2017, 17, 32. [Google Scholar] [CrossRef][Green Version]
- Gezer, S.; Avcl, A.; Türktan, M. Cusum analysis for learning curve of videothoracoscopic lobectomy. Open Med. 2016, 11, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Karl, A.; Buchner, A.; Becker, A.; Staehler, M.; Seitz, M.; Khoder, W.; Schneevoigt, B.; Weninger, E.; Rittler, P.; Grimm, T.; et al. A new concept for early recovery after surgery for patients undergoing radical cystectomy for bladder cancer: Results of a prospective randomized study. J. Urol. 2014, 191, 335–340. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The american joint committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Hayn, M.H.; Hussain, A.; Mansour, A.M.; Andrews, P.E.; Carpentier, P.; Castle, E.; Dasgupta, P.; Rimington, P.; Thomas, R.; Khan, S.; et al. The learning curve of robot-assisted radical cystectomy: Results from the international robotic cystectomy consortium. Eur. Urol. 2010, 58, 197–202. [Google Scholar] [CrossRef]
- Porreca, A.; Chessa, F.; Romagnoli, D.; Salvaggio, A.; Cafarelli, A.; Borghesi, M.; Bianchi, L.; Dandrea, M.; D’Agostino, D.; Dente, D.; et al. Robot assisted radical cystectomy with totally intracorporeal urinary diversion: Initial, single-surgeon’s experience after a modified modular training. Minerva Urol. Nefrol. 2018, 70, 193–201. [Google Scholar] [CrossRef]
- Abboudi, H.; Khan, M.S.; Guru, K.A.; Froghi, S.; De Win, G.; Van Poppel, H.; Dasgupta, P.; Ahmed, K. Learning curves for urological procedures: A systematic review. BJU Int. 2014, 114, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Dell’Oglio, P.; Mazzone, E.; Lambert, E.; Vollemaere, J.; Goossens, M.; Larcher, A.; Van Der Jeugt, J.; Devos, G.; Poelaert, F.; Uvin, P.; et al. The Effect of Surgical Experience on Perioperative and Oncological Outcomes After Robot-assisted Radical Cystectomy with Intracorporeal Urinary Diversion: Evidence from a Referral Centre with Extensive Experience in Robotic Surgery. Eur. Urol. Focus 2021, 7, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Catto, J.W.F.; Khetrapal, P.; Ambler, G.; Sarpong, R.; Khan, M.S.; Tan, M.; Feber, A.; Dixon, S.; Goodwin, L.; Williams, N.R.; et al. Robot-assisted radical cystectomy with intracorporeal urinary diversion versus open radical cystectomy (iROC): Protocol for a randomised controlled trial with internal feasibility study. BMJ Open 2018, 8, e020500. [Google Scholar] [CrossRef]
- Chen, J.; Remulla, D.; Nguyen, J.H.; Aastha, D.; Liu, Y.; Dasgupta, P.; Hung, A.J. Current status of artificial intelligence applications in urology and their potential to influence clinical practice. BJU Int. 2019, 124, 567–577. [Google Scholar] [CrossRef]

| Age (years) | 62 (57/66) |
| Body Mass Index (kg/m2) | 26 (24/27) |
| Haemoglobin (Hb) preop (g/dl) | 13.6 (11.8/14.5) |
| Hb Drop (g/dl) | −2.7 (−3.7/−2.0) |
| Number of lymphnodes | 32 (24/38) |
| Operative Time (min) | 330 (300/378) |
| Lenght of stay (days) | 10 (9/14) |
| pT3 > 3° | 28/100 (28%) |
| pN+ | 22/100 (22%) |
| Neoadiuvant Chemotherapy | 37/100 (37%) |
| Complications | 26 Patients |
|---|---|
| Clavien I Mild Hypoxemia Catheter obstruction Other | 9/100 3/100 3/100 3/100 |
| Clavien II Fever Anemia requiring trasfusion Acute respiratory failure | 23/100 15/100 6/100 2/100 |
| Clavien IIIa Urine leakage requiring nephrostomy placement | 7/100 |
| Clavien IIIb Bowel Leakage | 9/100 |
| Clavien IV Sepsis and acute kidney failure | 1/100 |
| Clavien V | 0/100 |
| Ureteral stent placement | 15/100 |
| Reimplantation | 13/100 |
| Nephrostomy placement | 4/100 |
| Fever | 2/100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, R.; Mastroianni, R.; Tuderti, G.; Ferriero, M.; Brassetti, A.; Anceschi, U.; Guaglianone, S.; De Nunzio, C.; Cicione, A.; Tubaro, A.; et al. Benchmarking PASADENA Consensus along the Learning Curve of Robotic Radical Cystectomy with Intracorporeal Neobladder: CUSUM Based Assessment. J. Clin. Med. 2021, 10, 5969. https://doi.org/10.3390/jcm10245969
Lombardo R, Mastroianni R, Tuderti G, Ferriero M, Brassetti A, Anceschi U, Guaglianone S, De Nunzio C, Cicione A, Tubaro A, et al. Benchmarking PASADENA Consensus along the Learning Curve of Robotic Radical Cystectomy with Intracorporeal Neobladder: CUSUM Based Assessment. Journal of Clinical Medicine. 2021; 10(24):5969. https://doi.org/10.3390/jcm10245969
Chicago/Turabian StyleLombardo, Riccardo, Riccardo Mastroianni, Gabriele Tuderti, Mariaconsiglia Ferriero, Aldo Brassetti, Umberto Anceschi, Salvatore Guaglianone, Cosimo De Nunzio, Antonio Cicione, Andrea Tubaro, and et al. 2021. "Benchmarking PASADENA Consensus along the Learning Curve of Robotic Radical Cystectomy with Intracorporeal Neobladder: CUSUM Based Assessment" Journal of Clinical Medicine 10, no. 24: 5969. https://doi.org/10.3390/jcm10245969
APA StyleLombardo, R., Mastroianni, R., Tuderti, G., Ferriero, M., Brassetti, A., Anceschi, U., Guaglianone, S., De Nunzio, C., Cicione, A., Tubaro, A., Gallucci, M., & Simone, G. (2021). Benchmarking PASADENA Consensus along the Learning Curve of Robotic Radical Cystectomy with Intracorporeal Neobladder: CUSUM Based Assessment. Journal of Clinical Medicine, 10(24), 5969. https://doi.org/10.3390/jcm10245969







