Characterization of Spinal Sensorimotor Network Using Transcutaneous Spinal Stimulation during Voluntary Movement Preparation and Performance
Abstract
1. Introduction
2. Methods
2.1. Participants
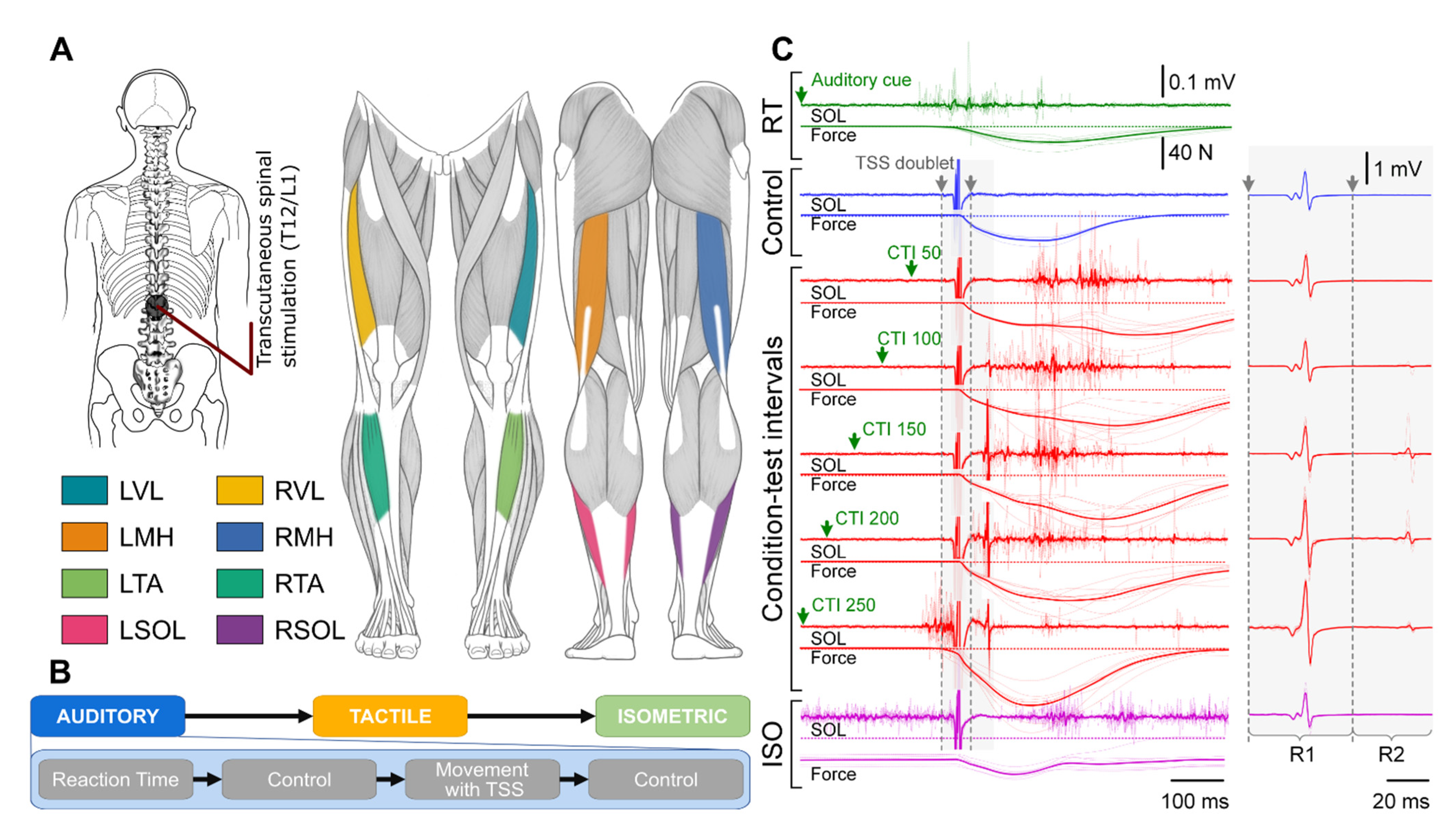
2.2. Experimental Setup
2.3. Experimental Procedure
2.4. Statistical Analysis
3. Results
3.1. Reaction Time
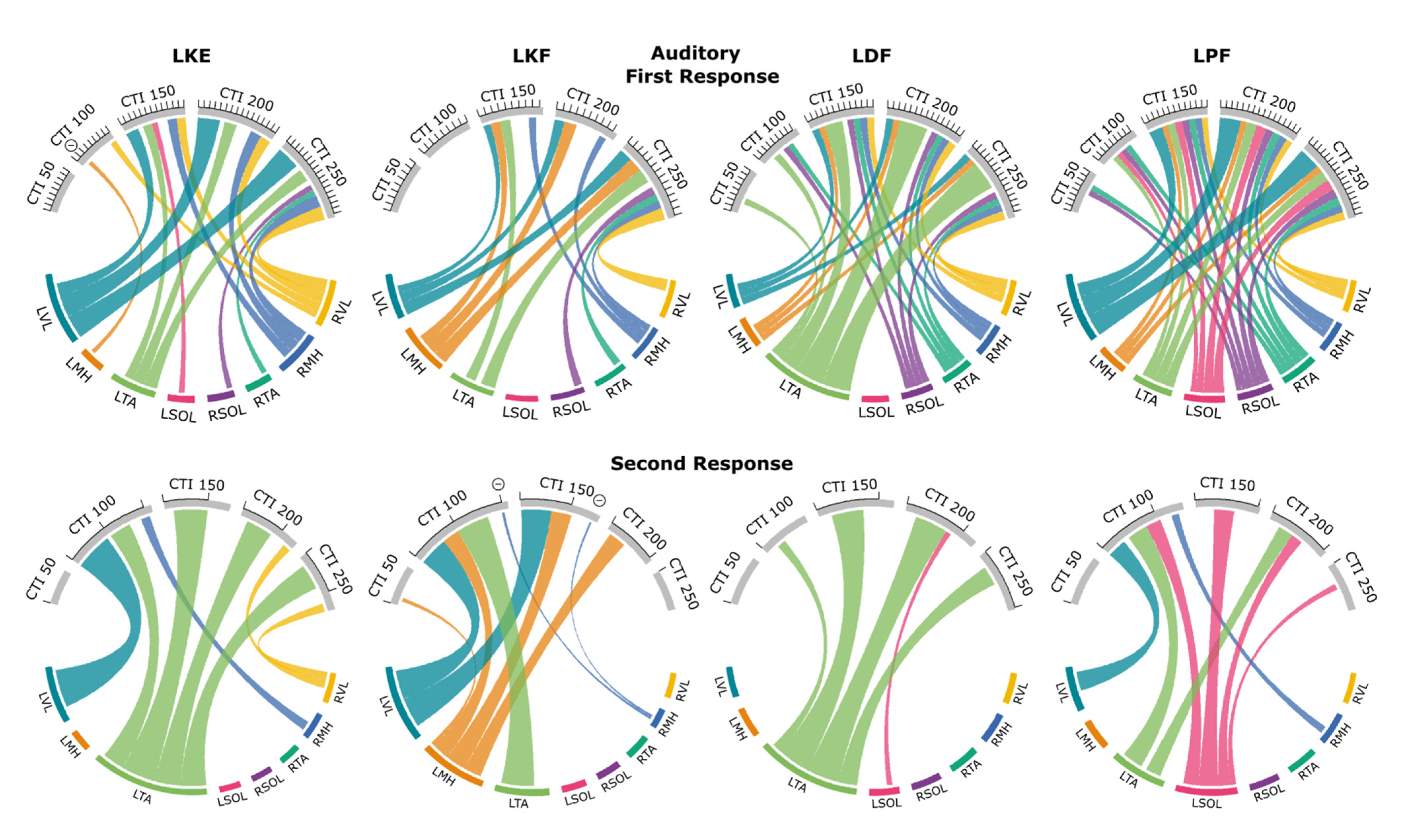
3.2. Auditory Condition
3.2.1. First Responses (R1s)
3.2.2. Second Responses (R2s)
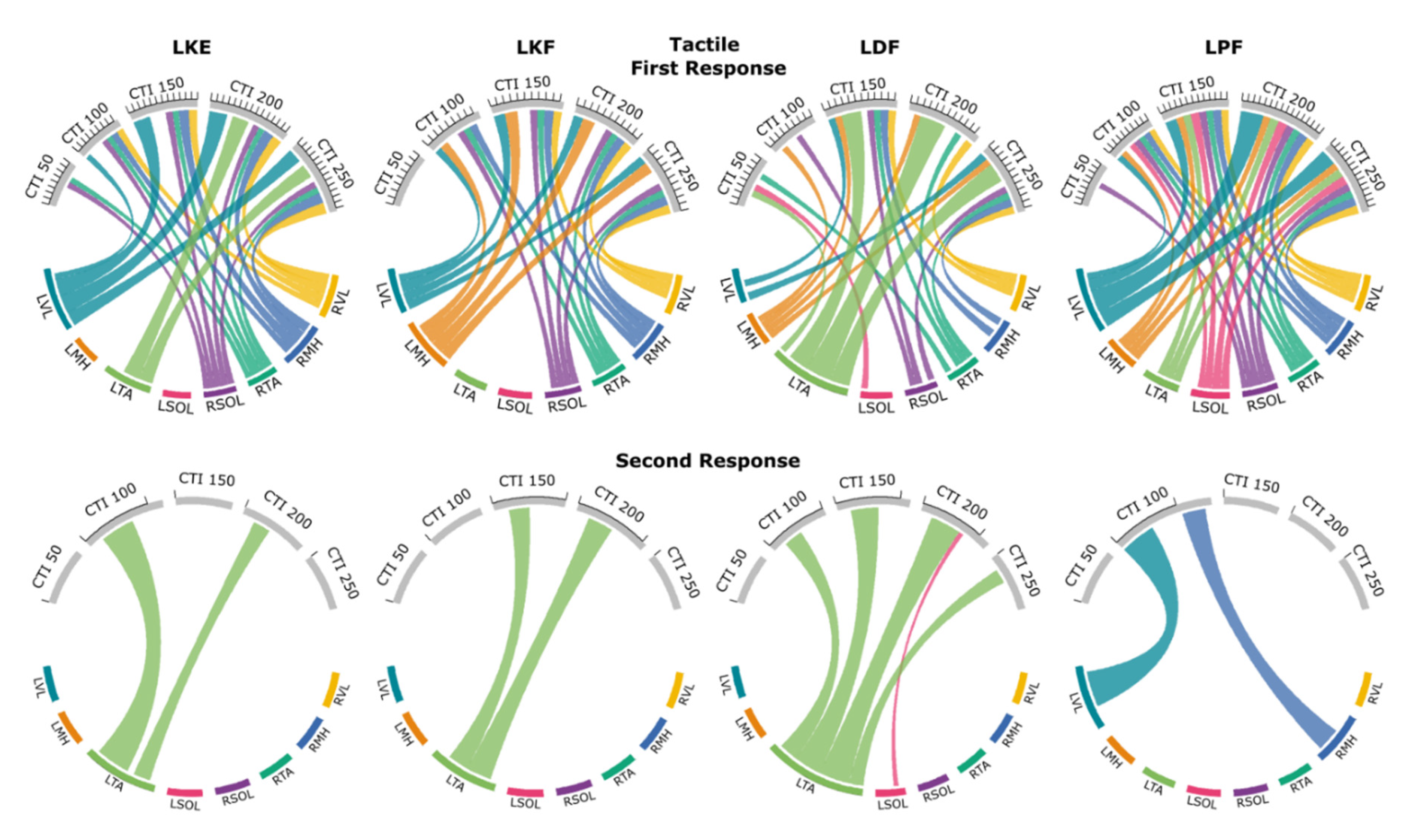
3.3. Tactile Condition
3.3.1. First Responses (R1s)
3.3.2. Second Responses (R2s)
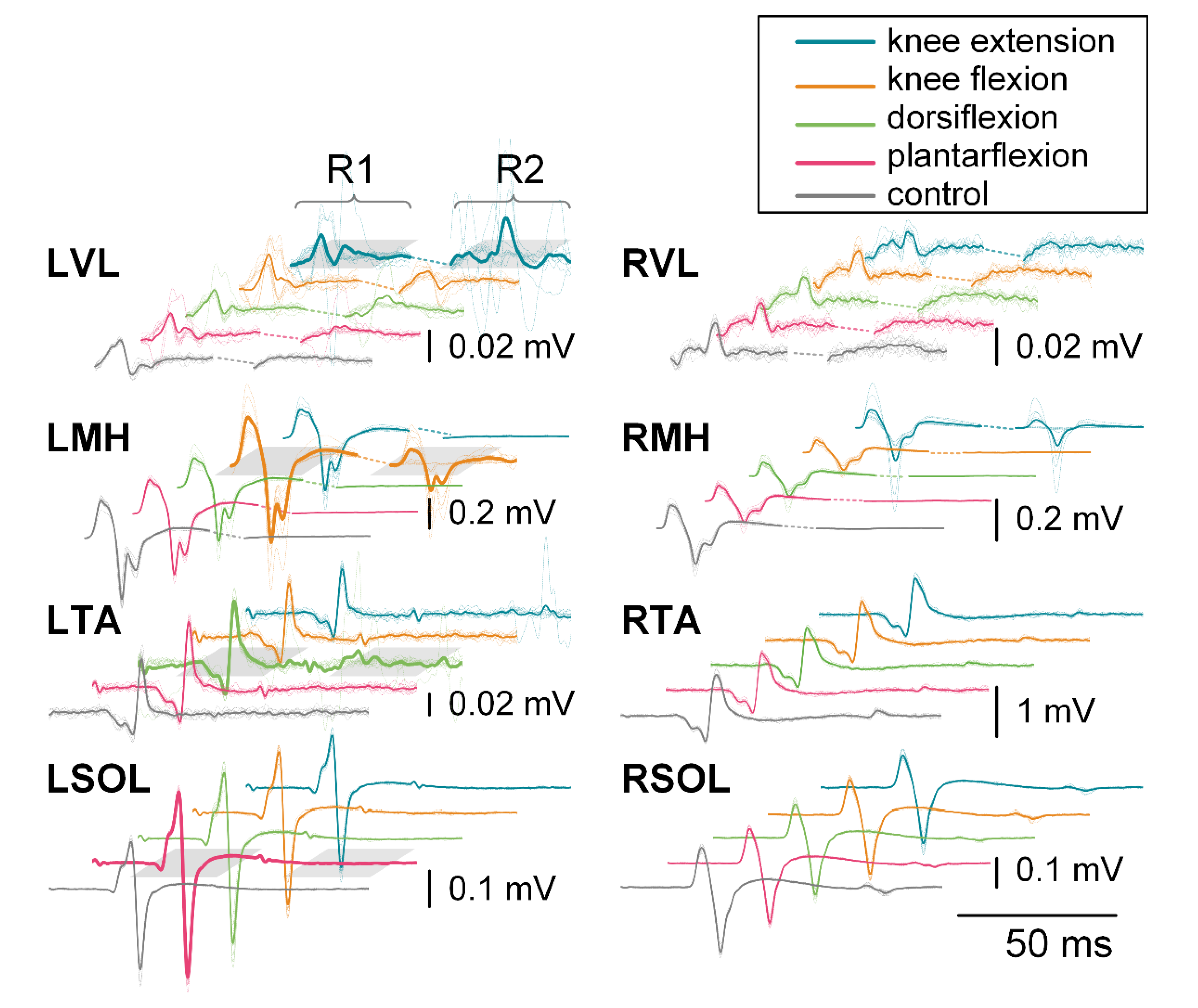
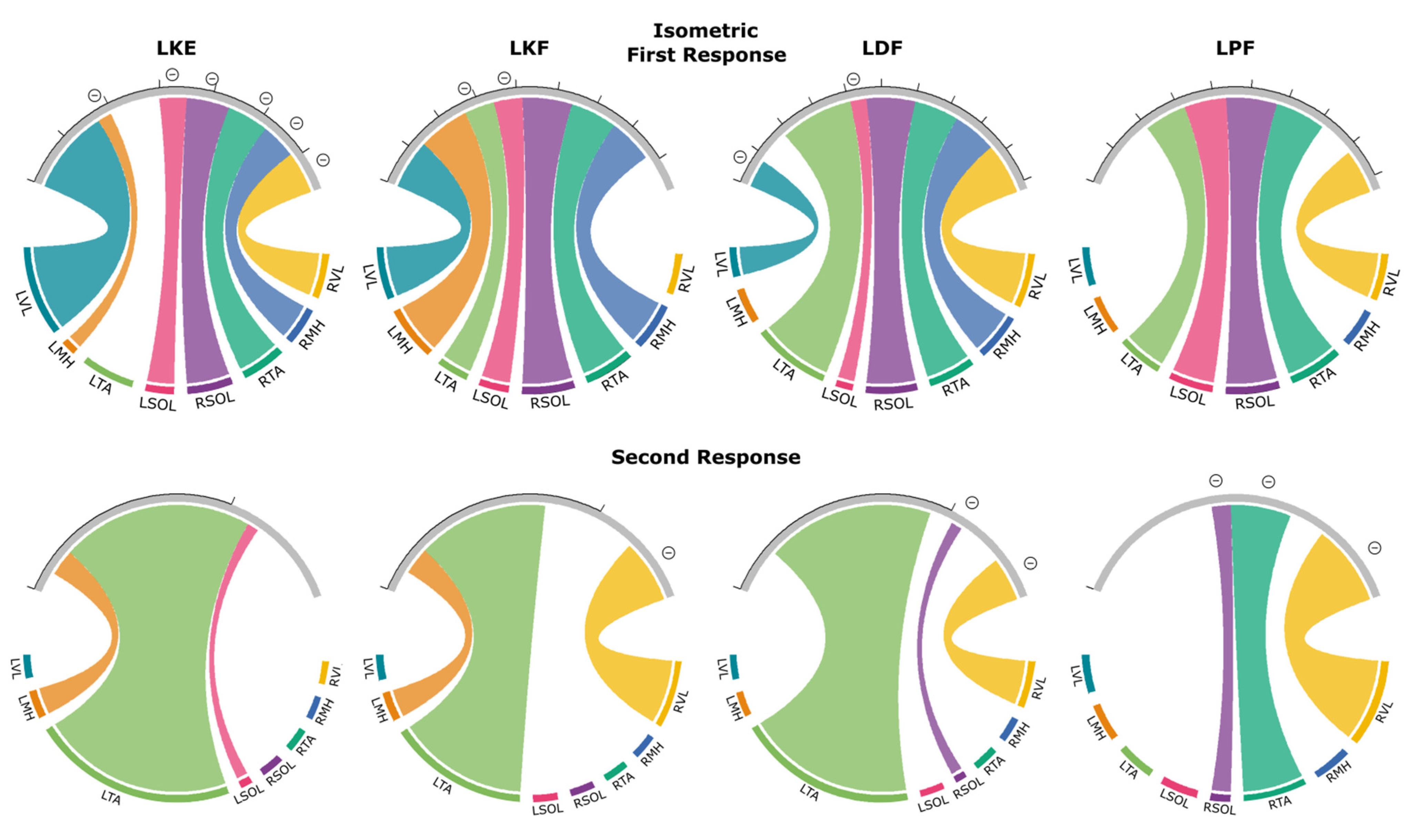
3.4. Isometric Contraction Response
3.4.1. First Responses (R1s)
3.4.2. Second Responses (R2s)
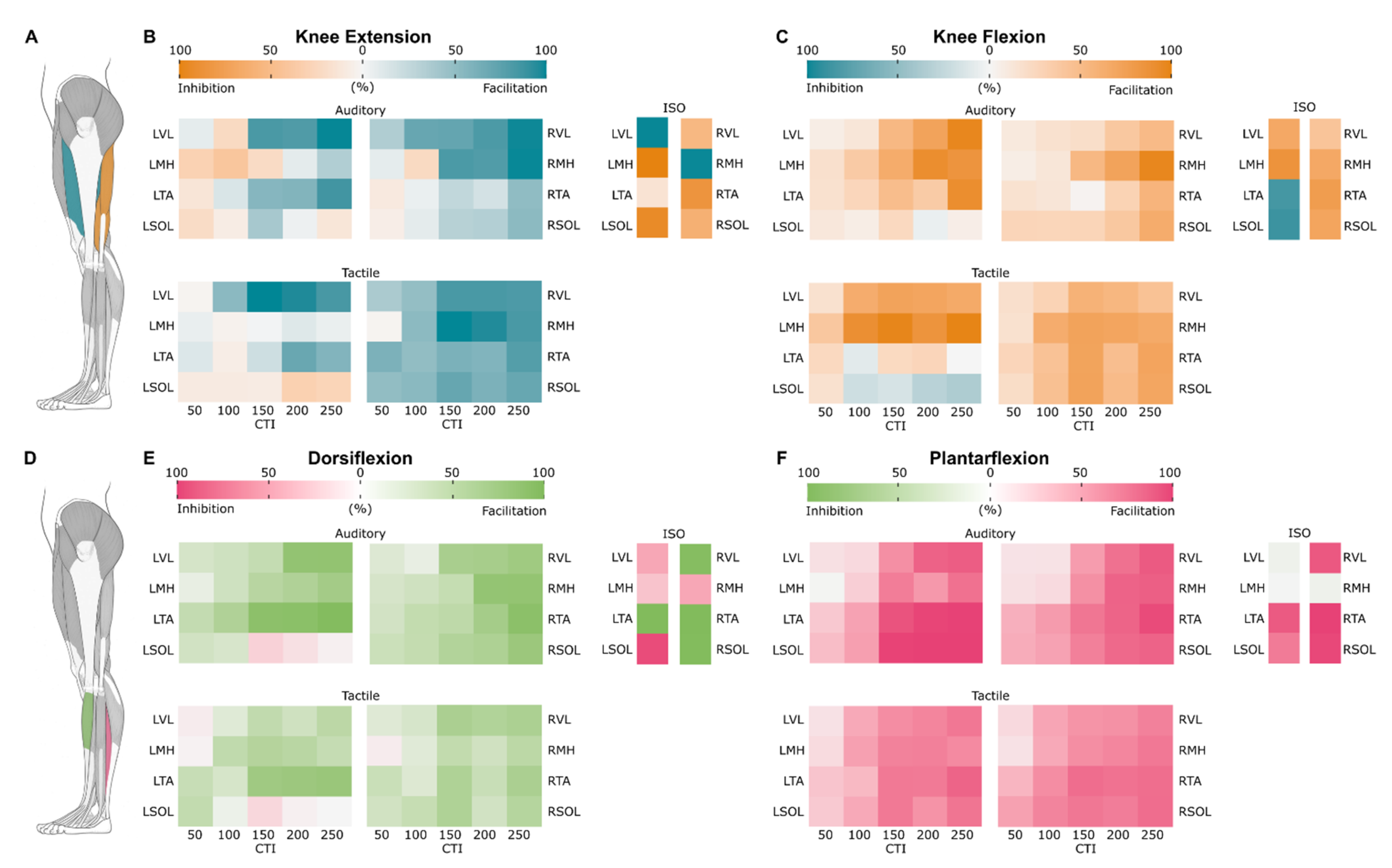
3.5. Probability Maps of Modulated Responses
3.5.1. Knee Extension
3.5.2. Knee Flexion
3.5.3. Dorsiflexion
3.5.4. Plantarflexion
4. Discussion
4.1. Modulation of Spinally Evoked Motor Potentials Prior to the Movements
4.2. Modulation of Spinally Evoked Motor Potentials during Voluntary Contractions
4.3. Probability of Modulated Responses in Agonists and Antagonists
4.4. Modulation of Second Response Prior to and during the Movements
4.5. Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dobkin, B.H.; Havton, L.A. Basic advances and new avenues in therapy of spinal cord injury. Annu. Rev. Med. 2004, 55, 255–282. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, V.R.; Roy, R.R. Physiology of motor deficits and the potential of motor recovery after a spinal cord injury. In The Physiology of Exercise in Spinal Cord Injury; Taylor, J.A., Ed.; Springer: Boston, MA, USA, 2016; pp. 13–35. [Google Scholar]
- Kakulas, B.A. Pathology of spinal injuries. Cent. Nerv. Syst. Trauma 1984, 1, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, M.R. Residual motor functions in spinal cord injury. Adv. Neurol. 1988, 47, 138–155. [Google Scholar] [PubMed]
- Sherwood, A.M.; Dimitrijevic, M.R.; McKay, W.B. Evidence of subclinical brain influence in clinically complete spinal cord injury: Discomplete sci. J. Neurol. Sci. 1992, 110, 90–98. [Google Scholar] [CrossRef]
- McKay, W.B.; Lim, H.K.; Priebe, M.M.; Stokic, D.S.; Sherwood, A.M. Clinical neurophysiological assessment of residual motor control in post-spinal cord injury paralysis. Neurorehabilit. Neural Repair 2004, 18, 144–153. [Google Scholar] [CrossRef]
- Mayr, W.; Krenn, M.; Dimitrijevic, M.R. Motor control of human spinal cord disconnected from the brain and under external movement. Adv. Exp. Med. Biol. 2016, 957, 159–171. [Google Scholar] [PubMed]
- Taccola, G.; Sayenko, D.; Gad, P.; Gerasimenko, Y.; Edgerton, V.R. And yet it moves: Recovery of volitional control after spinal cord injury. Prog. Neurobiol. 2018, 160, 64–81. [Google Scholar] [CrossRef]
- Edgerton, V.R.; Courtine, G.; Gerasimenko, Y.P.; Lavrov, I.; Ichiyama, R.M.; Fong, A.J.; Cai, L.L.; Otoshi, C.K.; Tillakaratne, N.J.; Burdick, J.W.; et al. Training locomotor networks. Brain Res. Rev. 2008, 57, 241–254. [Google Scholar] [CrossRef]
- Fong, A.J.; Roy, R.R.; Ichiyama, R.M.; Lavrov, I.; Courtine, G.; Gerasimenko, Y.; Tai, Y.C.; Burdick, J.; Edgerton, V.R. Recovery of control of posture and locomotion after a spinal cord injury: Solutions staring us in the face. Prog. Brain Res. 2009, 175, 393–418. [Google Scholar]
- Courtine, G.; Gerasimenko, Y.; van den Brand, R.; Yew, A.; Musienko, P.; Zhong, H.; Song, B.; Ao, Y.; Ichiyama, R.M.; Lavrov, I.; et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 2009, 12, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, S.; Frigon, A. Recovery of locomotion after spinal cord injury: Some facts and mechanisms. Annu. Rev. Neurosci. 2011, 34, 413–440. [Google Scholar] [CrossRef]
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of over-ground walking after chronic motor complete spinal cord injury. N. Engl. J. Med. 2018, 379, 1244–1250. [Google Scholar] [CrossRef]
- Gill, M.L.; Grahn, P.J.; Calvert, J.S.; Linde, M.B.; Lavrov, I.A.; Strommen, J.A.; Beck, L.A.; Sayenko, D.G.; Van Straaten, M.G.; Drubach, D.I.; et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat. Med. 2018, 24, 1677–1682. [Google Scholar] [CrossRef]
- Wagner, F.B.; Mignardot, J.B.; Le Goff-Mignardot, C.G.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seanez, I.; Caban, M.; Pirondini, E.; et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, Y.P.; Lu, D.C.; Modaber, M.; Zdunowski, S.; Gad, P.; Sayenko, D.G.; Morikawa, E.; Haakana, P.; Ferguson, A.R.; Roy, R.R.; et al. Noninvasive reactivation of motor descending control after paralysis. J. Neurotrauma 2015, 32, 1968–1980. [Google Scholar] [CrossRef] [PubMed]
- Sayenko, D.G.; Rath, M.; Ferguson, A.R.; Burdick, J.W.; Havton, L.A.; Edgerton, V.R.; Gerasimenko, Y.P. Self-assisted standing enabled by non-invasive spinal stimulation after spinal cord injury. J. Neurotrauma 2019, 36, 1435–1450. [Google Scholar] [CrossRef] [PubMed]
- Rattay, F.; Minassian, K.; Dimitrijevic, M.R. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 2. Quantitative analysis by computer modeling. Spinal Cord 2000, 38, 473–489. [Google Scholar] [CrossRef]
- Ladenbauer, J.; Minassian, K.; Hofstoetter, U.S.; Dimitrijevic, M.R.; Rattay, F. Stimulation of the human lumbar spinal cord with implanted and surface electrodes: A computer simulation study. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 637–645. [Google Scholar] [CrossRef]
- Minassian, K.; Jilge, B.; Rattay, F.; Pinter, M.M.; Binder, H.; Gerstenbrand, F.; Dimitrijevic, M.R. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: Electromyographic study of compound muscle action potentials. Spinal Cord 2004, 42, 401–416. [Google Scholar] [CrossRef]
- Murg, M.; Binder, H.; Dimitrijevic, M.R. Epidural electric stimulation of posterior structures of the human lumbar spinal cord: 1. Muscle twitches—A functional method to define the site of stimulation. Spinal Cord 2000, 38, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Sayenko, D.G.; Atkinson, D.A.; Dy, C.J.; Gurley, K.M.; Smith, V.L.; Angeli, C.; Harkema, S.J.; Edgerton, V.R.; Gerasimenko, Y.P. Spinal segment-specific transcutaneous stimulation differentially shapes activation pattern among motor pools in humans. J. Appl. Physiol. 2015, 118, 1364–1374. [Google Scholar] [CrossRef]
- Minassian, K.; McKay, W.B.; Binder, H.; Hofstoetter, U.S. Targeting lumbar spinal neural circuitry by epidural stimulation to restore motor function after spinal cord injury. Neurotherapeutics 2016, 13, 284–294. [Google Scholar] [CrossRef]
- Minassian, K.; Persy, I.; Rattay, F.; Pinter, M.M.; Kern, H.; Dimitrijevic, M.R. Human lumbar cord circuitries can be activated by extrinsic tonic input to generate locomotor-like activity. Hum. Mov. Sci. 2007, 26, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Danner, S.M.; Hofstoetter, U.S.; Freundl, B.; Binder, H.; Mayr, W.; Rattay, F.; Minassian, K. Human spinal locomotor control is based on flexibly organized burst generators. Brain 2015, 138, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; Danner, S.M.; Freundl, B.; Binder, H.; Mayr, W.; Rattay, F.; Minassian, K. Periodic modulation of repetitively elicited monosynaptic reflexes of the human lumbosacral spinal cord. J. Neurophysiol. 2015, 114, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G.; et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef]
- Dimitrijevic, M.; Kakulas, B.; Vrbova, G. Recent Achievements in Restorative Neurology: Progressive Neuromuscular Diseases; Karger: New York, NY, USA, 1986. [Google Scholar]
- Gerasimenko, Y.; Sayenko, D.; Gad, P.; Liu, C.T.; Tillakaratne, N.J.K.; Roy, R.R.; Kozlovskaya, I.; Edgerton, V.R. Feed-forwardness of spinal networks in posture and locomotion. Neuroscientist 2017, 23, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Sayenko, D.G.; Atkinson, D.A.; Mink, A.M.; Gurley, K.M.; Edgerton, V.R.; Harkema, S.J.; Gerasimenko, Y.P. Vestibulospinal and corticospinal modulation of lumbosacral network excitability in human subjects. Front. Physiol. 2018, 9, 1746. [Google Scholar] [CrossRef]
- Atkinson, D.A.; Sayenko, D.G.; D’Amico, J.M.; Mink, A.; Lorenz, D.J.; Gerasimenko, Y.P.; Harkema, S. Interlimb conditioning of lumbosacral spinally evoked motor responses after spinal cord injury. Clin. Neurophysiol. 2020, 131, 1519–1532. [Google Scholar] [CrossRef]
- Eichenberger, A.; Rüegg, D. Relation between the specific h reflex facilitation preceding a voluntary movement and movement parameters in man. J. Physiol. 1984, 347, 545–559. [Google Scholar] [CrossRef]
- Gottlieb, G.L.; Agarwal, G.C.; Stark, L. Interactions between voluntary and postural mechanisms of human motor system. J. Neurophysiol. 1970, 33, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Pierrot-Deseilligny, E.; Lacert, P. Amplitude and variability of monosynaptic reflexes prior to various voluntary movements in normal and spastic man. In Human Reflexes, Pathophysiology of Motor Systems, Methodology of Human Reflexes; Karger Publishers: London, UK, 1973; Volume 3, pp. 538–549. [Google Scholar]
- Hultborn, H.; Meunier, S.; Pierrot-Deseilligny, E.; Shindo, M. Changes in presynaptic inhibition of ia fibres at the onset of voluntary contraction in man. J. Physiol. 1987, 389, 757–772. [Google Scholar] [CrossRef]
- Ivanchenko, Y.Z. Anticipatory changes in the soleus h reflex in humans related to the movements of the contralateral foot in upright stance. Neurophysiology 2014, 46, 134–138. [Google Scholar] [CrossRef]
- Minassian, K.; Persy, I.; Rattay, F.; Dimitrijevic, M.R.; Hofer, C.; Kern, H. Posterior root-muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve 2007, 35, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Courtine, G.; Harkema, S.J.; Dy, C.J.; Gerasimenko, Y.P.; Dyhre-Poulsen, P. Modulation of multisegmental monosynaptic responses in a variety of leg muscles during walking and running in humans. J. Physiol. 2007, 582, 1125–1139. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Binder, H.; Minassian, K. Recovery cycles of posterior root-muscle reflexes evoked by transcutaneous spinal cord stimulation and of the h reflex in individuals with intact and injured spinal cord. PLoS ONE 2019, 14, e0227057. [Google Scholar] [CrossRef]
- Andrews, J.C.; Stein, R.B.; Roy, F.D. Post-activation depression in the human soleus muscle using peripheral nerve and transcutaneous spinal stimulation. Neurosci. Lett. 2015, 589, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Roy, F.D.; Gibson, G.; Stein, R.B. Effect of percutaneous stimulation at different spinal levels on the activation of sensory and motor roots. Exp. Brain Res. 2012, 223, 281–289. [Google Scholar] [CrossRef] [PubMed]
- McKay, W.B.; Lee, D.C.; Lim, H.K.; Holmes, S.A.; Sherwood, A.M. Neurophysiological examination of the corticospinal system and voluntary motor control in motor-incomplete human spinal cord injury. Exp. Brain Res. 2005, 163, 379–387. [Google Scholar] [CrossRef]
- Eccles, J.C.; Eccles, R.M.; Magni, F. Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. J. Physiol. 1961, 159, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, F.; Hultborn, H.; Illert, M. Integration in spinal neuronal systems. Compr. Physiol. 2011, 2, 509–595. [Google Scholar]
- Calancie, B.; Broton, J.G.; Klose, K.J.; Traad, M.; Difini, J.; Ayyar, D.R. Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal cord injury in man. Electroencephalogr. Clin. Neurophysiol. 1993, 89, 177–186. [Google Scholar] [CrossRef]
- Moreno-Lopez, Y.; Bichara, C.; Delbecq, G.; Isope, P.; Cordero-Erausquin, M. The corticospinal tract primarily modulates sensory inputs in the mouse lumbar cord. Elife 2021, 10, e65304. [Google Scholar] [CrossRef] [PubMed]
- Lemon, R. Recent advances in our understanding of the primate corticospinal system. F1000Research 2019, 8, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, M.; Masugi, Y.; Sasaki, A.; Sayenko, D.G.; Nakazawa, K. On the reflex mechanisms of cervical transcutaneous spinal cord stimulation in human subjects. J. Neurophysiol. 2019, 121, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; Minassian, K.; Hofer, C.; Mayr, W.; Rattay, F.; Dimitrijevic, M.R. Modification of reflex responses to lumbar posterior root stimulation by motor tasks in healthy subjects. Artif. Organs 2008, 32, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Nakagawa, K.; Masugi, Y.; Nakazawa, K. Intra-limb modulations of posterior root-muscle reflexes evoked from the lower-limb muscles during isometric voluntary contractions. Exp. Brain Res. 2021, 239, 3035–3043. [Google Scholar] [CrossRef]
- Iles, J.; Pardoe, J. Changes in transmission in the pathway of heteronymous spinal recurrent inhibition from soleus to quadriceps motor neurons during movement in man. Brain 1999, 122, 1757–1764. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meunier, S.; Pierrot-Deseilligny, E.; Simonetta, M. Pattern of monosynaptic heteronymous ia connections in the human lower limb. Exp. Brain Res. 1993, 96, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Wilmink, R.J.; Nichols, T.R. Distribution of heterogenic reflexes among the quadriceps and triceps surae muscles of the cat hind limb. J. Neurophysiol. 2003, 90, 2310–2324. [Google Scholar] [CrossRef] [PubMed]
- Koceja, D.M.; Trimble, M.H.; Earles, D.R. Inhibition of the soleus h-reflex in standing man. Brain Res. 1993, 629, 155–158. [Google Scholar] [CrossRef]
- Masani, K.; Sayenko, D.G.; Vette, A.H. What triggers the continuous muscle activity during upright standing? Gait Posture 2013, 37, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A. Biomechanics and Motor Control of Human Movement, 4th ed.; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Crone, C.; Nielsen, J. Methodological implications of the post activation depression of the soleus h-reflex in man. Exp. Brain Res. 1989, 78, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Poon, D.E.; Roy, F.D.; Gorassini, M.A.; Stein, R.B. Interaction of paired cortical and peripheral nerve stimulation on human motor neurons. Exp. Brain Res. 2008, 188, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Matthews, P. Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise. J. Physiol. 1996, 492, 597–628. [Google Scholar] [CrossRef] [PubMed]
- Windhorst, U. On the role of recurrent inhibitory feedback in motor control. Prog. Neurobiol. 1996, 49, 517–587. [Google Scholar] [CrossRef]
- Stein, R.B.; Thompson, A.K. Muscle reflexes in motion: How, what, and why? Exerc. Sport Sci. Rev. 2006, 34, 145–153. [Google Scholar] [CrossRef]
- Hultborn, H.; Illert, M.; Nielsen, J.; Paul, A.; Ballegaard, M.; Wiese, H. On the mechanism of the post-activation depression of the h-reflex in human subjects. Exp. Brain Res. 1996, 108, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Katz, R.; Morin, C.; Pierrot-Deseilligny, E.; Hibino, R. Conditioning of h reflex by a preceding subthreshold tendon reflex stimulus. J. Neurol. Neurosurg. Psychiatry 1977, 40, 575–580. [Google Scholar] [CrossRef]
- Burke, D.; Adams, R.W.; Skuse, N.F. The effects of voluntary contraction on the h reflex of human limb muscles. Brain 1989, 112 Pt 2, 417–433. [Google Scholar] [CrossRef]
- Hultborn, H.; Nielsen, J.B. Modulation of transmitter release from ia afferents by their preceding activity—A “post-activation depression”. In Presynaptic Inhibition and Neural Control; Oxford University Press: Oxford, UK, 1998; pp. 178–191. [Google Scholar]
- Iles, J.F. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of ia afferents from the human lower limb. J. Physiol. 1996, 491 Pt 1, 197–207. [Google Scholar] [CrossRef]
- Andrews, J.C.; Stein, R.B.; Roy, F.D. Reduced postactivation depression of soleus h reflex and root evoked potential after transcranial magnetic stimulation. J. Neurophysiol. 2015, 114, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Kirshblum, S.C.; Waring, W.; Biering-Sorensen, F.; Burns, S.P.; Johansen, M.; Schmidt-Read, M.; Donovan, W.; Graves, D.; Jha, A.; Jones, L.; et al. Reference for the 2011 revision of the international standards for neurological classification of spinal cord injury. J. Spinal Cord Med. 2011, 34, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Van Middendorp, J.J.; Hosman, A.J.; Donders, A.R.; Pouw, M.H.; Ditunno, J.F., Jr.; Curt, A.; Geurts, A.C.; Van de Meent, H. A clinical prediction rule for ambulation outcomes after traumatic spinal cord injury: A longitudinal cohort study. Lancet 2011, 377, 1004–1010. [Google Scholar] [CrossRef]
- Zorner, B.; Blanckenhorn, W.U.; Dietz, V.; Curt, A. Clinical algorithm for improved prediction of ambulation and patient stratification after incomplete spinal cord injury. J. Neurotrauma 2010, 27, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Scivoletto, G.; Di Donna, V. Prediction of walking recovery after spinal cord injury. Brain Res. Bull. 2009, 78, 43–51. [Google Scholar] [CrossRef]
- Freund, P.; Seif, M.; Weiskopf, N.; Friston, K.; Fehlings, M.G.; Thompson, A.J.; Curt, A. Mri in traumatic spinal cord injury: From clinical assessment to neuroimaging biomarkers. Lancet Neurol. 2019, 18, 1123–1135. [Google Scholar] [CrossRef]
- Jo, H.J.; Perez, M.A. Corticospinal-motor neuronal plasticity promotes exercise-mediated recovery in humans with spinal cord injury. Brain 2020, 143, 1368–1382. [Google Scholar] [CrossRef]
- Cioni, B.; Dimitrijevic, M.R.; McKay, W.B.; Sherwood, A.M. Voluntary supraspinal suppression of spinal reflex activity in paralyzed muscles of spinal cord injury patients. Exp. Neurol. 1986, 93, 574–583. [Google Scholar] [CrossRef]
- Dimitrijevic, M.; McKay, W.; Sarjanovic, I.; Sherwood, A.; Svirtlit, L.; Vrbova, G. Co-activation of ipsi-and contralateral muscle groups during contraction of ankle dorsiflexors. J. Neurol. Sci. 1992, 109, 49–55. [Google Scholar] [CrossRef]
- Barolat, G.; Myklebust, J.B.; Wenninger, W. Enhancement of voluntary motor function following spinal cord stimulation--case study. Appl. Neurophysiol. 1986, 49, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Pinter, M.; Gerstenbrand, F.; Dimitrijevic, M. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 3. Control of spasticity. Spinal Cord 2000, 38, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; McKay, W.B.; Tansey, K.E.; Mayr, W.; Kern, H.; Minassian, K. Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J. Spinal Cord Med. 2014, 37, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; Freundl, B.; Danner, S.M.; Krenn, M.J.; Mayr, W.; Binder, H.; Minassian, K. Transcutaneous spinal cord stimulation induces temporary attenuation of spasticity in individuals with spinal cord injury. J. Neurotrauma 2020, 37, 481–493. [Google Scholar] [CrossRef]
- Sayenko, D.G.; Angeli, C.; Harkema, S.J.; Edgerton, V.R.; Gerasimenko, Y.P. Neuromodulation of evoked muscle potentials induced by epidural spinal-cord stimulation in paralyzed individuals. J. Neurophysiol. 2014, 111, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.S.; Manson, G.A.; Grahn, P.J.; Sayenko, D.G. Preferential activation of spinal sensorimotor networks via lateralized transcutaneous spinal stimulation in neurologically intact humans. J. Neurophysiol. 2019, 122, 2111–2118. [Google Scholar] [CrossRef]
- Grahn, P.J.; Lavrov, I.A.; Sayenko, D.G.; Van Straaten, M.G.; Gill, M.L.; Strommen, J.A.; Calvert, J.S.; Drubach, D.I.; Beck, L.A.; Linde, M.B.; et al. Enabling task-specific volitional motor functions via spinal cord neuromodulation in a human with paraplegia. Mayo Clin. Proc. 2017, 92, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Angeli, C.A.; Edgerton, V.R.; Gerasimenko, Y.P.; Harkema, S.J. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 2014, 137, 1394–1409. [Google Scholar] [CrossRef]
- Darrow, D.; Balser, D.; Netoff, T.I.; Krassioukov, A.; Phillips, A.; Parr, A.; Samadani, U. Epidural spinal cord stimulation facilitates immediate restoration of dormant motor and autonomic supraspinal pathways after chronic neurologically complete spinal cord injury. J. Neurotrauma 2019, 36, 2325–2336. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steele, A.G.; Atkinson, D.A.; Varghese, B.; Oh, J.; Markley, R.L.; Sayenko, D.G. Characterization of Spinal Sensorimotor Network Using Transcutaneous Spinal Stimulation during Voluntary Movement Preparation and Performance. J. Clin. Med. 2021, 10, 5958. https://doi.org/10.3390/jcm10245958
Steele AG, Atkinson DA, Varghese B, Oh J, Markley RL, Sayenko DG. Characterization of Spinal Sensorimotor Network Using Transcutaneous Spinal Stimulation during Voluntary Movement Preparation and Performance. Journal of Clinical Medicine. 2021; 10(24):5958. https://doi.org/10.3390/jcm10245958
Chicago/Turabian StyleSteele, Alexander G., Darryn A. Atkinson, Blesson Varghese, Jeonghoon Oh, Rachel L. Markley, and Dimitry G. Sayenko. 2021. "Characterization of Spinal Sensorimotor Network Using Transcutaneous Spinal Stimulation during Voluntary Movement Preparation and Performance" Journal of Clinical Medicine 10, no. 24: 5958. https://doi.org/10.3390/jcm10245958
APA StyleSteele, A. G., Atkinson, D. A., Varghese, B., Oh, J., Markley, R. L., & Sayenko, D. G. (2021). Characterization of Spinal Sensorimotor Network Using Transcutaneous Spinal Stimulation during Voluntary Movement Preparation and Performance. Journal of Clinical Medicine, 10(24), 5958. https://doi.org/10.3390/jcm10245958






