Occult Infection with Hepatitis C Virus: Looking for Clear-Cut Boundaries and Methodological Consensus
Abstract
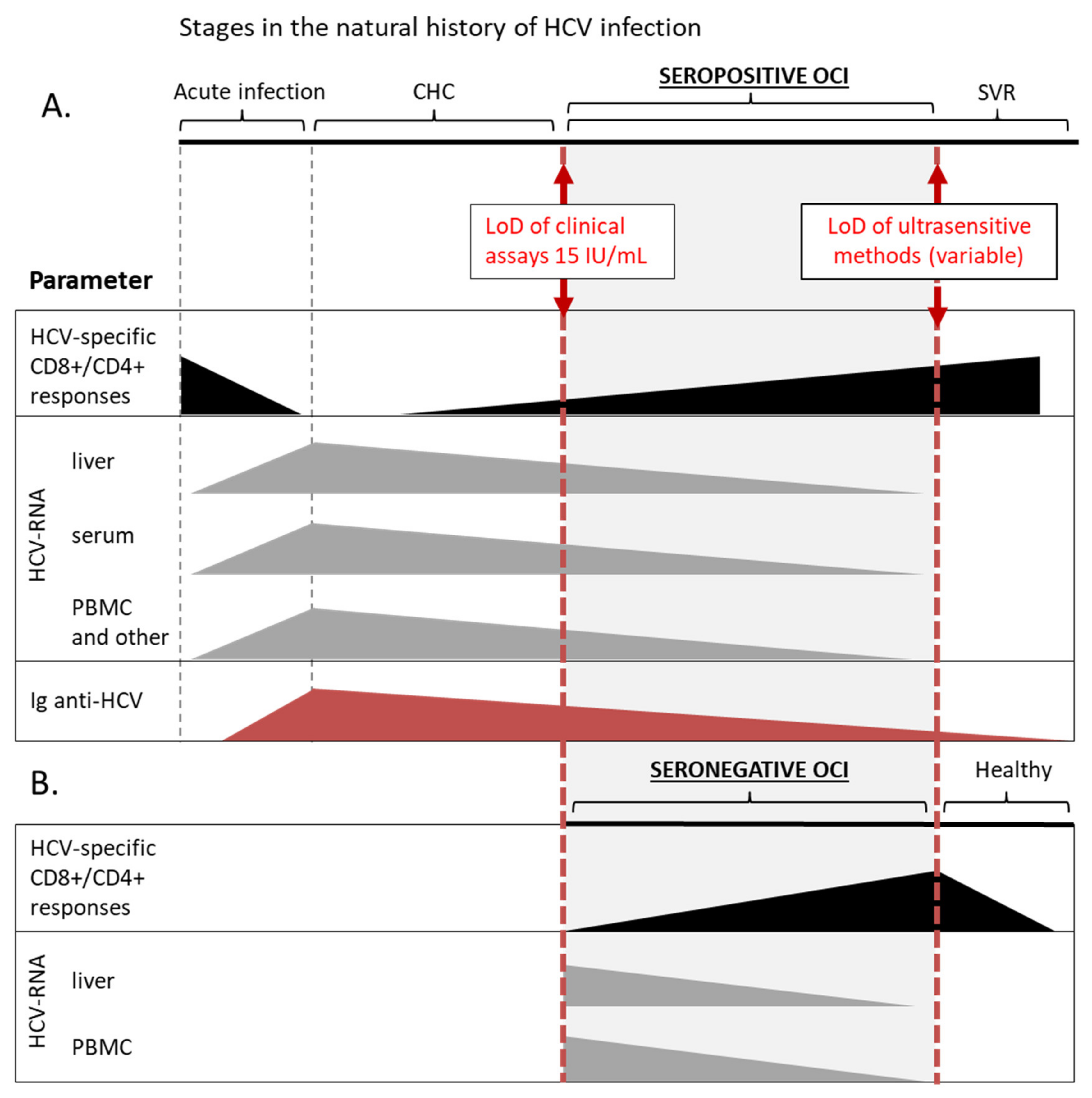
1. Introduction
2. Consequences of HCV Lymphotropism
3. Mechanisms of Occult HCV Persistence
4. Immune Landscape of OCI
5. Clinical Consequences of OCI
6. Epidemiological Significance of OCI
7. Inconsistencies in Approach to Detect Occult Infection
7.1. Patients Selection
Immunosuppression in OCI and CHC
| Ref. | Study Group | n | Patient Material | RNA Isolation Method | HCV-RNA Detection Method | Method LoD | Equivalent of RNA Amount per Reaction | OCI Prevalence |
|---|---|---|---|---|---|---|---|---|
| [37] | Seronegative hemodialysis patients | 515 | PBMC | AccuPrep viral RNA extraction kit | nested RT-PCR, total HCV-RNA, and HCV-RNA (−) strand | ns | ns | 95 (18.4%), [58 (11.3%) with HCV-RNA (−)] |
| [81] | Seronegative hemodialysis patients | 62 | PBMC, | Automated extraction; QIAamp Viral RNA Mini kit (Qiagen) | qRT-PCR probe-based Artus1HCV-RG RT-PCR Kit (Qiagen) | ns | ns | 3 (4.8%) |
| [120] | Seronegative predialysis patients CrCl < 60 mL/min/1.73 m2 | 91 | PBMC, serum | ns | RT-PCR | ns | 100ng | 15 (16.5%) |
| [38] | Seronegative patients with beta-thalassemia major | 181 | PBMC | Proba-NK RNA Extraction Kit (DNA-Technology, Russia) | nested RT-PCR | ns | ns | 6 (3.3%) |
| [79] | Seropositive patients with beta-thalassemia major | 48 | PBMC, plasma | High Pure Viral Nucleic Acid Kit (Roche Diagnostics) | nested RT-PCR | ns | 62.5 ng | 3 (6.3%) |
| [39] | Seronegative hemophilia patients with normal LE | 450 | PBMC | AccuPrep Viral RNA Extraction Kit (Bioneer Corp., South Korea) | nested RT-PCR | ns | ns | 46 (10.2%) |
| [108] | Liver and/or kidney transplant recipients with SVR to DAAs and elevated LE, immunosuppressants- ns | 7 | PBMC (n=7); liver biopsy (n=4) | RNeasy mini kit (Qiagen) | nested RT-PCR (Superscript III one-step PCR) | 2 IU/mL (5 copies/mL) for RT-PCR | RNA from 5 × 106 PBMC, or 5–30 mg liver tissue | No OCI |
| [53] | Patients with SVR to DAAs | 42 | mitogen-stimulated PBMC | TriReagent (Ambion, USA) | real time RT-PCR, total HCV-RNA and HCV-RNA (−) strand | ≤1.5 IU (≤5 vge/µg RNA for RT-PCR | 500 ng | 31 (74%), [29 (69%) with HCV-RNA (−)] |
| [54] | Patients with SVR to DAAs | 1280 | PBMC | Automated extraction (QIAamp1 RNA kits, Qiagen) | qRT-PCR probe-based Artus1HCV-RG RT-PCR Kit (Qiagen) | 0.19 IU/μL (0.23 vge/mL) for qRT-PCR | ns | 50 (3.9%) |
| [121] | Patients with SVR to IFN-based therapy | 52 | whole blood | PAXgene Blood RNA Kit (Qiagen) | nested RT-PCR | 0.74 IU/mL of blood | ns | 2 (3.8%) |
| [55] | Immunocompetent patients with SVR (various treatment regimens) | 60 DAA 50 IFN 30 SR | paired liver biopsies (n=110); PBMC (n=89) | RNeasy plus mini kit (Qiagen) | Liver biopsies: RNAscope in situ hybridization; PBMC: qRT-PCR (PCR-fluorescence probing, KHB, China) | ns | ns | 9 (15%) DAA 5 (10%) IFN+R 2 (6.7%) SR |
| [122] | HIV co-infected patients after SVR to anti-HCV therapy | 123 | serum, PBMC | Automated extraction; PBMC: RNeasy plus universal kit, serum: QIAmp miniElute virus spin kit (Qiagen) | nested RT-digital droplet PCR | ns | ns | 1 (0.8%) |
| [77] | IDUs (seropositive and seronegative) HCV-RNA negative in serum | 126 | PBMC, plasma | High Pure Viral Nucleic Acid Kit (Roche Diagnostics) | nested RT-PCR | 50 IU/mL plasma | 125 ng | 11 (8.7%) |
| [89] | Healthy blood donors | 1037 | plasma, PBMC | QIAamp Viral RNA Mini kit-plasma, RNAeasy kit-PBMC (Qiagen) | nested RT-PCR | ns | ns | 35 (3.4%) |
7.2. Type of Sample
7.3. Testing Serial Samples
7.4. Processing Patients Material and RNA Extraction Technique
7.5. Sensitive Detection Method
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Recommendations and Guidance on Hepatitis C Virus Self-Testing; World Health Organization: Geneva, Switzerland, 2021; p. 32. [Google Scholar]
- Comarmond, C.; Cacoub, P.; Saadoun, D. Treatment of chronic hepatitis C-associated cryoglobulinemia vasculitis at the era of direct-acting antivirals. Ther. Adv. Gastroenterol. 2020, 13, 1756284820942617. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.Q.; MacParland, S.; Mulrooney, P.M.; Cooksley, H.; Naoumov, N.V.; Michalak, T.I. Hepatitis C Virus Persistence after Spontaneous or Treatment-Induced Resolution of Hepatitis, C. J. Virol. 2004, 78, 5867–5874. [Google Scholar] [CrossRef] [PubMed]
- Castillo, I.; Pardo, M.; Salas, C.; Graus, J.; Carreño, V.; Bartolomé, J.; Ortiz-Movilla, N.; Rodríguez-Iñigo, E.; De Lucas, S.; Jiménez-Heffernan, J.A.; et al. Occult Hepatitis C Virus Infection in Patients in Whom the Etiology of Persistently Abnormal Results of Liver-Function Tests Is Unknown. J. Infect. Dis. 2004, 189, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Radkowski, M.; Gallegos-Orozco, J.F.; Jablońska, J.; Colby, T.V.; Walewska-Zielecka, B.; Kubicka, J.; Wilkinson, J.; Adair, D.; Rakela, J.; Laskus, T. Persistence of hepatitis C virus in patients successfully treated for chronic hepatitis C. Hepatol. 2005, 41, 106–114. [Google Scholar] [CrossRef]
- Pham, T.N.; Michalak, T.I. Occult Hepatitis C Virus Infection and Its Relevance in Clinical Practice. J. Clin. Exp. Hepatol. 2011, 1, 185–189. [Google Scholar] [CrossRef]
- Russelli, G.; Pizzillo, P.; Iannolo, G.; Barbera, F.; Tuzzolino, F.; Liotta, R.; Traina, M.; Vizzini, G.; Gridelli, B.; Badami, E.; et al. HCV replication in gastrointestinal mucosa: Potential extra-hepatic viral reservoir and possible role in HCV infection recurrence after liver transplantation. PLoS ONE 2017, 12, e0181683. [Google Scholar] [CrossRef]
- Pal, S.; Sullivan, D.G.; Kim, S.; Lai, K.K.; Kae, J.; Cotler, S.J.; Carithers, R.L.; Wood, B.L.; Perkins, J.D.; Gretch, D.R. Productive Replication of Hepatitis C Virus in Perihepatic Lymph Nodes In Vivo: Implications of HCV Lymphotropism. Gastroenterology 2006, 130, 1107–1116. [Google Scholar] [CrossRef]
- Radkowski, M.; Kubicka, J.; Kisiel, E.; Cianciara, J.; Nowicki, M.; Rakela, J.; Laskus, T. Detection of active hepatitis C virus and hepatitis G virus/GB virus C replication in bone marrow in human subjects. Blood 2000, 95, 3986–3989. [Google Scholar] [CrossRef]
- Pham, T.N.; King, D.; MacParland, S.; McGrath, J.S.; Reddy, S.B.; Bursey, F.R.; Michalak, T.I. Hepatitis C Virus Replicates in the Same Immune Cell Subsets in Chronic Hepatitis C and Occult Infection. Gastroenterology 2008, 134, 812–822. [Google Scholar] [CrossRef]
- Goutagny, N.; Fatmi, A.; De Ledinghen, V.; Penin, F.; Couzigou, P.; Inchauspé, G.; Bain, C. Evidence of Viral Replication in Circulating Dendritic Cells during Hepatitis C Virus Infection. J. Infect. Dis. 2003, 187, 1951–1958. [Google Scholar] [CrossRef]
- Pawełczyk, A.; Kubisa, N.; Jabłońska, J.; Bukowska-Ośko, I.; Cortes, K.C.; Fic, M.; Laskus, T.; Radkowski, M. Detection of hepatitis C virus (HCV) negative strand RNA and NS3 protein in peripheral blood mononuclear cells (PBMC): CD3+, CD14+ and CD19+. Virol. J. 2013, 10, 346. [Google Scholar] [CrossRef]
- Skardasi, G.; Chen, A.Y.; Michalak, T.I. Authentic Patient-Derived Hepatitis C Virus Infects and Productively Replicates in Primary CD4+and CD8+T LymphocytesIn Vitro. J. Virol. 2018, 92, 01790. [Google Scholar] [CrossRef]
- Raghwani, J.; Rose, R.; Sheridan, I.; Lemey, P.; Suchard, M.A.; Santantonio, T.; Farci, P.; Klenerman, P.; Pybus, O.G. Exceptional Heterogeneity in Viral Evolutionary Dynamics Characterises Chronic Hepatitis C Virus Infection. PLOS Pathog. 2016, 12, e1005894. [Google Scholar] [CrossRef]
- Durand, T.; Di Liberto, G.; Colman, H.; Cammas, A.; Boni, S.; Marcellin, P.; Cahour, A.; Vagner, S.; Féray, C. Occult infection of peripheral B cells by hepatitis C variants which have low translational efficiency in cultured hepatocytes. Gut 2010, 59, 934–942. [Google Scholar] [CrossRef]
- Forton, D.M.; Karayiannis, P.; Mahmud, N.; Taylor-Robinson, S.D.; Thomas, H.C. Identification of Unique Hepatitis C Virus Quasispecies in the Central Nervous System and Comparative Analysis of Internal Translational Efficiency of Brain, Liver, and Serum Variants. J. Virol. 2004, 78, 5170–5183. [Google Scholar] [CrossRef]
- Navas, S.; Martín, J.; Quiroga, J.A.; Castillo, I.; Carreño, V. Genetic Diversity and Tissue Compartmentalization of the Hepatitis C Virus Genome in Blood Mononuclear Cells, Liver, and Serum from Chronic Hepatitis C Patients. J. Virol. 1998, 72, 1640–1646. [Google Scholar] [CrossRef]
- Radkowski, M.; Wilkinson, J.; Nowicki, M.; Adair, D.; Vargas, H.E.; Ingui, C.; Rakela, J.; Laskus, T. Search for Hepatitis C Virus Negative-Strand RNA Sequences and Analysis of Viral Sequences in the Central Nervous System: Evidence of Replication. J. Virol. 2002, 76, 600–608. [Google Scholar] [CrossRef]
- Ji, Y.; Dang, X.; Nguyen, L.N.T.; Nguyen, L.N.; Zhao, J.; Cao, D.; Khanal, S.; Schank, M.; Wu, X.Y.; Morrison, Z.D.; et al. Topological DNA damage, telomere attrition and T cell senescence during chronic viral infections. Immun. Ageing 2019, 16, 12. [Google Scholar] [CrossRef]
- Kondo, Y.; Machida, K.; Liu, H.M.; Ueno, Y.; Kobayashi, K.; Wakita, T.; Shimosegawa, T.; Lai, M.M.C. Hepatitis C Virus Infection of T Cells Inhibits Proliferation and Enhances Fas-Mediated Apoptosis by Down-Regulating the Expression of CD44 Splicing Variant. J. Infect. Dis. 2009, 199, 726–736. [Google Scholar] [CrossRef]
- Kondo, Y.; Ueno, Y.; Kakazu, E.; Kobayashi, K.; Shiina, M.; Tamai, K.; Machida, K.; Inoue, J.; Wakui, Y.; Fukushima, K.; et al. Lymphotropic HCV strain can infect human primary naïve CD4+ cells and affect their proliferation and IFN-γ secretion activity. J. Gastroenterol. 2010, 46, 232–241. [Google Scholar] [CrossRef]
- MacParland, S.A.; Chen, A.Y.; Corkum, C.P.; Pham, T.N.; Michalak, T.I. Patient-derived hepatitis C virus inhibits CD4+ but not CD8+ T lymphocyte proliferation in primary T cells. Virol. J. 2015, 12, 93. [Google Scholar] [CrossRef]
- Bhattarai, N.; McLinden, J.H.; Xiang, J.; Kaufman, T.M.; Stapleton, J.T. Conserved Motifs within Hepatitis C Virus Envelope (E2) RNA and Protein Independently Inhibit T Cell Activation. PLoS Pathog. 2015, 11, e1005183. [Google Scholar] [CrossRef]
- Domínguez-Villar, M.; Muñoz-Suano, A.; Anaya-Baz, B.; Aguilar, S.; Novalbos, J.P.; Giron, J.A.; Rodríguez-Iglesias, M.; Garcia-Cozar, F. Hepatitis C virus core protein up-regulates anergy-related genes and a new set of genes, which affects T cell homeostasis. J. Leukoc. Biol. 2007, 82, 1301–1310. [Google Scholar] [CrossRef]
- Yao, Z.Q.; King, E.; Prayther, D.; Yin, D.; Moorman, J. T Cell Dysfunction by Hepatitis C Virus Core Protein Involves PD-1/PDL-1 Signaling. Viral Immunol. 2007, 20, 276–287. [Google Scholar] [CrossRef]
- Kondo, Y.; Ninomiya, M.; Kimura, O.; Machida, K.; Funayama, R.; Nagashima, T.; Kobayashi, K.; Kakazu, E.; Kato, T.; Nakayama, K.; et al. HCV Infection Enhances Th17 Commitment, Which Could Affect the Pathogenesis of Autoimmune Diseases. PLoS ONE 2014, 9, e98521. [Google Scholar] [CrossRef]
- Dai, B.; Chen, A.Y.; Corkum, C.P.; Peroutka, R.J.; Landon, A.; Houng, S.A.; Muniandy, P.; Zhang, Y.; Lehrmann, E.; Mazan-Mamczarz, K.; et al. Hepatitis C virus upregulates B-cell receptor signaling: A novel mechanism for HCV-associated B-cell lymphoproliferative disorders. Oncogene 2015, 35, 2979–2990. [Google Scholar] [CrossRef]
- Rosa, D.; Saletti, G.; De Gregorio, E.; Zorat, F.; Comar, C.; D’Oro, U.; Nuti, S.; Houghton, M.; Barnaba, V.; Pozzato, G.; et al. Activation of naive B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders. Proc. Natl. Acad. Sci. USA 2005, 102, 18544–18549. [Google Scholar] [CrossRef]
- Machida, K.; Cheng, K.T.-N.; Sung, V.M.-H.; Shimodaira, S.; Lindsay, K.L.; Levine, A.M.; Lai, M.-Y. Hepatitis C virus induces a mutator phenotype: Enhanced mutations of immunoglobulin and protooncogenes. Proc. Natl. Acad. Sci. USA 2004, 101, 4262–4267. [Google Scholar] [CrossRef]
- Cuéllar, M.C.R.; García-Lozano, J.R.; Sánchez, B.; Praena-Fernandez, J.M.; Sierra, C.M.; Núñez-Roldán, A.; Aguilar-Reina, J. Lymphomagenesis-related gene expression in B cells from sustained virological responders with occult hepatitis C virus infection. J. Viral Hepat. 2016, 23, 606–613. [Google Scholar] [CrossRef]
- Comarmond, C.; Lorin, V.; Marques, C.; Maciejewski-Duval, A.; Joher, N.; Planchais, C.; Touzot, M.; Biard, L.; Hieu, T.; Quiniou, V.; et al. TLR9 signalling in HCV-associated atypical memory B cells triggers Th1 and rheumatoid factor autoantibody responses. J. Hepatol. 2019, 71, 908–919. [Google Scholar] [CrossRef]
- Chigbu, D.I.; Loonawat, R.; Sehgal, M.; Patel, D.; Jain, P. Hepatitis C Virus Infection: Host–Virus Interaction and Mechanisms of Viral Persistence. Cells 2019, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Klepper, A.; Eng, F.J.; Doyle, E.H.; El-Shamy, A.; Rahman, A.H.; Fiel, M.I.; Avino, G.C.; Lee, M.; Ye, F.; Roayaie, S.; et al. Hepatitis C virus double-stranded RNA is the predominant form in human liver and in interferon-treated cells. Hepatology 2016, 66, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Elmasry, S.; Wadhwa, S.; Bang, B.-R.; Cook, L.; Chopra, S.; Kanel, G.; Kim, B.; Harper, T.; Feng, Z.; Jerome, K.R.; et al. Detection of Occult Hepatitis C Virus Infection in Patients Who Achieved a Sustained Virologic Response to Direct-Acting Antiviral Agents for Recurrent Infection After Liver Transplantation. Gastroenterology 2017, 152, 550–553.e8. [Google Scholar] [CrossRef] [PubMed]
- Laskus, T.; Radkowski, M.; Jablonska, J.; Kibler, K.; Wilkinson, J.; Adair, D.; Rakela, J. Human immunodeficiency virus facilitates infection/replication of hepatitis C virus in native human macrophages. Blood 2004, 103, 3854–3859. [Google Scholar] [CrossRef]
- O’Brien, T.R.; Jackson, S.S. What Have We Learned from Studies of IFN-λ Variants and Hepatitis C Virus Infection? J. Interf. Cytokine Res. 2019, 39, 618–626. [Google Scholar] [CrossRef]
- Ayadi, A.; Nafari, A.H.; Sakhaee, F.; Rajabi, K.; Ghaderi, Y.; Jamnani, F.R.; Vaziri, F.; Siadat, S.D.; Fateh, A. Host genetic factors and clinical parameters influencing the occult hepatitis C virus infection in patients on chronic hemodialysis: Is it still a controversial infection? Hepatol. Res. 2019, 49, 605–616. [Google Scholar] [CrossRef]
- Ayadi, A.; Nafari, A.H.; Irani, S.; Mohebbi, E.; Mohebbi, F.; Sakhaee, F.; Vaziri, F.; Siadat, S.D.; Fateh, A. Occult hepatitis C virus infection in patients with beta-thalassemia major: Is it a neglected and unexplained phenomenon? J. Cell. Biochem. 2019, 120, 11908–11914. [Google Scholar] [CrossRef]
- Nafari, A.H.; Ayadi, A.; Noormohamadi, Z.; Sakhaee, F.; Vaziri, F.; Siadat, S.D.; Fateh, A. Occult hepatitis C virus infection in hemophilia patients and its correlation with interferon lambda 3 and 4 polymorphisms. Infect. Genet. Evol. 2020, 79, 104144. [Google Scholar] [CrossRef]
- Bukowska-Ośko, I.; Cortés, K.C.; Pawełczyk, A.; Płoski, R.; Fic, M.; Perlejewski, K.; Demkow, U.; Berak, H.; Horban, A.; Laskus, T.; et al. Analysis of Genotype 1b Hepatitis C Virus IRES in Serum and Peripheral Blood Mononuclear Cells in Patients Treated with Interferon and Ribavirin. BioMed Res. Int. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Pham, T.N.Q.; Mercer, S.E.; Michalak, T.I. Chronic hepatitis C and persistent occult hepatitis C virus infection are characterized by distinct immune cell cytokine expression profiles. J. Viral Hepat. 2009, 16, 547–556. [Google Scholar] [CrossRef]
- Radkowski, M.; Opoka-Kegler, J.; Cortes, K.C.; Bukowska-Ośko, I.; Perlejewski, K.; Pawełczyk, A.; Laskus, T. Evidence for immune activation in patients with residual hepatitis C virus RNA long after successful treatment with IFN and ribavirin. J. Gen. Virol. 2014, 95, 2004–2009. [Google Scholar] [CrossRef]
- Mousa, N.; Eldars, W.; Eldegla, H.; Fouda, O.; Gad, Y.; Abousamra, N.; Elmasry, E.; Arafa, M. Cytokine Profiles and Hepatic Injury in Occult Hepatitis C Versus Chronic Hepatitis C Virus Infection. Int. J. Immunopathol. Pharmacol. 2014, 27, 87–96. [Google Scholar] [CrossRef]
- Pham, T.N.Q.; Mulrooney-Cousins, P.M.; Mercer, S.E.; MacParland, S.A.; Inglot, M.; Zalewska, M.; Simon, K.; Michalak, T.I. Antagonistic expression of hepatitis C virus and alpha interferon in lymphoid cells during persistent occult infection. J. Viral Hepat. 2007, 14, 537–548. [Google Scholar] [CrossRef]
- Quiroga, J.A.; Llorente, S.; Castillo, I.; Rodríguez-Iñigo, E.; Pardo, M.; Carreño, V. Cellular Immune Responses Associated with Occult Hepatitis C Virus Infection of the Liver. J. Virol. 2006, 80, 10972–10979. [Google Scholar] [CrossRef][Green Version]
- Roque-Cuéllar, M.C.; Sánchez, B.; García-Lozano, J.R.; Praena-Fernandez, J.M.; Márquez-Galán, J.L.; Roldán, A.N.; Aguilar-Reina, J. Hepatitis C virus-specific cellular immune responses in sustained virological responders with viral persistence in peripheral blood mononuclear cells. Liver Int. 2013, 34, e80–e88. [Google Scholar] [CrossRef]
- Roque-Cuéllar, M.C.; Sánchez, B.; García-Lozano, J.R.; Garrido-Serrano, A.; Sayago, M.; Praena-Fernández, J.M.; Roldán, A.N.; Aguilar-Reina, J. Expression of CD81, SR-BI and LDLR in lymphocytes and monocytes from patients with classic and occult hepatitis C virus infection. J. Med. Virol. 2012, 84, 1727–1736. [Google Scholar] [CrossRef]
- Gardini, A.C.; Foschi, F.G.; Conti, F.; Petracci, E.; Vukotic, R.; Marisi, G.; Buonfiglioli, F.; Vitale, G.; Ravaioli, F.; Gitto, S.; et al. Immune inflammation indicators and ALBI score to predict liver cancer in HCV-patients treated with direct-acting antivirals. Dig. Liver Dis. 2019, 51, 681–688. [Google Scholar] [CrossRef]
- Gardini, A.C.; Conti, F.; Foschi, F.G.; Brillanti, S.; Andreone, P.; Mazzella, G.; Ravaioli, F.; Buonfiglioli, F.; Bolondi, L.; Crespi, C.; et al. Imbalance of Neutrophils and Lymphocyte Counts Can Be Predictive of Hepatocellular Carcinoma Occurrence in Hepatitis C-related Cirrhosis Treated with Direct-acting Antivirals. Gastroenterology 2018, 154, 2281–2282. [Google Scholar] [CrossRef]
- Debes, J.D.; van Tilborg, M.; Groothuismink, Z.M.; Hansen, B.E.; Wiesch, J.S.Z.; von Felden, J.; de Knegt, R.J.; Boonstra, A. Levels of Cytokines in Serum Associate with Development of Hepatocellular Carcinoma in Patients With HCV Infection Treated With Direct-Acting Antivirals. Gastroenterology 2018, 154, 515–517.e3. [Google Scholar] [CrossRef]
- Meissner, E.G.; Wu, D.; Osinusi, A.; Bon, D.; Virtaneva, K.; Sturdevant, D.; Porcella, S.; Wang, H.; Herrmann, E.; McHutchison, J.; et al. Endogenous intrahepatic IFNs and association with IFN-free HCV treatment outcome. J. Clin. Investig. 2014, 124, 3352–3363. [Google Scholar] [CrossRef]
- Alao, H.; Cam, M.; Keembiyehetty, C.; Zhang, F.; Serti, E.; Suarez, D.; Park, H.; Fourie, N.H.; Wright, E.C.; Henderson, W.; et al. Baseline Intrahepatic and Peripheral Innate Immunity are Associated with Hepatitis C Virus Clearance During Direct-Acting Antiviral Therapy. Hepatology 2018, 68, 2078–2088. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska, A.; Lorenc, B.; Cheba, M.; Bielawski, K.P.; Sikorska, K. Neutrocyte-to-lymphocyte ratio predicts the presence of a replicative hepatitis C virus strand after therapy with direct-acting antivirals. Clin. Exp. Med. 2019, 19, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Mekky, M.A.; Sayed, H.I.; Abdelmalek, M.O.; Saleh, M.A.; Osman, O.A.; Osman, H.A.; Morsy, K.H.; Hetta, H.F. Prevalence and predictors of occult hepatitis C virus infection among Egyptian patients who achieved sustained virologic response to sofosbuvir/daclatasvir therapy: A multi-center study. Infect. Drug Resist. 2019, 12, 273–279. [Google Scholar] [CrossRef]
- Wang, Y.; Rao, H.; Chi, X.; Li, B.; Liu, H.; Wu, L.; Zhang, H.; Liu, S.; Zhou, G.; Li, N.; et al. Detection of residual HCV-RNA in patients who have achieved sustained virological response is associated with persistent histological abnormality. EBioMedicine 2019, 46, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, S.; Liu, Z.-H.; Qi, W.-Q.; Zhang, Q.; Zhang, Y.-G.; Sun, D.-R.; Xu, Y.; Wang, H.-G.; Li, Z.-X.; et al. Regulatory polymorphism of CXCL10 rs1439490 in seronegative occult hepatitis C virus infection. World J. Gastroenterol. 2018, 24, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A. Impact of a sustained virological response on the long-term outcome of hepatitis C. Liver Int. 2011, 31, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M.; López-Alcorocho, J.M.; Rodríguez-Iñigo, E.; Castillo, I.; Carreno, V. Comparative study between occult hepatitis C virus infection and chronic hepatitis C. J. Viral Hepat. 2007, 14, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Berasain, C.; Betés, M.; Panizo, A.; Ruiz, J.; Herrero, J.I.; Civeira, M.; Prieto, J. Pathological and virological findings in patients with persistent hypertransaminasaemia of unknown aetiology. Gut 2000, 47, 429–435. [Google Scholar] [CrossRef]
- Bokharaei-Salim, F.; Keyvani, H.; Monavari, S.H.R.; Alavian, S.M.; Madjd, Z.; Toosi, M.N.; Alizadeh, A.H.M. Occult hepatitis C virus infection in Iranian patients with cryptogenic liver disease. J. Med. Virol. 2011, 83, 989–995. [Google Scholar] [CrossRef]
- Yaghobi, R.; Kazemi, M.J.; Geramizadeh, B.; Hosseini, S.A.M.; Moayedi, J. Significance of Occult Hepatitis C Virus Infection in Liver Transplant Patients with Cryptogenic Cirrhosis. Exp. Clin. Transplant. 2020, 18, 206–209. [Google Scholar] [CrossRef]
- Keyvani, H.; Bokharaei-Salim, F.; Monavari, S.H.; Esghaei, M.; Toosi, M.N.; Fakhim, S.; Sadigh, Z.-A.; Alavian, S.M. Occult Hepatitis C Virus Infection in Candidates for Liver Transplant With Cryptogenic Cirrhosis. Zahedan J. Res. Med Sci. 2013, 13, e11290. [Google Scholar] [CrossRef]
- Finkelmeier, F.; Dultz, G.; Peiffer, K.-H.; Kronenberger, B.; Krauss, F.; Zeuzem, S.; Sarrazin, C.; Vermehren, J.; Waidmann, O. Risk of de novo Hepatocellular Carcinoma after HCV Treatment with Direct-Acting Antivirals. Liver Cancer 2018, 7, 190–204. [Google Scholar] [CrossRef]
- Comar, M.; Molin, G.D.; D’Agaro, P.; Crocè, S.L.; Tiribelli, C.; Campello, C. HBV, HCV, and TTV detection by in situ polymerase chain reaction could reveal occult infection in hepatocellular carcinoma: Comparison with blood markers. J. Clin. Pathol. 2006, 59, 526–529. [Google Scholar] [CrossRef]
- Esaki, T.; Suzuki, N.; Yokoyama, K.; Iwata, K.; Irie, M.; Anan, A.; Nakane, H.; Yoshikane, M.; Nishizawa, S.; Ueda, S.; et al. Hepatocellular Carcinoma in a Patient with Liver Cirrhosis Associated with Negative Serum HCV Tests but Positive Liver Tissue HCV RNA. Intern. Med. 2004, 43, 279–282. [Google Scholar] [CrossRef]
- Hanafy, A.S.; Seleem, W.M.; Basha, M.; Marei, A.M. Residual hepatitis C virus in peripheral blood mononuclear cell as a risk factor for hepatocellular carcinoma after achieving a sustained virological response: A dogma or fiction. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1275–1282. [Google Scholar] [CrossRef]
- Manickam, C.; Martinot, A.J.; Jones, R.A.; Varner, V.; Reeves, R.K. Hepatic immunopathology during occult hepacivirus re-infection. Virology 2017, 512, 48–55. [Google Scholar] [CrossRef]
- Castillo, I.; Martinez-Ara, J.; Olea, T.; Bartolomé, J.; Madero, R.; Hernández, E.; Bernis, C.; Aguilar, A.A.; Quiroga, J.; Carreño, V.; et al. High prevalence of occult hepatitis C virus infection in patients with primary and secondary glomerular nephropathies. Kidney Int. 2014, 86, 619–624. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Y.; Wang, S.; Zou, W. Detection of the hepatitis C virus antigen in kidney tissue from infected patients with various glomerulonephritis. Nephrol. Dial. Transplant. 2009, 24, 2745–2751. [Google Scholar] [CrossRef][Green Version]
- Bataille, S.; Kaplanski, G.; Boucraut, J.; Halfon, P.; Camus, C.; Daniel, L.; Burtey, S.; Berland, Y.; Dussol, B. Membranoproliferative Glomerulonephritis and Mixed Cryoglobulinemia after Hepatitis C Virus Infection Secondary to Glomerular NS3 Viral Antigen Deposits. Am. J. Nephrol. 2012, 35, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wu, D.; Wang, T.; Li, T.; Xu, S.; Chen, F.; Jin, X.; Lou, G. Detection of viral antigens in renal tissue of glomerulonephritis patients without serological evidence of hepatitis B virus and hepatitis C virus infection. Int. J. Infect. Dis. 2013, 17, e535–e538. [Google Scholar] [CrossRef]
- Casato, M.; Lilli, D.; Donato, G.; Granata, M.; Conti, V.; Del Giudice, G.; Rivanera, D.; Scagnolari, C.; Antonelli, G.; Fiorilli, M. Occult hepatitis C virus infection in type II mixed cryoglobulinaemia. J. Viral Hepat. 2003, 10, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Sikorska-Wiśniewska, M.; Sikorska, K.; Wróblewska, A.; Liberek, T.; Perkowska-Ptasińska, A.; Dębska-Ślizień, A. Recurrence of Cryoglobulinemia Secondary to Hepatitis C in a Patient with HCV RNA (−) Negative in the Serum. Case Rep. Nephrol. Dial. 2021, 11, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Giannini, C.; Giannelli, F.; Zignego, A.L. Association between mixed cryoglobulinemia, translocation (14;18), and persistence of occult HCV lymphoid infection after treatment. Hepatology 2006, 43, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, A.; Mohamed, E.; Shalaby, A.; Eissa, N.; El-Dabaa, D.S.; Sallam, E.; Kamel, A.M.; Abdelaziz, M.M.; El-Afifi, H.; Abdel-Moneim, A.M. Occult hepatitis C virus infection in patients with malignant lymphoproliferative disorders. Int. J. Immunopathol. Pharmacol. 2020, 34. [Google Scholar] [CrossRef]
- Kisiel, E.; Radkowski, M.; Pawelczyk, A.; Horban, A.; Stańczak, J.; Bukowska-Ośko, I.; Cortés, K.C.; Kazmierczak, J.; Popiel, M.; Laskus, T. Seronegative hepatitis C virus infection in patients with lymphoproliferative disorders. J. Viral Hepat. 2013, 21, 424–429. [Google Scholar] [CrossRef]
- Sheikh, M.; Bokharaei-Salim, F.; Monavari, S.H.; Ataei-Pirkooh, A.; Esghaei, M.; Moradi, N.; Babaei, R.; Fakhim, A.; Keyvani, H. Molecular diagnosis of occult hepatitis C virus infection in Iranian injection drug users. Arch. Virol. 2018, 164, 349–357. [Google Scholar] [CrossRef]
- Donyavi, T.; Bokharaei-Salim, F.; Khanaliha, K.; Sheikh, M.; Bastani, M.N.; Moradi, N.; Babaei, R.; Habib, Z.; Fakhim, A.; Esghaei, M. High prevalence of occult hepatitis C virus infection in injection drug users with HIV infection. Arch. Virol. 2019, 164, 2493–2504. [Google Scholar] [CrossRef]
- Kahyesh-Esfandiary, R.; Sadigh, Z.; Esghaei, M.; Bastani, M.; Donyavi, T.; Najafi, A.; Fakhim, A.; Bokharaei-Salim, F. Detection of HCV genome in peripheral blood mononuclear cells of Iranian seropositive and HCV RNA negative in plasma of patients with beta-thalassemia major: Occult HCV infection. J. Med Virol. 2019, 91, 107–114. [Google Scholar] [CrossRef]
- Barril, G.; Castillo, I.; Arenas, M.D.; Espinosa, M.; Garcia-Valdecasas, J.; Garcia-Fernandez, N.; González-Parra, E.; Alcazar, J.M.; Sánchez, C.; Diez-Baylón, J.C.; et al. Occult Hepatitis C Virus Infection among Hemodialysis Patients. J. Am. Soc. Nephrol. 2008, 19, 2288–2292. [Google Scholar] [CrossRef]
- Abdelmoemen, G.; Khodeir, S.A.; Saif, S.A.-; Kobtan, A.; Abd-Elsalam, S. Prevalence of occult hepatitis C virus among hemodialysis patients in Tanta university hospitals: A single-center study. Environ. Sci. Pollut. Res. 2017, 25, 5459–5464. [Google Scholar] [CrossRef]
- De Marco, L.; Manzini, P.; Trevisan, M.; Gillio-Tos, A.; Danielle, F.; Balloco, C.; Pizzi, A.; De Filippo, E.; D’Antico, S.; Violante, B.; et al. Prevalence and Follow-Up of Occult HCV Infection in an Italian Population Free of Clinically Detectable Infectious Liver Disease. PLoS ONE 2012, 7, e43541. [Google Scholar] [CrossRef]
- Hedayati-Moghaddam, M.R.; Soltanian, H.; Ahmadi-Ghezeldasht, S. Occult hepatitis C virus infection in the Middle East and Eastern Mediterranean countries: A systematic review and meta-analysis. World J. Hepatol. 2021, 13, 242–260. [Google Scholar] [CrossRef]
- Gatserelia, L.; Sharvadze, L.; Karchava, M.; Dolmazashvili, E.; Tsertsvadze, T. Occurrence of occult HCV infection among Hiv infected patients in Georgia. Georgian Med. News 2014, 226, 37–41. [Google Scholar]
- Bokharaei-Salim, F.; Keyvani, H.; Esghaei, M.; Zare-Karizi, S.; Dermenaki-Farahani, S.-S.; Hesami-Zadeh, K.; Fakhim, S. Prevalence of occult hepatitis C virus infection in the Iranian patients with human immunodeficiency virus infection. J. Med Virol. 2016, 88, 1960–1966. [Google Scholar] [CrossRef]
- Jamshidi, S.; Bokharaei-Salim, F.; Esghaei, M.; Bastani, M.; Garshasbi, S.; Chavoshpour, S.; Dehghani-Dehej, F.; Fakhim, S.; Khanaliha, K. Occult HCV and occult HBV coinfection in Iranian human immunodeficiency virus-infected individuals. J. Med. Virol. 2020, 92, 3354–3364. [Google Scholar] [CrossRef]
- De Marco, L.; Gillio-Tos, A.; Fiano, V.; Ronco, G.; Krogh, V.; Palli, D.; Panico, S.; Tumino, R.; Vineis, P.; Merletti, F.; et al. Occult HCV Infection: An Unexpected Finding in a Population Unselected for Hepatic Disease. PLoS ONE 2009, 4, e8128. [Google Scholar] [CrossRef]
- Quiroga, J.A.; Avellon, A.; Bartolomé, J.; Andréu, M.; Flores, E.; González, M.I.; González, R.; Pérez, S.; Richart, L.A.; Castillo, I.; et al. Detection of hepatitis C virus (HCV) core–specific antibody suggests occult HCV infection among blood donors. Transfusion 2016, 56, 1883–1890. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, M.D.L.L.; Noguez, U.; Arroyo-Anduiza, C.I.; Mata-Marin, J.A.; Benitez-Arvizu, G.; Portillo-López, M.L.; Ocaña-Mondragón, A. Prevalence and risk factors of Occult Hepatitis C infections in blood donors from Mexico City. PLoS ONE 2018, 13, e0205659. [Google Scholar] [CrossRef]
- Lin, H.; Chen, X.; Zhu, S.; Mao, P.; Zhu, S.; Liu, Y.; Huang, C.; Sun, J.; Zhu, J. Prevalence of Occult Hepatitis C Virus Infection among Blood Donors in Jiangsu, China. Intervirology 2016, 59, 204–210. [Google Scholar] [CrossRef]
- Abdelwahab, S.F. Cellular immune response to hepatitis-C-virus in subjects without viremia or seroconversion: Is it important? Infect. Agents Cancer 2016, 11, 23. [Google Scholar] [CrossRef][Green Version]
- Roque-Cuéllar, M.C.; Sánchez, B.; García-Lozano, J.R.; Praena-Fernandez, J.M.; Roldán, A.N.; Aguilar-Reina, J. Cellular immune responses and occult infection in seronegative heterosexual partners of chronic hepatitis C patients. J. Viral Hepat. 2011, 18, e541–e549. [Google Scholar] [CrossRef]
- MacParland, S.A.; Pham, T.N.Q.; Guy, C.S.; Michalak, T.I. Hepatitis C virus persisting after clinically apparent sustained virological response to antiviral therapy retains infectivity in vitro. Hepatology 2009, 49, 1431–1441. [Google Scholar] [CrossRef]
- Veerapu, N.S.; Park, S.-H.; Tully, D.C.; Allen, T.; Rehermann, B. Trace amounts of sporadically reappearing HCV RNA can cause infection. J. Clin. Investig. 2014, 124, 3469–3478. [Google Scholar] [CrossRef]
- Katayama, K.; Kumagai, J.; Komiya, Y.; Mizui, M.; Yugi, H.; Kishimoto, S.; Yamanaka, R.; Tamatsukuri, S.; Tomoguri, T.; Miyakawa, Y.; et al. Titration of Hepatitis C Virus in Chimpanzees for Determining the Copy Number Required for Transmission. Intervirology 2004, 47, 57–64. [Google Scholar] [CrossRef]
- Barreiro, P.; Vispo, E.; Maida, I.; Aguilera, A.; Fernández-Montero, J.V.; de Mendoza, C.; Labarga, P.; Soriano, V. Very late HCV relapse following triple therapy for hepatitis C. Antivir. Ther. 2014, 19, 723–724. [Google Scholar] [CrossRef]
- Hara, K.; Rivera, M.M.; Koh, C.; DeMino, M.; Page, S.; Nagabhyru, P.R.; Rehermann, B.; Liang, T.J.; Hoofnagle, J.H.; Heller, T. Sequence Analysis of Hepatitis C Virus From Patients With Relapse After a Sustained Virological Response: Relapse or Reinfection? J. Infect. Dis. 2014, 209, 38–45. [Google Scholar] [CrossRef][Green Version]
- Boschi, C.; Colson, P.; Tissot-Dupont, H.; Bernit, E.; Botta-Fridlund, D.; Aherfi, S. Hepatitis C Virus Relapse 78 Weeks After Completion of Successful Direct-Acting Therapy. Clin. Infect. Dis. 2017, 65, 1051–1053. [Google Scholar] [CrossRef]
- Cortés-Mancera, F.M.; Restrepo, J.C.; Osorio, G.; Hoyos, S.; Correa, G.; Navas, M.C. Occult hepatitis C virus infection in a re-transplanted patient with liver failure of unknown etiology. Rev. Col. Gastroenterol. 2010, 25, 76. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-99572010000100016&lng=en (accessed on 29 October 2021).
- Castillo, I.; Bartolomé, J.; Quiroga, J.A.; Barril, G.; Carreño, V. Hepatitis C virus infection in the family setting of patients with occult hepatitis C. J. Med. Virol. 2009, 81, 1198–1203. [Google Scholar] [CrossRef]
- Quiroga, J.A.; Castillo, I.; Llorente, S.; Bartolomé, J.; Barril, G.; Carreño, V. Identification of serologically silent occult hepatitis C virus infection by detecting immunoglobulin G antibody to a dominant HCV core peptide epitope. J. Hepatol. 2009, 50, 256–263. [Google Scholar] [CrossRef]
- Castillo, I.; Bartolomé, J.; Quiroga, J.A.; Barril, G.; Carreño, V. Diagnosis of occult hepatitis C without the need for a liver biopsy. J. Med. Virol. 2010, 82, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Barril, G.; Quiroga, J.A.; Arenas, M.D.; Espinosa, M.; Garcia-Fernandez, N.; Cigarrán, S.; Herrero, J.A.; Del Peso, G.; Caro, P.; Agudo, R.G.; et al. Impact of Isolated Hepatitis C Virus (HCV) Core-Specific Antibody Detection and Viral RNA Amplification among HCV-Seronegative Dialysis Patients at Risk for Infection. J. Clin. Microbiol. 2014, 52, 3053–3056. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Michalak, T.I.; Pham, T.N. Anti-HCV core antibody: A potential new marker of occult and otherwise serologically silent HCV infection. J. Hepatol. 2009, 50, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Bernardin, F.; Tobler, L.; Walsh, I.; Williams, J.D.; Busch, M.; Delwart, E. Clearance of hepatitis C virus RNA from the peripheral blood mononuclear cells of blood donors who spontaneously or therapeutically control their plasma viremia. Hepatology 2007, 47, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Nicot, F.; Kamar, N.; Mariamé, B.; Rostaing, L.; Pasquier, C.; Izopet, J. No evidence of occult hepatitis C virus (HCV) infection in serum of HCV antibody-positive HCV RNA-negative kidney-transplant patients. Transpl. Int. 2009, 23, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Baid-Agrawal, S.; Schindler, R.; Reinke, P.; Staedtler, A.; Rimpler, S.; Malik, B.; Frei, U.; Berg, T. Prevalence of occult hepatitis C infection in chronic hemodialysis and kidney transplant patients. J. Hepatol. 2014, 60, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Del Bello, A.; Abravanel, F.; Alric, L.; Lavayssiere, L.; Lhomme, S.; Bellière, J.; Izopet, J.; Kamar, N. No evidence of occult hepatitis C or E virus infections in liver-transplant patients with sustained virological response after therapy with direct acting agents. Transpl. Infect. Dis. 2019, 21, e13093. [Google Scholar] [CrossRef]
- Bartolomé, J.; Castillo, I.; Quiroga, J.A.; Carreño, V. Underestimation of occult hepatitis C virus infection in chronic haemodialysis and kidney transplant patients. J. Hepatol. 2014, 61, 1184–1185. [Google Scholar] [CrossRef][Green Version]
- Coppola, N.; Pisaturo, M.; Guastafierro, S.; Tonziello, G.; Sica, A.; Sagnelli, C.; Ferrara, M.G.; Sagnelli, E. Absence of occult hepatitis C virus infection in patients under immunosupressive therapy for oncohematological diseases. Hepatology 2011, 54, 1487–1489. [Google Scholar] [CrossRef]
- Kamar, N.; Nicot, F.; Izopet, J.; Rostaing, L. Occult Hepatitis C Virus Infection in Hemodialysis Patients: Examining the Evidence. Am. J. Kidney Dis. 2009, 54, 10–12. [Google Scholar] [CrossRef]
- Lee, H.L.; Bae, S.H.; Jang, B.; Hwang, S.; Yang, H.; Nam, H.C.; Sung, P.S.; Lee, S.W.; Jang, J.W.; Choi, J.Y.; et al. Reactivation of Hepatitis C Virus and Its Clinical Outcomes in Patients Treated with Systemic Chemotherapy or Immunosuppressive Therapy. Gut Liver 2017, 11, 870–877. [Google Scholar] [CrossRef]
- Schramm, F.; Soulier, E.; Royer, C.; Weitten, T.; Fafi-Kremer, S.; Brignon, N.; Meyer, N.; Ellero, B.; Woehl-Jaegle, M.; Meyer, C.; et al. Frequent Compartmentalization of Hepatitis C Virus with Leukocyte-Related Amino Acids in the Setting of Liver Transplantation. J. Infect. Dis. 2008, 198, 1656–1666. [Google Scholar] [CrossRef]
- Kahraman, A.; Witzke, O.; Scherag, A.; Pütter, C.; Miller, M.; Dechêne, A.; Ross, S.R.; Gerken, G.; Hilgard, P. Impact of immuno-suppressive therapy on hepatitis C infection after renal transplantation. Clin. Nephrol. 2011, 75, 16. [Google Scholar] [PubMed]
- Sagnelli, E.; Pisaturo, M.; Sagnelli, C.; Coppola, N. Rituximab-Based Treatment, HCV Replication, and Hepatic Flares. Clin. Dev. Immunol. 2012, 2012, 1–5. [Google Scholar] [CrossRef]
- Torres, H.A.; Hosry, J.; Mahale, P.; Economides, M.P.; Jiang, Y.; Lok, A.S. Hepatitis C virus reactivation in patients receiving cancer treatment: A prospective observational study. Hepatol. 2018, 67, 36–47. [Google Scholar] [CrossRef]
- Nakagawa, M.; Sakamoto, N.; Tanabe, Y.; Koyama, T.; Itsui, Y.; Takeda, Y.; Chen, C.; Kakinuma, S.; Oooka, S.; Maekawa, S.; et al. Suppression of Hepatitis C Virus Replication by Cyclosporin A Is Mediated by Blockade of Cyclophilins. Gastroenterology 2005, 129, 1031–1041. [Google Scholar] [CrossRef]
- Stöhr, S.; Costa, R.; Sandmann, L.; Westhaus, S.; Pfaender, S.; Anggakusuma, D.E.; Meuleman, P.; Vondran, F.W.R.; Manns, M.P. Host cell mTORC1 is required for HCV RNA replication. Gut 2016, 65, 2017–2028. [Google Scholar] [CrossRef]
- Ciesek, S.; Steinmann, E.; Iken, M.; Ott, M.; Helfritz, F.A.; Wappler, I.; Manns, M.P.; Wedemeyer, H.; Pietschmann, T. Glucocorticosteroids Increase Cell Entry by Hepatitis C Virus. Gastroenterology 2010, 138, 1875–1884. [Google Scholar] [CrossRef]
- Sette, L.H.B.C.; Lopes, E.P.A.; Guedes Dos Anjos, N.C.; Valente, L.M.; Vieira de Oliveira, S.A.; Lucena-Silva, N. High prevalence of occult hepatitis C infection in predialysis patients. World J. Hepatol. 2019, 11, 09–118. [Google Scholar] [CrossRef]
- Lybeck, C.; Brenndörfer, E.D.; Sällberg, M.; Montgomery, S.M.; Aleman, S.; Duberg, A.S. Long-term follow-up after cure from chronic hepatitis C virus infection shows occult hepatitis and a risk of hepatocellular carcinoma in noncirrhotic patients. Eur. J. Gastroenterol. Hepatol. 2019, 31, 506–513. [Google Scholar] [CrossRef]
- Frías, M.; Rivero-Juárez, A.; Téllez, F.; Palacios, R.; Jiménez-Arranz, Á.; Pineda, J.A.; Merino, D.; Gómez-Vidal, M.A.; Pérez-Camacho, I.; Camacho, Á.; et al. Evaluation of hepatitis C viral RNA persistence in HIV-infected patients with long-term sustained virological response by droplet digital PCR. Sci. Rep. 2019, 29, 12507. [Google Scholar] [CrossRef]
- Pham, T.N.Q.; MacParland, S.A.; Coffin, C.S.; Lee, S.S.; Bursey, F.R.; Michalak, T.I. Mitogen-induced upregulation of hepatitis C virus expression in human lymphoid cells. J. Gen. Virol. 2005, 86, 657–666. [Google Scholar] [CrossRef]
- Cuypers, H.T.; Bresters, D.; Winkel, I.N.; Reesink, H.W.; Weiner, A.J.; Houghton, M.; van der Poel, C.L.; Lelie, P.N. Storage conditions of blood samples and primer selection affect the yield of cDNA polymerase chain reaction products of hepatitis C virus. J. Clin. Microbiol. 1992, 30, 3220–3224. [Google Scholar] [CrossRef]
- Castillo, I.; Rodríguez-Iñigo, E.; Bartolomé, J.; de Lucas, S.; Ortíz-Movilla, N.; López-Alcorocho, J.M.; Pardo, M.; Carreño, V. Hepatitis C virus replicates in peripheral blood mononuclear cells of patients with occult hepatitis C virus infection. Gut 2005, 54, 682–685. [Google Scholar] [CrossRef]
- Castillo, I.; Bartolomé, J.; Quiroga, J.A.; Barril, G.; Carreño, V. Long-term virological follow up of patients with occult hepatitis C virus infection. Liver Int. 2011, 31, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Dennin, R.H.; Wo, J.E. DNA sequences homologous to hepatitis C virus (HCV) in the extrachromosomal circular DNA in peripheral blood mononuclear cells of HCV-negative subjects. J. Zhejiang Univ. Sci. B 2019, 20, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Colucci, G.; Gutekunst, K. Development of a quantitative PCR assay for monitoring HCV viraemia levels in patients with chronic hepatitis C. J. Viral. Hepat. 1997, 4, 75–78. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróblewska, A.; Bielawski, K.P.; Sikorska, K. Occult Infection with Hepatitis C Virus: Looking for Clear-Cut Boundaries and Methodological Consensus. J. Clin. Med. 2021, 10, 5874. https://doi.org/10.3390/jcm10245874
Wróblewska A, Bielawski KP, Sikorska K. Occult Infection with Hepatitis C Virus: Looking for Clear-Cut Boundaries and Methodological Consensus. Journal of Clinical Medicine. 2021; 10(24):5874. https://doi.org/10.3390/jcm10245874
Chicago/Turabian StyleWróblewska, Anna, Krzysztof Piotr Bielawski, and Katarzyna Sikorska. 2021. "Occult Infection with Hepatitis C Virus: Looking for Clear-Cut Boundaries and Methodological Consensus" Journal of Clinical Medicine 10, no. 24: 5874. https://doi.org/10.3390/jcm10245874
APA StyleWróblewska, A., Bielawski, K. P., & Sikorska, K. (2021). Occult Infection with Hepatitis C Virus: Looking for Clear-Cut Boundaries and Methodological Consensus. Journal of Clinical Medicine, 10(24), 5874. https://doi.org/10.3390/jcm10245874






