Omentin Is Independently Associated with Stroke Severity and Ipsilateral Carotid Artery Stenosis in Patients with Acute Cerebral Ischemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Regulations
2.2. Setting and Eligibility Criteria
2.3. Data Collection
2.4. Sample Collection and Measurements
2.5. Blinding
2.6. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montellano, F.A.; Ungethum, K.; Ramiro, L.; Nacu, A.; Hellwig, S.; Fluri, F.; Whiteley, W.N.; Bustamante, A.; Montaner, J.; Heuschmann, P.U. Role of blood-based biomarkers in ischemic stroke prognosis: A systematic review. Stroke 2021, 52, 543–551. [Google Scholar] [CrossRef]
- Katsanos, A.H.; Makris, K.; Stefani, D.; Koniari, K.; Gialouri, E.; Lelekis, M.; Chondrogianni, M.; Zompola, C.; Dardiotis, E.; Rizos, I.; et al. Plasma glial fibrillary acidic protein in the differential diagnosis of intracerebral hemorrhage. Stroke 2017, 48, 2586–2588. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.B.; Furie, K.L. Molecular biomarkers in stroke diagnosis and prognosis. Biomark. Med. 2009, 3, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gao, Y. Clinical relevance of serum omentin-1 levels as a biomarker of prognosis in patients with acute cerebral infarction. Brain Behav. 2020, 10, e01678. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.L.; Khetani, S.A.; Zahner, G.J.; Spaulding, K.A.; Schaller, M.S.; Gasper, W.J.; Hills, N.K.; Schafer, A.L.; Grenon, S.M. Serum resistin is associated with impaired endothelial function and a higher rate of adverse cardiac events in patients with peripheral artery disease. J. Vasc. Surg. 2019, 69, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Chan, L.; Zhou, S.W. Omentin: Linking metabolic syndrome and cardiovascular disease. Curr. Vasc. Pharmacol. 2014, 12, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zuo, P.; Cao, L.; Gao, Z.; Ke, K. Omentin-1 is associated with carotid plaque instability among Ischemic stroke patients. J. Atheroscler. Thromb. 2018, 25, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Tsiotra, P.C.; Halvatsiotis, P.; Patsouras, K.; Maratou, E.; Salamalekis, G.; Raptis, S.A.; Dimitriadis, G.; Boutati, E. Circulating adipokines and mRNA expression in adipose tissue and the placenta in women with gestational diabetes mellitus. Peptides 2018, 101, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Chen, J.; Wu, Q.; Liu, X.; Li, M.; Li, Z.; Gao, Y. Serum levels of omentin-1 association with early diagnosis, lesion volume and severity of acute ischemic stroke. Cytokine 2018, 111, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, G.; Gui, Y.; Guo, Z.; Ren, R.; Sun, Y.; Song, J. Serum vaspin as a predictor of severity and prognosis in acute ischemic stroke patients. Nutr. Neurosci. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wada, J. Vaspin: A novel serpin with insulin-sensitizing effects. Expert Opin. Investig. Drugs 2008, 17, 327–333. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Di Raimondo, D.; Casuccio, A.; Guercio, G.; Del Cuore, A.; Puleo, M.G.; Della Corte, V.; Bellia, C.; Caronia, A.; Maida, C.; et al. Endothelial function, adipokine serum levels and white matter hyperintesities in subjects with diabetic foot syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 3920–3930. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Cheng, K.K.; Vanhoutte, P.M.; Lam, K.S.; Xu, A. Vascular effects of adiponectin: Molecular mechanisms and potential therapeutic intervention. Clin. Sci. 2008, 114, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.M.; Wang, S.; Wen, X.; Han, X.R.; Wang, Y.J.; Shen, M.; Fan, S.H.; Zhang, Z.F.; Shan, Q.; Li, M.Q.; et al. Impact of serum omentin-1 levels on functional prognosis in nondiabetic patients with ischemic stroke. Am. J. Transl. Res. 2019, 11, 1854–1863. [Google Scholar]
- Menzel, J.; di Giuseppe, R.; Biemann, R.; Wittenbecher, C.; Aleksandrova, K.; Pischon, T.; Fritsche, A.; Schulze, M.B.; Boeing, H.; Isermann, B.; et al. Omentin-1 and risk of myocardial infarction and stroke: Results from the EPIC-Potsdam cohort study. Atherosclerosis 2016, 251, 415–421. [Google Scholar] [CrossRef]
- Yu, D.; Huang, B.; Wu, B.; Xiao, J. Association of serum vaspin, apelin, and visfatin levels and stroke risk in a Chinese case-control study. Medicine 2021, 100, e25184. [Google Scholar] [CrossRef]
- Lin, J.; Yan, G.T.; Wang, L.H.; Hao, X.H.; Zhang, K.; Xue, H. Leptin fluctuates in intestinal ischemia-reperfusion injury as inflammatory cytokine. Peptides 2004, 25, 2187–2193. [Google Scholar] [CrossRef]
- Dagonnier, M.; Donnan, G.A.; Davis, S.M.; Dewey, H.M.; Howells, D.W. Acute stroke biomarkers: Are we there yet? Front. Neurol. 2021, 12, 619721. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gotzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Initiative, S. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Lambadiari, V.; Gastounioti, A.; Gkekas, C.; Giannakopoulos, T.G.; Koulia, K.; Maratou, E.; Alepaki, M.; Kakisis, J.; Karakitsos, P.; et al. The relationship of novel adipokines, RBP4 and omentin-1, with carotid atherosclerosis severity and vulnerability. Atherosclerosis 2014, 235, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Safouris, A.; Krogias, C.; Sharma, V.K.; Katsanos, A.H.; Faissner, S.; Roussopoulou, A.; Zompola, C.; Kneiphof, J.; Kargiotis, O.; Deftereos, S.; et al. Statin pretreatment and microembolic signals in large artery atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1415–1422. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Katsanos, A.H.; Sharma, V.K.; Krogias, C.; Mikulik, R.; Vadikolias, K.; Mijajlovic, M.; Safouris, A.; Zompola, C.; Faissner, S.; et al. Statin pretreatment is associated with better outcomes in large artery atherosclerotic stroke. Neurology 2016, 86, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Roussopoulou, A.; Tsivgoulis, G.; Krogias, C.; Lazaris, A.; Moulakakis, K.; Georgiadis, G.S.; Mikulik, R.; Kakisis, J.D.; Zompola, C.; Faissner, S.; et al. Safety of urgent endarterectomy in acute non-disabling stroke patients with symptomatic carotid artery stenosis: An international multicenter study. Eur. J. Neurol. 2019, 26, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 Update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American heart association/American Stroke association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- North American Symptomatic Carotid Endarterectomy Trial Collaborators; Barnett, H.J.M.; Taylor, D.W.; Haynes, R.B.; Sackett, D.L.; Peerless, S.J.; Ferguson, G.G.; Fox, A.J.; Rankin, R.N.; Hachinski, V.C.; et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 1991, 325, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Yurdakul, M.; Tola, M. Doppler criteria for identifying proximal vertebral artery stenosis of 50% or more. J. Ultrasound Med. 2011, 30, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Fotiadis, G.; Lambadiari, V.; Maratou, E.; Dimitriadis, G.; Liapis, C.D. Serum levels of novel adipokines in patients with acute ischemic stroke: Potential contribution to diagnosis and prognosis. Peptides 2014, 57, 12–16. [Google Scholar] [CrossRef]
- Kataoka, Y.; Shibata, R.; Ohashi, K.; Kambara, T.; Enomoto, T.; Uemura, Y.; Ogura, Y.; Yuasa, D.; Matsuo, K.; Nagata, T.; et al. Omentin prevents myocardial ischemic injury through AMP-activated protein kinase-and Akt-dependent mechanisms. J. Am. Coll. Cardiol. 2014, 63, 2722–2733. [Google Scholar] [CrossRef] [PubMed]
- Gu, N.; Dong, Y.; Tian, Y.; Di, Z.; Liu, Z.; Chang, M.; Jia, X.; Qian, Y.; Zhang, W. Anti-apoptotic and angiogenic effects of intelectin-1 in rat cerebral ischemia. Brain Res. Bull. 2017, 130, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Niersmann, C.; Carstensen-Kirberg, M.; Maalmi, H.; Holleczek, B.; Roden, M.; Brenner, H.; Herder, C.; Schottker, B. Higher circulating omentin is associated with increased risk of primary cardiovascular events in individuals with diabetes. Diabetologia 2020, 63, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Rashad, N.M.; Ahmed, H.S.; Ashour, W.M.R.; Yousef, M.S. Association of vaspin gene expression and its serum level on the risk of ischemic stroke in type 2 diabetic Egyptian patients: Prospective case-control study. Biotechnol. Appl. Biochem. 2020, 67, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Kannenberg, J.M.; Niersmann, C.; Huth, C.; Carstensen-Kirberg, M.; Wittenbecher, C.; Schulze, M.; Bluher, M.; Rathmann, W.; Peters, A.; et al. Independent and opposite associations of serum levels of omentin-1 and adiponectin with increases of glycaemia and incident type 2 diabetes in an older population: KORA F4/FF4 study. Eur. J. Endocrinol. 2017, 177, 277–286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Katsnelson, M.; Rundek, T. Obesity paradox and stroke: Noticing the (fat) man behind the curtain. Stroke 2011, 42, 3331–3332. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, M.; Janowska, J.; Chudek, J. Potential beneficial effect of some adipokines positively correlated with the adipose tissue content on the cardiovascular system. Int. J. Cardiol. 2016, 222, 581–589. [Google Scholar] [CrossRef]
- Feldmann, E.; Daneault, N.; Kwan, E.; Ho, K.J.; Pessin, M.S.; Langenberg, P.; Caplan, L.R. Chinese-white differences in the distribution of occlusive cerebrovascular disease. Neurology 1990, 40, 1541–1545. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, N. Ethnic differences in cardiovascular disease. Heart 2003, 89, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak-Kabzinska, A.; Marek, B.; Borgiel-Marek, H.; Kajdaniuk, D.; Kos-Kudla, B. Assessing the blood concentration of new adipocytokines in patients with ischaemic stroke. Endokrynol. Pol. 2020, 71, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Cura, H.S.; Ozdemir, H.H.; Demir, C.F.; Bulut, S.; Ilhan, N.; Inci, M.F. Investigation of vaspin level in patients with acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2014, 23, 453–456. [Google Scholar] [CrossRef] [PubMed]


| Variables | Patients with ACI (n = 135) |
|---|---|
| Age (years), mean (SD) | 59 ± 10 |
| Sex | |
| Male, n (%) | 92 (68) |
| Type of ACI | |
| TIA, n (%) | 30 (22) |
| Neurological deficit on admission | |
| NIHSSadm, median (IQR) | 3 (1–7) |
| Comorbidities, n (%) | |
| History of atrial fibrillation | 15 (11) |
| Diabetes | 38 (28) |
| Hypertension | 70 (52) |
| Dyslipidaemia | 76 (56) |
| Congestive heart failure | 6 (4) |
| History of stroke/TIA | 36 (27) |
| History of myocardial infarction | 26 (19) |
| Current smoking | 65 (48) |
| Alcohol misuse | 18 (13) |
| Peripheral artery disease | 3 (2) |
| TOAST classification | |
| Large-artery atherosclerosis, n (%) | 28 (21) |
| Cardioembolism, n (%) | 35 (26) |
| Small-vessel occlusion, n (%) | 6 (4) |
| Stroke of other determined aetiology, n (%) | 12 (9) |
| Stroke of undetermined aetiology, n (%) | 54 (40) |
| Ipsilateral carotid artery stenosis ≥ 50%, n (%) | 18 (13) |
| Contralateral carotid artery stenosis ≥ 50%, n (%) | 15 (11) |
| Vertebral artery stenosis ≥ 50%, n (%) | 3 (2) |
| Left atrial enlargement moderate or severe, n (%) | 12 (9) |
| Valvular disease, n (%) | 7 (5) |
| Atrial fibrillation on Holter monitor, n (%) | 23 (17) |
| CRP mg/L, mean (SD) | 8.2 ± 10.9 |
| Omentin ng/mL, mean (SD) | 10.2 ± 6.3 |
| Vaspin ng/mL, mean (SD) | 146.6 ± 177.1 |
| Early neurological deterioration, n (%) | 8 (6) |
| NIHSSdis median (IQR) | 1 (0–3) |
| Recurrent stroke within 3 months, n (%) | 7 (5) |
| Death within 3 months, n (%) | 6 (4) |
| Simple Linear Regression | Multiple Linear Regression | |||||
|---|---|---|---|---|---|---|
| Variable | LRC | 95% CI | p-value | LRC | 95% CI | p-value |
| Age | 0.175 | 0.075, 0.274 | 0.001 | 0.170 | 0.063, 0.277 | 0.002 |
| Male sex | 0.786 | −1.513, 3.085 | 0.500 | - | - | |
| NIHSSadm | 0.325 | 0.141, 0.509 | 0.001 | 0.290 | 0.063, 0.516 | 0.013 |
| TIA | −0.285 | −5.459, −0.237 | 0.033 | −0.017 | −3.668, 1.634 | 0.449 |
| History of atrial fibrillation | 5.261 | 1.865, 8.658 | 0.033 | 3.72 | −0.030, 7.471 | 0.052 |
| Diabetes | 0.801 | −1.551, 3.153 | 0.501 | - | - | |
| Hypertension | 2.507 | 0.425, 4.588 | 0.019 | 0.968 | −1.349, 3.286 | 0.409 |
| Dyslipidaemia | −1.614 | −3.737, 0.501 | 0.135 | - | - | |
| Congestive heart failure | 5.980 | 0.519, 11.439 | 0.032 | −1.771 | −8.728, 5.186 | 0.615 |
| History of stroke/TIA | 0.080 | −2.329, 2.450 | 0.947 | - | - | |
| History of myocardial infarction | 3.941 | 1.320, 6.561 | 0.004 | 2.157 | −0.875, 5.189 | 0.161 |
| Current smoking | 0.464 | −1.234, 2.161 | 0.590 | - | - | |
| Alcohol misuse | 1.484 | −1.672, 4.641 | 0.354 | - | - | |
| Peripheral artery disease | 0.612 | −6.523, 7.747 | 0.865 | - | - | |
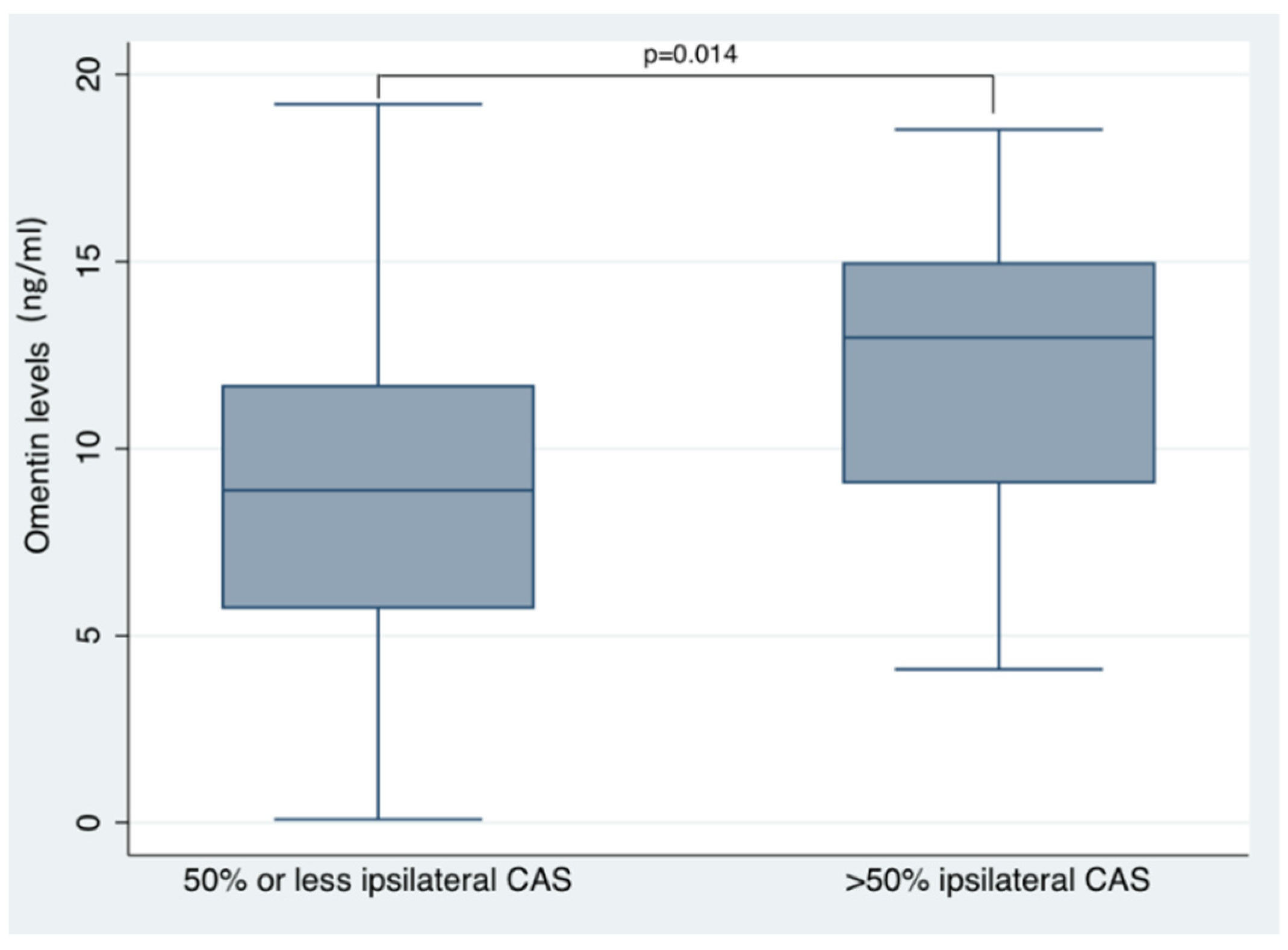
| Ipsilateral carotid artery stenosis ≥ 50% | 3.905 | 0.813, 6.997 | 0.014 | 3.411 | 0.194, 6.628 | 0.038 |
| Contralateral carotid artery stenosis ≥ 50% | 1.716 | −1.715, 5.147 | 0.324 | - | - | |
| Vertebral artery stenosis ≥ 50% | −3.582 | −10.660, 3.500 | 0.319 | - | - | |
| Left atrial enlargement | 3.108 | −0.235, 6.452 | 0.068 | 2.844 | −0.375, 6.063 | 0.082 |
| Valvular disease | −4.811 | −11.946, 2.324 | 0.183 | - | - | |
| Atrial fibrillation on Holter monitor | 2.483 | −0.242, 5.209 | 0.074 | 1.046 | −1.788, 3.881 | 0.465 |
| CRP | −0.031 | −0.167, 0.105 | 0.647 | - | - | |
| Early neurological deterioration | 0.677 | −3.763, 5.118 | 0.763 | - | - | |
| NIHSSdis | 0.307 | 0.035, 0.580 | 0.027 | −0.387 | −0.813, 0.04 | 0.075 |
| Recurrent stroke within 3 months | −0.771 | −5.898, 4.357 | 0.767 | - | - | |
| Death within 3 months | 1.759 | −4.757, 8.276 | 0.594 | - | - | |
| Simple Linear Regression | |||
|---|---|---|---|
| Variable | LRC | 95% CI | p-Value |
| Age | 1.721 | −1.400, 4.842 | 0.277 |
| Male sex | 19.442 | −50.072, 88.956 | 0.581 |
| NIHSSadm | −0.308 | −6.952, 6.337 | 0.927 |
| TIA | −15.700 | −85.749, 54.350 | 0.658 |
| History of atrial fibrillation | 9.640 | −97.736, 117.016 | 0.859 |
| Diabetes | −18.963 | −90.150, 52.223 | 0.599 |
| Hypertension | 39.458 | −25.330, 104.250 | 0.230 |
| Dyslipidaemia | 58.887 | −6.492, 124.267 | 0.077 |
| Congestive heart failure | 23.013 | −158.334, 204.361 | 0.802 |
| History of stroke/TIA | −24.251 | −96.379, 47.877 | 0.507 |
| History of myocardial infarction | 53.163 | −30.235, 136.561 | 0.209 |
| Current smoking | 4.246 | −50.447, 58.939 | 0.878 |
| Alcohol misuse | −5.309 | −103.451, 92.832 | 0.915 |
| Peripheral artery disease | −35.744 | −244.056, 172.566 | 0.735 |
| Ipsilateral carotid artery stenosis ≥ 50% | −41.671 | −142.947, 59.605 | 0.417 |
| Contralateral carotid artery stenosis ≥50% | 8.704 | −99.933, 117.341 | 0.874 |
| History of vertebral artery stenosis ≥ 50% | 119.294 | −133.935, 372.522 | 0.353 |
| Left atrial enlargement | −20.432 | −123.491, 82.626 | 0.695 |
| Valvular disease | −78.747 | −263.383, 105.888 | 0.387 |
| Atrial fibrillation on Holter monitor | −55.052 | −164.022, 53.919 | 0.318 |
| CRP | 0.3694 | −3.657, 4.395 | 0.855 |
| Early neurological deterioration | −50.136 | −198.607, 98.334 | 0.505 |
| NIHSSdis | −9.122 | −19.371, 1.126 | 0.080 |
| Recurrent stroke within 3 months | −19.876 | −169.624, 129.873 | 0.793 |
| Death within 3 months | −130.753 | −353.731, 92.225 | 0.247 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chondrogianni, M.; Lambadiari, V.; Katsanos, A.H.; Stefanou, M.I.; Palaiodimou, L.; Triantafyllou, A.S.; Karagiannis, G.; Konstantakos, V.; Ioakeimidis, M.; Triantafyllou, S.; et al. Omentin Is Independently Associated with Stroke Severity and Ipsilateral Carotid Artery Stenosis in Patients with Acute Cerebral Ischemia. J. Clin. Med. 2021, 10, 5797. https://doi.org/10.3390/jcm10245797
Chondrogianni M, Lambadiari V, Katsanos AH, Stefanou MI, Palaiodimou L, Triantafyllou AS, Karagiannis G, Konstantakos V, Ioakeimidis M, Triantafyllou S, et al. Omentin Is Independently Associated with Stroke Severity and Ipsilateral Carotid Artery Stenosis in Patients with Acute Cerebral Ischemia. Journal of Clinical Medicine. 2021; 10(24):5797. https://doi.org/10.3390/jcm10245797
Chicago/Turabian StyleChondrogianni, Maria, Vaia Lambadiari, Aristeidis H. Katsanos, Maria Ioanna Stefanou, Lina Palaiodimou, Alexandros Stavros Triantafyllou, Georgios Karagiannis, Vasileios Konstantakos, Michael Ioakeimidis, Sokratis Triantafyllou, and et al. 2021. "Omentin Is Independently Associated with Stroke Severity and Ipsilateral Carotid Artery Stenosis in Patients with Acute Cerebral Ischemia" Journal of Clinical Medicine 10, no. 24: 5797. https://doi.org/10.3390/jcm10245797
APA StyleChondrogianni, M., Lambadiari, V., Katsanos, A. H., Stefanou, M. I., Palaiodimou, L., Triantafyllou, A. S., Karagiannis, G., Konstantakos, V., Ioakeimidis, M., Triantafyllou, S., Zompola, C., Liantinioti, C., Pappa, A., Rizos, I., Voumvourakis, K., Tsivgoulis, G., & Boutati, E. (2021). Omentin Is Independently Associated with Stroke Severity and Ipsilateral Carotid Artery Stenosis in Patients with Acute Cerebral Ischemia. Journal of Clinical Medicine, 10(24), 5797. https://doi.org/10.3390/jcm10245797








