Relationship between Serum Kallistatin and Afamin and Anthropometric Factors Associated with Obesity and of Being Overweight in Patients after Myocardial Infarction and without Myocardial Infarction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Sampling and Laboratory Tests
2.3. Anthropometric Measurements
2.4. Other Variables
2.5. Statistics Analysis
3. Results
3.1. Baseline Characteristics of the Participants
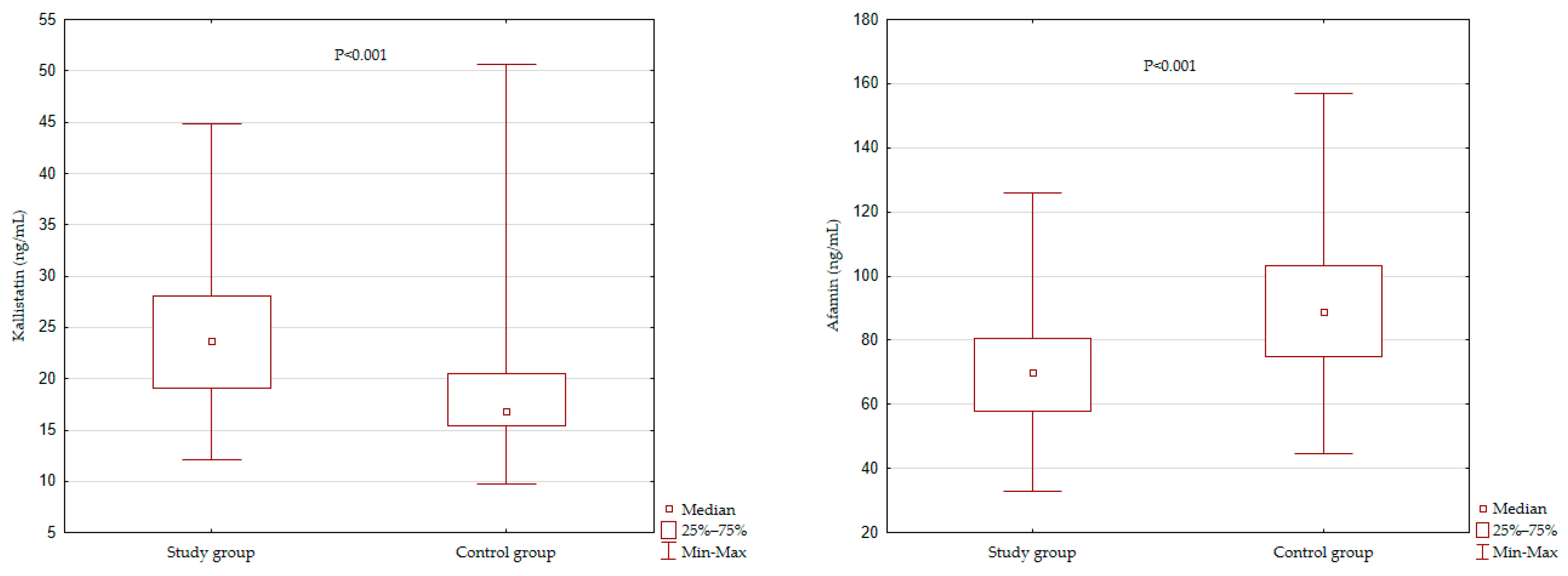
3.2. Kallistatin and Afamin Levels among the Study Groups
3.3. Correlations between the Concentration of Kallistatin and Afamin and Anthropometric Measurements
3.4. Relationship between the Concentration of Kallistatin and Afamin and Anthropometric Measurements—Multivariate Models
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, J.Y.; Lloyd-Jones, D.M.; Khan, S.S. Association of body mass index with mortality in cardiovascular disease: New insights into the obesity paradox from multiple perspectives. Trends Cardiovasc Med. 2019, 29, 220–225. [Google Scholar] [CrossRef]
- Chien, K.L.; Hsu, H.C.; Chen, Y.C.; Su, T.C.; Lee, Y.T.; Chen, M.F. Association between sequence variant of c.553 G > T in the apolipoprotein A5 gene and metabolic syndrome, insulin resistance, and carotid atherosclerosis. Transl. Res. 2009, 154, 133–141. [Google Scholar] [CrossRef]
- Su, X.; Peng, D. Emerging functions of adipokines in linking the development of obesity and cardiovascular diseases. Mol. Biol. Rep. 2020, 47, 7991–8006. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.; Liu, J.; Blaszczak, A.; Wright, V.; Jalilvand, A.; Needleman, B.; Noria, S.; Renton, D.; Hsueh, W. Adipocyte DIO2 expression increases in human obesity but is not related to systemic insulin sensitivity. J. Diabetes. Res. 2018, 15, 2464652. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef] [Green Version]
- Chao, J.; Tillman, D.M.; Wang, M.Y.; Margolius, H.S.; Chao, L. Identification of a new tissue-kallikrein-binding protein. Biochem. J. 1986, 239, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, J.; Guo, Y.; Chao, L. Protective role of endogenous kallistatin in vascular injury and senescence by inhibiting oxidative stress and inflammation. Oxid. Med. Cell Longev. 2018, 2018, 4138560. [Google Scholar] [CrossRef]
- Gao, L.; Yin, H.S.; Smith, R., Jr.; Chao, L.; Chao, J. Role of kallistatin in prevention of cardiac remodeling after chronic myocardial infarction. Lab. Investig. 2008, 88, 1157–1166. [Google Scholar] [CrossRef]
- Yao, Y.; Li, B.; Liu, C.; Fu, C.; Li, P.; Guo, Y.; Ma, G.; Liu, N.; Chao, L.; Chao, J. Reduced plasma kallistatin is associated with the severity of coronary artery disease, and kallistatin treatment attenuates atherosclerotic plaque formation in mice. J. Am. Heart Assoc. 2018, 7, e009562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kheiripour, N.; Khodamoradi, Z.; Ranjbar, A.; Borzouei, S. The positive effect of short-term nano-curcumin therapy on insulin resistance and serum levels of afamin in patients with metabolic syndrome. Avicenna J. Phytomed. 2021, 11, 146–153. [Google Scholar] [PubMed]
- Köninger, A.; Mathan, A.; Mach, P.; Frank, M.; Schmidt, B.; Schleussner, E.; Kimmig, R.; Gellhaus, A.; Dieplinger, H. Is afamin a novel biomarker for gestational diabetes mellitus? A pilot study. Reprod. Biol. Endocrinol. 2018, 16, 30. [Google Scholar] [CrossRef]
- Kronenberg, F.; Kollerits, B.; Kiechl, S.; Lamina, C.; Kedenko, L.; Meisinger, C.; Willeit, J.; Huth, C.; Wietzorrek, G.; Altmann, M.E.; et al. Plasma concentrations of afamin are associated with the prevalence and development of metabolic syndrome. Circ. Cardiovasc. Genet. 2014, 7, 822–829. [Google Scholar] [CrossRef] [Green Version]
- Polkowska, A.; Pasierowska, I.E.; Pasławska, M.; Pawluczuk, E.; Bossowski, A. Assessment of serum concentrations of adropin, afamin, and neudesin in children with type 1 diabetes. Biomed. Res. Int. 2019, 2019, 6128410. [Google Scholar] [CrossRef] [Green Version]
- Zdrojewski, T.; Jankowski, P.; Bandosz, P.; Bartuś, S.; Chwojnicki, K.; Drygas, W.; Gaciong, Z.; Hoffman, P.; Kalarus, Z.; Kaźmierczak, J.; et al. A new version of cardiovascular risk assessment system and risk charts calibrated for Polish population. Kardiol. Pol. 2015, 73, 958–961. [Google Scholar] [CrossRef] [Green Version]
- Boekholdt, S.M.; Arsenault, B.J.; Mora, S.; Pedersen, T.R.; LaRosa, J.C.; Nestel, P.J.; Simes, R.J.; Durrington, P.; Hitman, G.A.; Welch, K.M.; et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: A meta-analysis. JAMA. 2012, 307, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Physical Status: The Use an Interpretation of Anthropometry: Report of a WHO Expert Committee. WHO Tech Rep Ser 854, Geneva. 1995, pp. 1–452. Available online: https://apps.who.int/iris/handle/10665/37003 (accessed on 10 November 2021).
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef]
- Bergman, R.N.; Stefanovski, D.; Buchanan, T.A.; Sumner, A.E.; Reynolds, J.C.; Sebring, N.G.; Xiang, A.H.; Watanabe, R.M. A better index of body adiposity. Obesity (Silver Spring) 2011, 19, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Kahn, H.S. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: A population-based comparison. BMC Cardiovasc. Disord. 2005, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; González-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernández-González, S.O.; Jacques-Camarena, O.; Rodríguez-Morán, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Ji, Z.; Cai, J.; Li, Y.; Ma, G. Clinical significance of serum kallistatin and enox1 levels in patients with coronary heart disease. Med. Princ. Pract. 2021, 30, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Sirchak, E.S.; Opalenyk, S.M.; Kurchak, N.Y. Kallistatin level in patients with combination of chronic pancreatitis and aterosclerosis. Wiad. Lek. 2018, 71, 315–318. [Google Scholar] [PubMed]
- Miao, R.Q.; Murakami, H.; Song, Q.; Chao, L.; Chao, J. Kallistatin stimulates vascular smooth muscle cell proliferation and migration in vitro and neointima formation in balloon-injured rat artery. Circ. Res. 2000, 86, 418–424. [Google Scholar] [CrossRef] [Green Version]
- Chao, J.; Bledsoe, G.; Chao, L. Protective role of kallistatin in vascular and organ injury. Hypertension 2016, 68, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Chao, J.; Kotak, I.; Guo, D.; Parikh, S.J.; Bhagatwala, J.; Dong, Y.; Patel, S.Y.; Houk, C.; Chao, L.; et al. Plasma kallistatin is associated with adiposity and cardiometabolic risk in apparently healthy African American adolescents. Metabolism 2013, 62, 642–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gateva, A.; Assyov, Y.; Velikova, T.; Kamenov, Z. Increased kallistatin levels in patients with obesity and prediabetes compared to normal glucose tolerance. Endocr. Res. 2017, 42, 163–168. [Google Scholar] [CrossRef]
- Frühbeck, G.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Valentí, V.; Moncada, R.; Becerril, S.; Unamuno, X.; Silva, C.; Salvador, J.; et al. Novel protective role of kallistatin in obesity by limiting adipose tissue low grade inflammation and oxidative stress. Metabolism 2018, 87, 123–135. [Google Scholar] [CrossRef]
- Ward, L.J.; Olausson, P.; Li, W.; Yuan, X.M. Proteomics and multivariate modelling reveal sex-specific alterations in distinct regions of human carotid atheroma. Biol. Sex Differ. 2018, 9, 54. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.S.S.; Mashkur, M.S.; Siadat, S.O.R.; Ali, H.A. Assessment of serum afamin and preptin levels as a potential diagnosis markers for cardiovascular patients undergoing catheterization. Medico-Legal Update 2020, 20, 479–484. [Google Scholar]
- Ahmed, H.H.; Abdel Hameed, E.R.; Shehata, M.A.; El Wakeel, M.A.; Elsawy, D.H.; Elshafie, A.I. Relation between afamin level and some inflammatory markers in obese children. Med. Res. J. 2015, 14, 1–6. [Google Scholar] [CrossRef]
- Oller Moreno, S.; Cominetti, O.; Núñez Galindo, A.; Irincheeva, I.; Corthésy, J.; Astrup, A.; Saris, W.H.M.; Hager, J.; Kussmann, M.; Dayon, L. The differential plasma proteome of obese and overweight individuals undergoing a nutritional weight loss and maintenance intervention. Proteomics Clin. Appl. 2018, 12, 1600150. [Google Scholar] [CrossRef] [PubMed]
- de Lorenzo, A.; Sorge, S.P.; Iacopino, L.; Andreoli, A.; de Luca, P.P.; Sasso, G.F. Fat-free mass by bioelectrical impedance vs dual-energy X-ray absorptiometry (DXA). Appl. Radiat. Isot. 1998, 49, 739–741. [Google Scholar] [CrossRef]
| Variables | Study Group (n = 80) | Control Group (n = 80) | p |
|---|---|---|---|
| Demographic and Clinical Data: | |||
| Age (years) | 53.34 ± 4.74 | 49.25 ± 6.23 | <0.001 |
| Men | 22 (27.5) | 28 (35) | 0.31 |
| In Relationship | 64 (80) | 69 (86.3) | 0.398 |
| Current Smoking | 25 (31.3) | 9 (11.3) | <0.001 |
| Family History of CVD—on the Mother’s Side | 46 (57.5) | 42 (52.5) | 0.634 |
| Family History of CVD—on the Father’s Side | 49 (61.3) | 39 (48.8) | 0.153 |
| Diabetes | 19 (24) | 0 (0) | <0.001 |
| Arterial Hypertensions | 54 (68) | 0 (0) | <0.001 |
| Anthropometric Variables: | |||
| BMI (kg/m2) | 28.89 ± 4.91 | 26.64 ± 4.04 | 0.002 |
| WC (cm) | 103.9 ± 12,48 | 93.36 ± 12.50 | <0.001 |
| HC (cm) | 105 ± 10,83 | 102.9 ± 7.72 | 0.15 |
| WHR | 0.99 ± 0.08 | 0.91 ± 0.09 | <0.001 |
| WHtR | 0.60 ± 0.07 | 0.54 ± 0.07 | <0.001 |
| FM (%) | 31.08 ±7.71 | 29.65 ± 7.05 | 0.22 |
| VAI | 2.12 ± 1.56 | 1.06 ± 0.7 | <0.001 |
| BAI | 28.63 ± 6.44 | 27.79 ± 4.73 | 0.35 |
| LAP | 60.9 (43.4–89.6) | 48.3 (21.8–60) | <0.001 |
| TyGindex | 4.8 (0.26) | 4.7 (0.27) | 0.001 |
| Variables | Log Kallistatin (ng/mL) | Log Afamin (ng/mL) | ||
|---|---|---|---|---|
| r | p | r | p | |
| Study Group: | ||||
| BMI | −0.156 | 0.17 | 0.13 | 0.231 |
| WC | −0.12 | 0.27 | 0.14 | 0.210 |
| HC | −0.12 | 0.31 | −0.03 | 0.812 |
| WHR | −0.05 | 0.67 | 0.25 | 0.026 |
| WHtR | −0.12 | 0.30 | 0.12 | 0.295 |
| FM | 0.02 | 0.89 | 0.09 | 0.41 |
| VAI | 0.13 | 0.23 | 0.20 | 0.078 |
| BAI | −0.08 | 0.48 | −0.04 | 0.742 |
| LAP | 0.11 | 0.31 | 0.28 | 0.013 |
| TyG index | 0.11 | 0.33 | 0.35 | 0.002 |
| Control Group: | ||||
| BMI | 0.15 | 0.19 | 0.54 | <0.001 |
| WC | 0.25 | 0.02 | 0.47 | <0.01 |
| HC | 0.12 | 0.30 | 0.38 | 0.001 |
| WHR | 0.25 | 0.03 | 0.34 | 0.002 |
| WHtR | 0.18 | 0.11 | 0.10 | <0.001 |
| FM% | −0.01 | 0.92 | 0.60 | <0.001 |
| VAI | 0.17 | 0.13 | 0.29 | 0.008 |
| BAI | −0.09 | 0.45 | 0.39 | 0.002 |
| LAP | 0.23 | 0.04 | 0.46 | <0.001 |
| TyG index | 0.33 | <0.001 | 0.54 | <0.001 |
| Variables | Log Kallistatin (ng/mL) | Log Afamin (ng/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Model A | Model B | Model A | Model B | |||||
| b (SE) | p | b (SE) | p | b (SE) | p | b (SE) | p | |
| Study Group: | ||||||||
| BMI | −0.16 (0.11) | 0.164 | −0.16 (0.11) | 0.165 | 0.13 (0.11) | 0.268 | 0.13 (0.11) | 0.249 |
| WC | −0.14 (0.12) | 0.240 | −0.14 (0.12) | 0.240 | 0.14 (0.12) | 0.230 | 0.15 (0.12) | 0.206 |
| HC | −0.13 (0.12) | 0.289 | −0.13 (0.12) | 0.288 | −0.05 (0.12) | 0.674 | −0.04 (0.12) | 0.727 |
| WHR | −0.08 (0.14) | 0.555 | −0.08 (0.14) | 0.557 | 0.36 (0.13) | 0.006 | 0.37 (0.13) | 0.006 |
| WHtR | −0.12 (0.12) | 0.294 | −0.12 (0.12) | 0.295 | 0.11 (0.12) | 0.352 | 0.11 (0.12) | 0.273 |
| FM% | 0.05 (0.16) | 0.746 | 0.05 (0.16) | 0.750 | 0.16 (0.16) | 0.326 | 0.17 (0.16) | 0.300 |
| VAI | 0.14 (0.11) | 0.237 | 0.16 (0.12) | 0.195 | 0.20 (0.11) | 0.083 | 0.19 (0.12) | 0.131 |
| BAI | −0.12 (0.14) | 0.410 | −0.12 (0.15) | 0.410 | −0.10 (0.15) | 0.507 | −0.09 (0.15) | 0.539 |
| LAP | 0.11 (0.11) | 0.323 | 0.13 (0.12) | 0.300 | 0.28 (0.11) | 0.013 | 0.27 (0.11) | 0.02 |
| TyG index | 0.11 (0.11) | 0.346 | 0.13 (0.12) | 0.31 | 0.36 (0.11) | 0.001 | 0.36 (0.12) | 0.002 |
| Control Group: | ||||||||
| BMI | 0.08(0.11) | 0.499 | 0.07(0.11) | 0.535 | 0.48(0.10) | <0.001 | 0.48(0.10) | <0.001 |
| WC | 0.14(0.12) | 0.267 | 0.13(0.13) | 0.291 | 0.39(0.11) | 0.001 | 0.39(0.12) | 0.001 |
| HC | 0.11(0.11) | 0.330 | 0.10(0.11) | 0.345 | 0.38(0.10) | 0.000 | 0.38(0.10) | <0.001 |
| WHR | 0.11(0.14) | 0.421 | 0.11(0.14) | 0.457 | 0.19(0.14) | 0.164 | 0.20(0.14) | 0.167 |
| WHtR | 0.13(0.11) | 0.240 | 0.13(0.11) | 0.265 | 0.33(0.10) | 0.002 | 0.33(0.11) | 0.002 |
| FM% | 0.31(0.14) | 0.024 | 0.31(0.14) | 0.028 | 0.60(0.12) | <0.001 | 0.63(0.12) | <0.001 |
| VAI | 0.10(0.11) | 0.349 | 0.10(0.11) | 0.358 | 0.23(0.11) | 0.031 | 0.23(0.11) | 0.032 |
| BAI | 0.15(0.13) | 0.262 | 0.15(0.13) | 0.282 | 0.39(0.12) | 0.003 | 0.39(0.13) | 0.003 |
| LAP | 0.16(0.11) | 0.151 | 0.16(0.11) | 0.160 | 0.40(0.10) | <0.001 | 0.40(0.10) | <0.001 |
| TyG index | 0.25(0.11) | 0.027 | 0.48(0.10) | <0.001 | 0.48(0.10) | <0.001 | 0.50(0.10) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowicki, G.J.; Ślusarska, B.; Polak, M.; Naylor, K.; Kocki, T. Relationship between Serum Kallistatin and Afamin and Anthropometric Factors Associated with Obesity and of Being Overweight in Patients after Myocardial Infarction and without Myocardial Infarction. J. Clin. Med. 2021, 10, 5792. https://doi.org/10.3390/jcm10245792
Nowicki GJ, Ślusarska B, Polak M, Naylor K, Kocki T. Relationship between Serum Kallistatin and Afamin and Anthropometric Factors Associated with Obesity and of Being Overweight in Patients after Myocardial Infarction and without Myocardial Infarction. Journal of Clinical Medicine. 2021; 10(24):5792. https://doi.org/10.3390/jcm10245792
Chicago/Turabian StyleNowicki, Grzegorz Józef, Barbara Ślusarska, Maciej Polak, Katarzyna Naylor, and Tomasz Kocki. 2021. "Relationship between Serum Kallistatin and Afamin and Anthropometric Factors Associated with Obesity and of Being Overweight in Patients after Myocardial Infarction and without Myocardial Infarction" Journal of Clinical Medicine 10, no. 24: 5792. https://doi.org/10.3390/jcm10245792
APA StyleNowicki, G. J., Ślusarska, B., Polak, M., Naylor, K., & Kocki, T. (2021). Relationship between Serum Kallistatin and Afamin and Anthropometric Factors Associated with Obesity and of Being Overweight in Patients after Myocardial Infarction and without Myocardial Infarction. Journal of Clinical Medicine, 10(24), 5792. https://doi.org/10.3390/jcm10245792









