Association of Insulin Resistance with Vascular Ageing in a General Caucasian Population: An EVA Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Variables and Measurement Instruments
2.3. Measurement of Arterial Stiffness
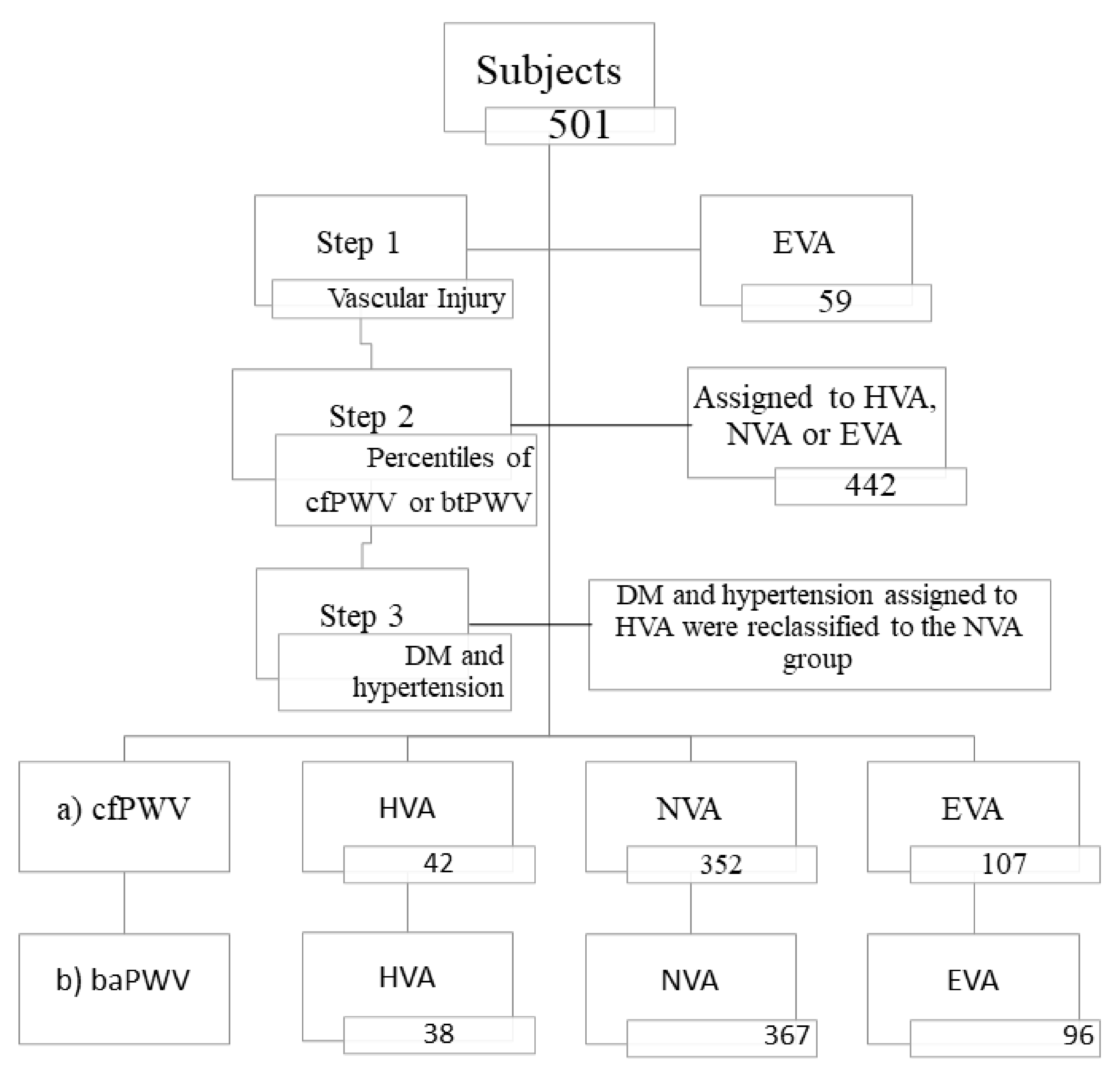
2.4. Definition of Vascular Ageing
- Step 1: the participants who presented with vascular injury in the carotid artery or peripheral arteriopathy, using the criteria established in the 2018 clinical practice guidelines of the European Societies of Hypertension and Cardiology for the treatment of arterial hypertension [38], were classified as early vascular ageing (EVA);
- Step 2: with the percentiles of arterial stiffness, we used two criteria: the 10th and 90th percentiles of cfPWV and the 10th and 90th percentiles of baPWV of the population studied by age and sex. The individuals with values of cfPWV or baPWV over p90 were considered EVA; those between p10 and p90 were classified as normal vascular ageing (NVA); and those with values below p10 were classified as healthy vascular ageing (HVA);
- Step 3: the individuals diagnosed with type 2 diabetes mellitus or hypertension were included in the HVA group; those with the criteria in the percentiles for cfPWV or those of baPWV were classified as NVA [39]. The distribution of participants fitting the two criteria in each of the groups is shown in Figure 2. The percentiles by age groups and sex of cfPWV and baPWV are shown in Supplementary Materials: Figure S1.
2.5. Insulin Resistance Indices
2.6. Measurement of Vascular Injury
2.7. Laboratory Analyses
2.8. Statistical Analysis
3. Results
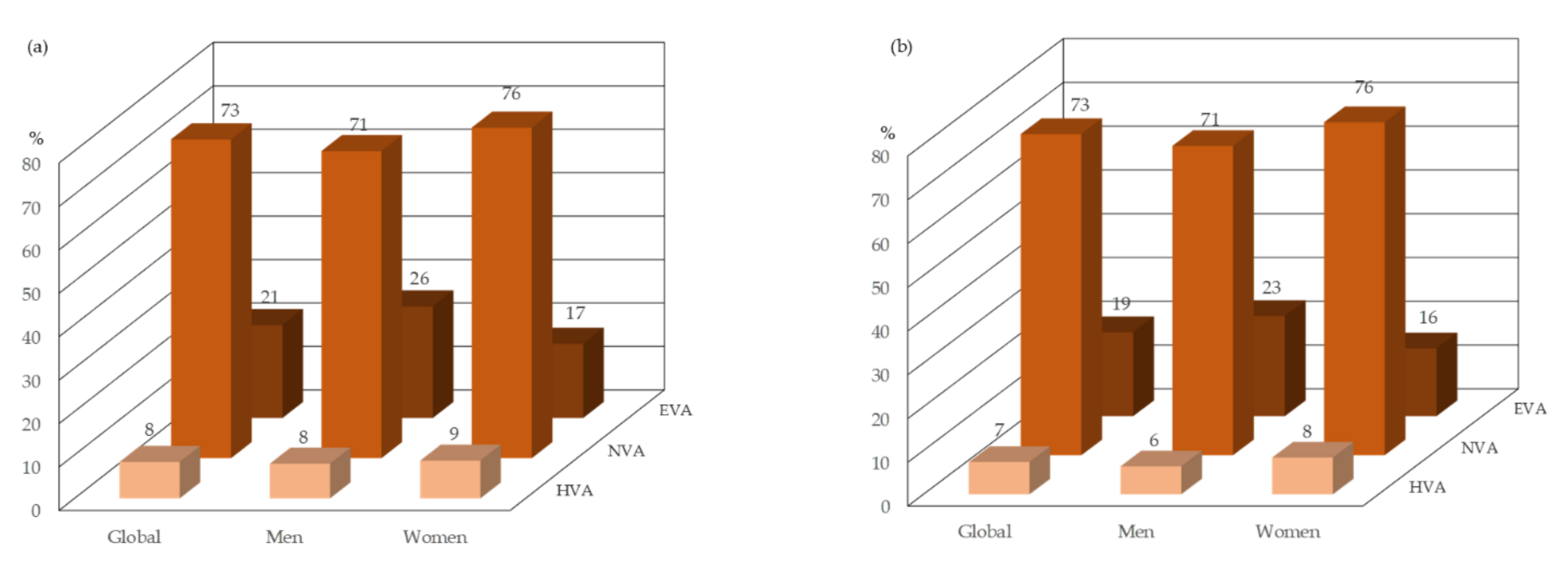
3.1. Baseline Characteristics
3.2. Relationship of the Insulin Resistance Indices with Vascular Ageing
3.3. Association of the Insulin Resistance Indices with Arterial Stiffness and Vascular Ageing
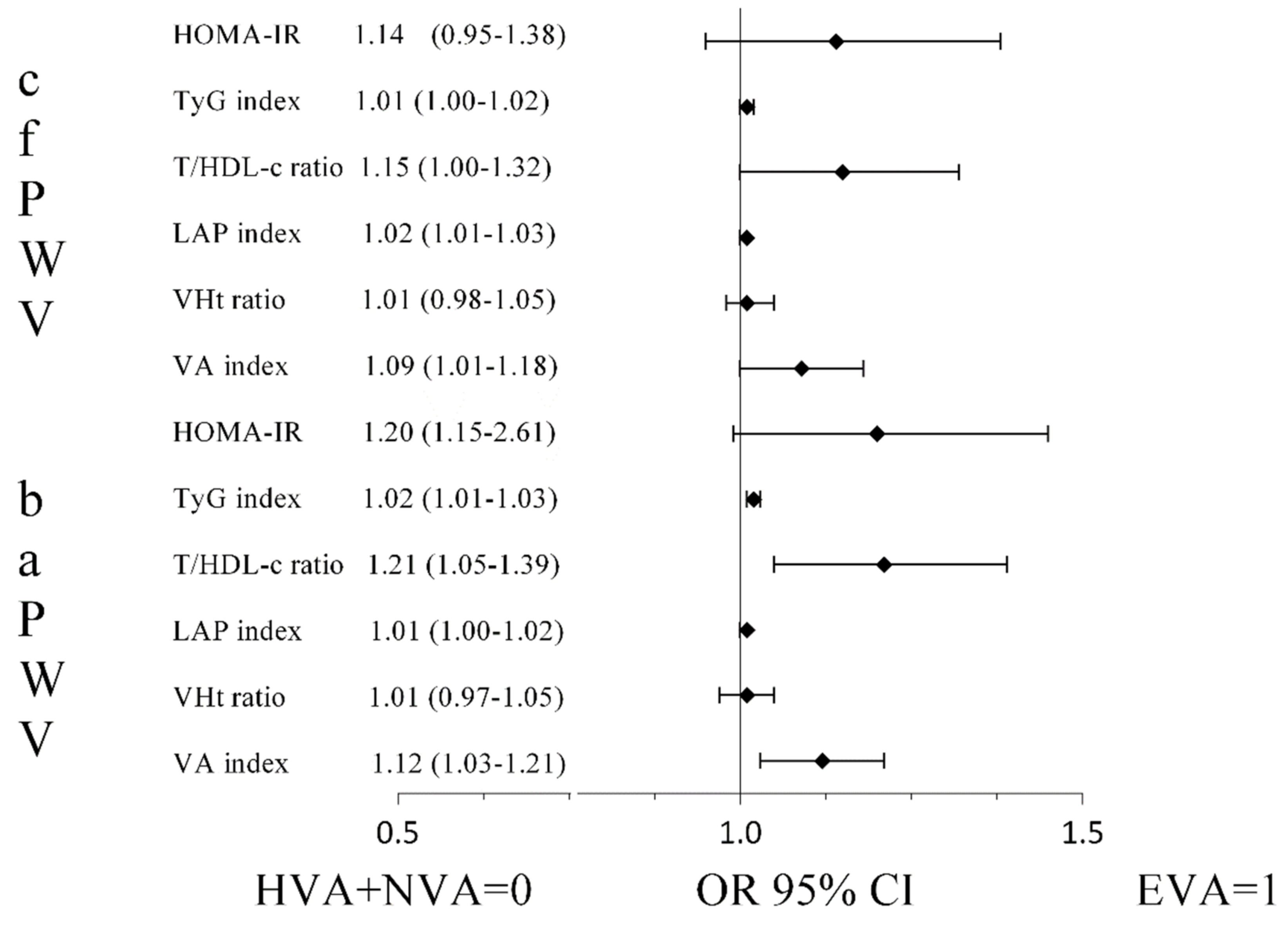
4. Discussion
5. Conclusions
6. Limits
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Strisciuglio, T.; Izzo, R.; Barbato, E.; Di Gioia, G.; Colaiori, I.; Fiordelisi, A.; Morisco, C.; Bartunek, J.; Franco, D.; Ammirati, G.; et al. Insulin Resistance Predicts Severity of Coronary Atherosclerotic Disease in Non-Diabetic Patients. J. Clin. Med. 2020, 9, 2144. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, L.B.; Bahrun, U.; Hatta, M.; Arif, M. Body Mass, Total Body Fat Percentage, and Visceral Fat Level Predict Insulin Resistance Better Than Waist Circumference and Body Mass Index in Healthy Young Male Adults in Indonesia. J. Clin. Med. 2018, 7, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janus, A.; Szahidewicz-Krupska, E.; Mazur, G.; Doroszko, A. Insulin Resistance and Endothelial Dysfunction Constitute a Common Therapeutic Target in Cardiometabolic Disorders. Mediat. Inflamm. 2016, 2016, 3634948. [Google Scholar] [CrossRef] [Green Version]
- Poon, A.K.; Meyer, M.L.; Tanaka, H.; Selvin, E.; Pankow, J.; Zeng, D.; Loehr, L.; Knowles, J.W.; Rosamond, W.; Heiss, G. Association of insulin resistance, from mid-life to late-life, with aortic stiffness in late-life: The Atherosclerosis Risk in Communities Study. Cardiovasc. Diabetol 2020, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- González-Clemente, J.M.; Cano, A.; Albert, L.; Giménez-Palop, O.; Romero, A.; Berlanga, E.; Vendrell, J.; Llauradó, G. Arterial Stiffness in Type 1 Diabetes: The Case for the Arterial Wall Itself as a Target Organ. J. Clin. Med. 2021, 10, 3616. [Google Scholar] [CrossRef] [PubMed]
- Gagliardino, J.J.; Salazar, M.R.; Espeche, W.G.; Tolosa Chapasian, P.E.; Gomez Garizoain, D.; Olano, R.D.; Stavile, R.N.; Balbín, E.; Martinez, C.; Leiva Sisnieguez, B.C.; et al. Arterial Stiffness: Its Relation with Prediabetes and Metabolic Syndrome and Possible Pathogenesis. J. Clin. Med. 2021, 10, 3251. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.K.; Lee, J.; Kim, H.S.; Kim, E.H.; Lee, M.J.; Yang, D.H.; Kang, J.W.; Jung, C.H.; Park, J.Y.; Kim, H.K.; et al. Triglyceride Glucose-Waist Circumference Better Predicts Coronary Calcium Progression Compared with Other Indices of Insulin Resistance: A Longitudinal Observational Study. J. Clin. Med. 2020, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Gast, K.B.; Tjeerdema, N.; Stijnen, T.; Smit, J.W.; Dekkers, O.M. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: Meta-analysis. PLoS ONE 2012, 7, e52036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancusi, C.; de Simone, G.; Best, L.G.; Wang, W.; Zhang, Y.; Roman, M.J.; Lee, E.T.; Howard, B.V.; Devereux, R.B. Myocardial mechano-energetic efficiency and insulin resistance in non-diabetic members of the Strong Heart Study cohort. Cardiovasc. Diabetol 2019, 18, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Li, S.; Liu, Y.; Bazzano, L.; He, J.; Mi, J.; Chen, W. Temporal relationship between inflammation and insulin resistance and their joint effect on hyperglycemia: The Bogalusa Heart Study. Cardiovasc. Diabetol 2019, 18, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvin, P.; Ward, G.; Walters, J.; Pestell, R.; Koschmann, M.; Vaag, A.; Martin, I.; Best, J.D.; Alford, F. A simple method for quantitation of insulin sensitivity and insulin release from an intravenous glucose tolerance test. Diabet. Med. 1992, 9, 921–928. [Google Scholar] [CrossRef]
- Bonora, E.; Targher, G.; Alberiche, M.; Bonadonna, R.C.; Saggiani, F.; Zenere, M.B.; Monauni, T.; Muggeo, M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000, 23, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; González-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernández-González, S.O.; Jacques-Camarena, O.; Rodríguez-Morán, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bikov, A.; Frent, S.M.; Meszaros, M.; Kunos, L.; Mathioudakis, A.G.; Negru, A.G.; Gaita, L.; Mihaicuta, S. Triglyceride-Glucose Index in Non-Diabetic, Non-Obese Patients with Obstructive Sleep Apnoea. J. Clin. Med. 2021, 10, 1932. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Reaven, G.M. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: Triglycerides × glucose versus triglyceride/high-density lipoprotein cholesterol. Metabolism 2011, 60, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Kahn, H.S. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: A population-based comparison. BMC Cardiovasc. Disord. 2005, 5, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foschi, F.G.; Conti, F.; Domenicali, M.; Giacomoni, P.; Borghi, A.; Bevilacqua, V.; Napoli, L.; Berardinelli, D.; Altini, M.; Cucchetti, A.; et al. External Validation of Surrogate Indices of Fatty Liver in the General Population: The Bagnacavallo Study. J. Clin. Med. 2021, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A. Visceral Adiposity Index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Yu, S.; Chi, C.; Fan, X.; Tang, J.; Ji, H.; Teliewubai, J.; Zhang, Y.; Xu, Y. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: The Northern Shanghai Study. Cardiovasc Diabetol. 2019, 18, 95. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Ahn, C.W.; Lee, B.K.; Kang, S.; Nam, J.S.; You, J.H.; Kim, M.J.; Kim, M.K.; Park, J.S. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc. Diabetol 2018, 17, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorentino, T.V.; Marini, M.A.; Succurro, E.; Andreozzi, F.; Sesti, G. Relationships of surrogate indexes of insulin resistance with insulin sensitivity assessed by euglycemic hyperinsulinemic clamp and subclinical vascular damage. BMJ Open Diabetes Res. Care 2019, 7, e000911. [Google Scholar] [CrossRef]
- Sánchez-García, A.; Rodríguez-Gutiérrez, R.; Mancillas-Adame, L.; González-Nava, V.; Díaz González-Colmenero, A.; Solis, R.C.; Álvarez-Villalobos, N.A.; González-González, J.G. Diagnostic Accuracy of the Triglyceride and Glucose Index for Insulin Resistance: A Systematic Review. Int. J. Endocrinol. 2020, 2020, 4678526. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Sequí-Domínguez, I.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Pozuelo-Carrascosa, D.P.; Nuñez de Arenas-Arroyo, S.; Martínez-Vizcaíno, V. Accuracy of Pulse Wave Velocity Predicting Cardiovascular and All-Cause Mortality. A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2080. [Google Scholar] [CrossRef]
- Ohkuma, T.; Ninomiya, T.; Tomiyama, H.; Kario, K.; Hoshide, S.; Kita, Y.; Inoguchi, T.; Maeda, Y.; Kohara, K.; Tabara, Y.; et al. Brachial-Ankle Pulse Wave Velocity and the Risk Prediction of Cardiovascular Disease: An Individual Participant Data Meta-Analysis. Hypertension 2017, 69, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.R.; Khunti, K.; Silverman, R.; Gray, L.J.; Srinivasan, B.; Lacy, P.S.; Williams, B.; Davies, M.J. Impact of metabolic indices on central artery stiffness: Independent association of insulin resistance and glucose with aortic pulse wave velocity. Diabetologia 2010, 53, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Boutouyrie, P.; Cunha, P.G.; Lacolley, P.; Nilsson, P.M. Concept of Extremes in Vascular Aging. Hypertension 2019, 74, 218–228. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, K.L.; Rossman, M.J.; Chonchol, M.; Seals, D.R. Strategies for Achieving Healthy Vascular Aging. Hypertension 2018, 71, 389–402. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wu, Y.Y.; Huang, X.M.; Yang, M.; Zha, B.B.; Wang, F.; Zha, Y.; Sheng, L.; Chen, Z.P.; Gu, Y. Ageing and type 2 diabetes in an elderly Chinese population: The role of insulin resistance and beta cell dysfunction. Eur. Rev. Med. Pharm. Sci. 2014, 18, 1790–1797. [Google Scholar]
- Niiranen, T.J.; Lyass, A.; Larson, M.G.; Hamburg, N.M.; Benjamin, E.J.; Mitchell, G.F.; Vasan, R.S. Prevalence, Correlates, and Prognosis of Healthy Vascular Aging in a Western Community-Dwelling Cohort: The Framingham Heart Study. Hypertension 2017, 70, 267–274. [Google Scholar] [CrossRef]
- Gomez-Marcos, M.A.; Martinez-Salgado, C.; Gonzalez-Sarmiento, R.; Hernandez-Rivas, J.M.; Sanchez-Fernandez, P.L.; Recio-Rodriguez, J.I.; Rodriguez-Sanchez, E.; García-Ortiz, L. Association between different risk factors and vascular accelerated ageing (EVA study): Study protocol for a cross-sectional, descriptive observational study. BMJ Open 2016, 6, e011031. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Sánchez, M.; Patino-Alonso, M.C.; Gómez-Sánchez, L.; Recio-Rodríguez, J.I.; Rodríguez-Sánchez, E.; Maderuelo-Fernández, J.A.; García-Ortiz, L.; Gómez-Marcos, M.A. Reference values of arterial stiffness parameters and their association with cardiovascular risk factors in the Spanish population. The EVA Study. Rev. Esp. Cardiol. (Engl. Ed.) 2020, 73, 43–52. [Google Scholar] [CrossRef]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; De Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace-Raso, F.U.; Protogerou, A.D.; et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens. 2012, 30, 445–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirai, K.; Hiruta, N.; Song, M.; Kurosu, T.; Suzuki, J.; Tomaru, T.; Miyashita, Y.; Saiki, A.; Takahashi, M.; Suzuki, K.; et al. Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: Theory, evidence and perspectives. J. Atheroscler. Thromb. 2011, 18, 924–938. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J. Hypertens. 2018, 36, 2284–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Sanchez, M.; Gomez-Sanchez, L.; Patino-Alonso, M.C.; Cunha, P.G.; Recio-Rodriguez, J.I.; Alonso-Dominguez, R.; Sanchez-Aguadero, N.; Rodriguez-Sanchez, E.; Maderuelo-Fernandez, J.A.; Garcia-Ortiz, L.; et al. Vascular aging and its relationship with lifestyles and other risk factors in the general Spanish population: Early Vascular Ageing Study. J. Hypertens. 2020, 38, 1110–1122. [Google Scholar] [CrossRef]
- Nilsson, P.M.; Laurent, S.; Cunha, P.G.; Olsen, M.H.; Rietzschel, E.; Franco, O.H.; Ryliškytė, L.; Strazhesko, I.; Vlachopoulos, C.; Chen, C.H.; et al. Characteristics of healthy vascular ageing in pooled population-based cohort studies: The global Metabolic syndrome and Artery REsearch Consortium. J. Hypertens. 2018, 36, 2340–2349. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Teliewubai, J.; Lu, Y.; Xiong, J.; Yu, S.; Chi, C.; Li, J.; Blacher, J.; Zhang, Y.; Xu, Y. Vascular aging and preclinical target organ damage in community-dwelling elderly: The Northern Shanghai Study. J. Hypertens. 2018, 36, 1391–1398. [Google Scholar] [CrossRef]
- Botto, F.; Obregon, S.; Rubinstein, F.; Scuteri, A.; Nilsson, P.M.; Kotliar, C. Frequency of early vascular aging and associated risk factors among an adult population in Latin America: The OPTIMO study. J. Hum. Hypertens. 2018, 32, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.G.; Cotter, J.; Oliveira, P.; Vila, I.; Boutouyrie, P.; Laurent, S.; Nilsson, P.M.; Scuteri, A.; Sousa, N. Pulse wave velocity distribution in a cohort study: From arterial stiffness to early vascular aging. J. Hypertens. 2015, 33, 1438–1445. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.; Lin, Y.; Luo, L.; Ye, P. Relationship between Central Arterial Stiffness and Insulin Resistance in Chinese Community-Dwelling Population without Diabetes Mellitus. Int. J. Endocrinol. 2017, 2017, 1073919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Zhan, A.; Huang, X.; Hu, L.; Zhou, W.; Wang, T.; Zhu, L.; Bao, H.; Cheng, X. Positive association between triglyceride glucose index and arterial stiffness in hypertensive patients: The China H-type Hypertension Registry Study. Cardiovasc. Diabetol 2020, 19, 139. [Google Scholar] [CrossRef] [PubMed]
- Won, K.B.; Park, G.M.; Lee, S.E.; Cho, I.J.; Kim, H.C.; Lee, B.K.; Chang, H.J. Relationship of insulin resistance estimated by triglyceride glucose index to arterial stiffness. Lipids Health Dis. 2018, 17, 268. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, L.; Liu, C. Overweightness, obesity and arterial stiffness in healthy subjects: A systematic review and meta-analysis of literature studies. Postgrad. Med. 2017, 129, 224–230. [Google Scholar] [CrossRef]
- Cunha, P.G.; Boutouyrie, P.; Nilsson, P.M.; Laurent, S. Early Vascular Ageing (EVA): Definitions and Clinical Applicability. Curr. Hypertens. Rev. 2017, 13, 8–15. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef] [Green Version]
- McEniery, C.M.; Wilkinson, I.B.; Johansen, N.B.; Witte, D.R.; Singh-Manoux, A.; Kivimaki, M.; Tabak, A.G.; Brunner, E.J.; Shipley, M.J. Nondiabetic Glucometabolic Status and Progression of Aortic Stiffness: The Whitehall II Study. Diabetes Care 2017, 40, 599–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, M.T.; Leite, N.C.; Cardoso, C.R.; Salles, G.F. Correlates of aortic stiffness progression in patients with type 2 diabetes: Importance of glycemic control: The Rio de Janeiro type 2 diabetes cohort study. Diabetes Care 2015, 38, 897–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Sun, H.; Shang, F.; Wu, H.; Shi, H.; Ren, L.; He, Y.; Zhang, M.; Peng, H. Association Between Glucose Metabolism And Vascular Aging In Chinese Adults: A Cross-Sectional Analysis In The Tianning Cohort Study. Clin. Interv. Aging. 2019, 14, 1937–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Global (501) | Men (248) | Women (251) | p Value | |
|---|---|---|---|---|
| Age, years | 55.90 ± 14.24 | 55.95 ± 14.31 | 55.85 ± 14.19 | 0.934 |
| Clinical measures, mean (SD) | ||||
| SBP, mmHg | 121 ± 23 | 126 ± 20 | 115 ± 25 | <0.001 |
| DBP, mmHg | 76 ± 10 | 77 ± 9 | 74 ± 10 | <0.001 |
| Total cholesterol, mg/dL | 195 ± 32 | 191 ± 32 | 197 ± 31 | 0.142 |
| LDL cholesterol, mg/dL | 116 ± 29 | 117 ± 30 | 114 ± 29 | 0.148 |
| HDL cholesterol, mg/dL | 59 ± 16 | 53 ± 14 | 64 ± 16 | <0.001 |
| Triglyceride, mg/dL | 103 ± 53 | 112 ± 54 | 94 ± 50 | <0.001 |
| Fasting glucose, mg/dL | 88 ± 17 | 90 ± 19 | 86 ± 16 | 0.013 |
| HbA1c, (%) | 5.49 ± 0.56 | 5.54 ± 0.63 | 5.44 ± 0.47 | <0.001 |
| Fasting insulin, μU/mL | 8.45 ± 4.66 | 8.78 ± 4.59 | 8.11 ± 4.71 | 0.110 |
| Weight, kg | 72 ± 14 | 79 ± 12 | 66 ± 12 | <0.001 |
| Height, cm | 165 ± 10 | 172 ± 7 | 159 ± 7 | <0.001 |
| BMI, kg/m2 | 26.52 ± 4.23 | 26.90 ± 3.54 | 26.14 ± 4.79 | 0.044 |
| Waist circumference, cm | 93.33 ± 12.00 | 98.76 ± 9.65 | 87.95 ± 11.68 | <0.001 |
| Arterial stiffness, mean (SD) | ||||
| cfPWV, m/s | 8.17 ± 2.53 | 8.58 ± 2.74 | 7.77 ± 2.24 | 0.043 |
| baPWV, m/s | 12.93 ± 2.68 | 13.16 ± 2.46 | 13.16 ± 2.46 | 0.064 |
| Cardiovascular Risk factors, n (%) | ||||
| Smoker | 90 (17.96) | 49 (9.80) | 41 8.20) | 0.353 |
| Hypertension | 147 (29.34) | 82 (32.93) | 65 (25.79) | 0.095 |
| Dyslipidaemia | 191 (38.12) | 95 (38.10) | 96 (38.20) | 0.989 |
| Normal glucose | 358 (71.42) | 174 (69.90) | 184 (73.00) | 0.314 |
| Prediabetes | 105 (21.00) | 49 (19.70) | 56 (22.20) | 0.459 |
| Diabetes mellitus | 38 (7.58) | 26 (10.40) | 12 (4.80) | 0.018 |
| Obesity | 94 (18.76) | 42 (16.90) | 52 (20.60) | 0.304 |
| Abdominal obesity | 193 (38.52) | 78 (31.30) | 115 (45.80) | 0.001 |
| Medication use, n (%) | ||||
| Antihypertensive drugs | 147 (29) | 82 (33) | 65 (26) | 0.095 |
| Lipid-lowering drugs | 102 (20) | 49 (20) | 53 (21) | 0.740 |
| Hypoglycaemic drugs | 35 (7) | 23 (9) | 12 (5) | 0.055 |
| Insulin resistance indexes, mean (SD) | ||||
| HOMA-IR | 1.87 ± 1.18 | 1.98 ± 1.18 | 1.77 ± 1.17 | 0.052 |
| TyG index | 10.12 ± 1.15 | 10.37 ± 1.06 | 9.86 ± 1.18 | <0.001 |
| T/HDL-c ratio | 2.01 ± 1.49 | 2.36 ± 1.59 | 1.66 ± 1.29 | <0.001 |
| LAP index | 43.10 ± 31.08 | 48.68 ± 30.51 | 37.39 ± 30.67 | <0.001 |
| WHt ratio | 0.57 ± 0.07 | 0.58 ± 0.06 | 0.56 ± 0.08 | 0.001 |
| VA index | 3.27 ± 2.44 | 3.31 ± 2.26 | 3.21 ± 2.61 | 0.656 |
| HVA (42) | NVA (253) | EVA (107) | p Value | |
|---|---|---|---|---|
| Global, mean (SD) | ||||
| HOMA-IR, *,¥,& | 1.36 ± 0.64 | 1.85 ± 1.14 | 2.17 ± 1.37 | 0.001 |
| TyG index, *,¥,& | 179 ± 30 | 197 ± 40 | 220 ± 70 | <0.001 |
| T/HDL-c ratio, ¥,& | 1.54 ± 0.99 | 1.95 ± 1.43 | 2.38 ± 1.73 | 0.003 |
| LAP index, *,¥,& | 26.26 ± 15.54 | 42.52 ± 31.20 | 51.62 ± 32.37 | <0.001 |
| WHt ratio, *,¥,& | 0.53 ± 0.06 | 0.57 ± 0.07 | 0.58 ± 0.08 | 0.019 |
| VA index, *,¥,& | 2.39 ± 1.31 | 3.20 ± 2.40 | 3.85 ± 2.79 | 0.004 |
| Men, mean (SD) | ||||
| HOMA-IR, *,¥ | 1.39 ± 0.80 | 1.98 ± 1.22 | 2.16 ± 1.13 | 0.038 |
| TyG, mg/dL, *,¥,& | 187 ± 28 | 204 ± 38 | 228 ± 73 | 0.001 |
| T/HDL-c ratio | 2.05 ± 1.18 | 2.28 ± 1.56 | 2.67 ± 1.74 | 0.165 |
| LAP index, *,¥ | 32.25 ± 15.43 | 47.73 ± 30.40 | 56.28 ± 32.25 | 0.007 |
| WHt ratio, *,¥ | 0.54 ± 0.04 | 0.58 ± 0.06 | 0.59 ± 0.06 | 0.005 |
| VA index | 2.77 ± 1.65 | 3.18 ± 2.22 | 3.86 ± 2.46 | 0.067 |
| Women, mean (SD) | ||||
| HOMA-IR, ¥,& | 1.33 ± 0.44 | 1.73 ± 1.05 | 2.18 ± 1.68 | 0.015 |
| TyG index, *, ¥,& | 172 ± 32 | 191 ± 41 | 209 ± 64 | 0.006 |
| T/HDL-c ratio, *,¥ | 1.07 ± 0.43 | 1.67 ± 1.25 | 1.94 ± 1.63 | 0.033 |
| LAP index, *,& | 20.83 ± 13.81 | 37.93 ± 31.25 | 44.69 ± 31.64 | 0.011 |
| WHt ratio, *,¥ | 0.51 ± 0.07 | 0.56 ± 0.08 | 0.58 ± 0.09 | 0.012 |
| VA index, *,& | 2.06 ± 0.82 | 3.21 ± 2.55 | 3.80 ± 3.23 | 0.039 |
| HVA (38) | NVA (367) | EVA (96) | p Value | |
|---|---|---|---|---|
| Global, mean (SD) | ||||
| HOMA-IR, *,¥,& | 1.23 ± 0.61 | 1.85 ± 1.13 | 2.20 ± 1.38 | <0.001 |
| TyG index, *,¥,& | 180 ± 28 | 198 ± 42 | 219 ± 68 | <0.001 |
| T/HDL-c ratio, ¥,& | 1.63 ± 1.16 | 1.93 ± 1.39 | 2.47 ± 1.84 | 0.002 |
| LAP index, *,& | 34.30 ± 23.60 | 41.66 ± 31.01 | 51.76 ± 32.27 | 0.004 |
| WHt ratio, ¥,& | 0.54 ± 0.06 | 0.56 ± 0.07 | 0.58 ± 0.07 | 0.019 |
| VA index, ¥,& | 2.63 ± 1.80 | 3.15 ± 2.31 | 3.95 ± 2.97 | 0.005 |
| Men, mean (SD) | ||||
| HOMA-IR, *,¥ | 1.15 ± 0.59 | 1.98 ± 1.20 | 2.20 ± 1.15 | 0.007 |
| TyG index, *,¥,& | 192 ± 23 | 204 ± 39 | 228 ± 74 | 0.002 |
| T/HDL-c ratio | 2.13 ± 1.20 | 2.27 ± 1.52 | 2.69 ± 1.86 | 0.186 |
| LAP index | 38.95 ± 21.16 | 47.12 ± 30.53 | 56.23 ± 31.57 | 0.061 |
| WHt ratio, *,¥ | 0.54 ± 0.05 | 0.58 ± 0.06 | 0.59 ± 0.06 | 0.010 |
| VA index | 2.96 ± 1.81 | 3.19 ± 2.16 | 3.82 ± 2.63 | 0.158 |
| Women, mean (SD) | ||||
| HOMA-IR, ¥,& | 1.29 ± 0.64 | 1.73 ± 1.06 | 2.19 ± 1.68 | 0.014 |
| TyG index, *,¥,& | 171 ± 29 | 192 ± 44 | 206 ± 56 | 0.017 |
| T/HDL-c ratio, ¥,& | 1.25 ± 0.99 | 1.61 ± 1.18 | 2.14 ± 1.77 | 0.019 |
| LAP index | 30.76 ± 25.22 | 36.63 ± 30.68 | 45.23 ± 32.57 | 0.162 |
| WHt ratio | 0.55 ± 0.07 | 0.55 ± 0.08 | 0.56 ± 0.09 | 0.685 |
| VA index, ¥,& | 2.39 ± 1.80 | 3.11 ± 2.45 | 4.13 ± 3.43 | 0.027 |
| β | 95% IC | p | |
|---|---|---|---|
| cfPWV, m/sec | |||
| HOMA-IR | 0.196 | (0.049–0.344) | 0.009 |
| TyG index | 0.012 | (0.004–0.020) | 0.004 |
| T/HDL-c ratio | 0.137 | (0.026–0.248) | 0.015 |
| LAP index | 0.011 | (0.005–0.016) | <0.001 |
| WHt ratio | 3.798 | (1.293–6.304) | 0.003 |
| VA index | 0.091 | (0.024–0.158) | 0.008 |
| baPWV, m/sec | |||
| HOMA-IR | 0.203 | (0.060–0.346) | 0.005 |
| TyG index | 0.007 | (0.003–0.011) | 0.002 |
| T/HDL-c ratio | 0.227 | (0.118–0.335) | <0.001 |
| LAP index | 0.009 | (0.004–0.014) | 0.001 |
| WHt ratio | 2.848 | (0.365–5.331) | 0.025 |
| VA index | 0.134 | (0.069–0.199) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Sánchez, L.; Gómez-Sánchez, M.; Lugones-Sánchez, C.; Tamayo-Morales, O.; González-Sánchez, S.; Rodríguez-Sánchez, E.; García-Ortiz, L.; Gómez-Marcos, M.A.; on behalf of the EVA Investigators. Association of Insulin Resistance with Vascular Ageing in a General Caucasian Population: An EVA Study. J. Clin. Med. 2021, 10, 5748. https://doi.org/10.3390/jcm10245748
Gómez-Sánchez L, Gómez-Sánchez M, Lugones-Sánchez C, Tamayo-Morales O, González-Sánchez S, Rodríguez-Sánchez E, García-Ortiz L, Gómez-Marcos MA, on behalf of the EVA Investigators. Association of Insulin Resistance with Vascular Ageing in a General Caucasian Population: An EVA Study. Journal of Clinical Medicine. 2021; 10(24):5748. https://doi.org/10.3390/jcm10245748
Chicago/Turabian StyleGómez-Sánchez, Leticia, Marta Gómez-Sánchez, Cristina Lugones-Sánchez, Olaya Tamayo-Morales, Susana González-Sánchez, Emiliano Rodríguez-Sánchez, Luis García-Ortiz, Manuel A. Gómez-Marcos, and on behalf of the EVA Investigators. 2021. "Association of Insulin Resistance with Vascular Ageing in a General Caucasian Population: An EVA Study" Journal of Clinical Medicine 10, no. 24: 5748. https://doi.org/10.3390/jcm10245748
APA StyleGómez-Sánchez, L., Gómez-Sánchez, M., Lugones-Sánchez, C., Tamayo-Morales, O., González-Sánchez, S., Rodríguez-Sánchez, E., García-Ortiz, L., Gómez-Marcos, M. A., & on behalf of the EVA Investigators. (2021). Association of Insulin Resistance with Vascular Ageing in a General Caucasian Population: An EVA Study. Journal of Clinical Medicine, 10(24), 5748. https://doi.org/10.3390/jcm10245748






