Different Stratification of Physiological Factors Affecting Cerebral Perfusion Pressure in Hypoxic-Ischemic Brain Injury after Cardiac Arrest According to Visible or Non-Visible Primary Brain Injury: A Retrospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
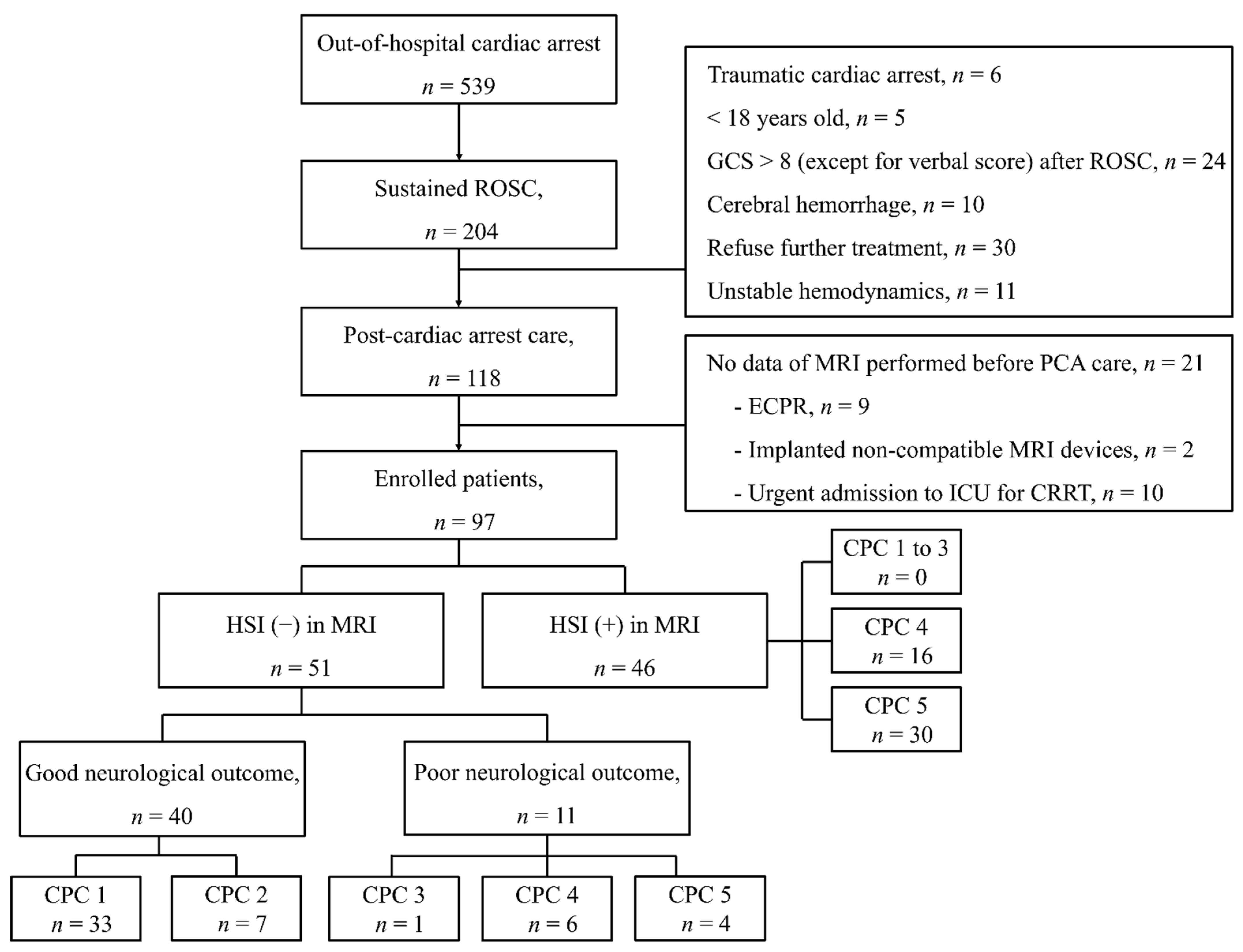
2.1. Study Setting, Design, and Participants
2.2. Targeted Temperature Management Protocol
2.3. Data Collection and Clinical Variables
2.4. Measurement of HSI in DWI
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
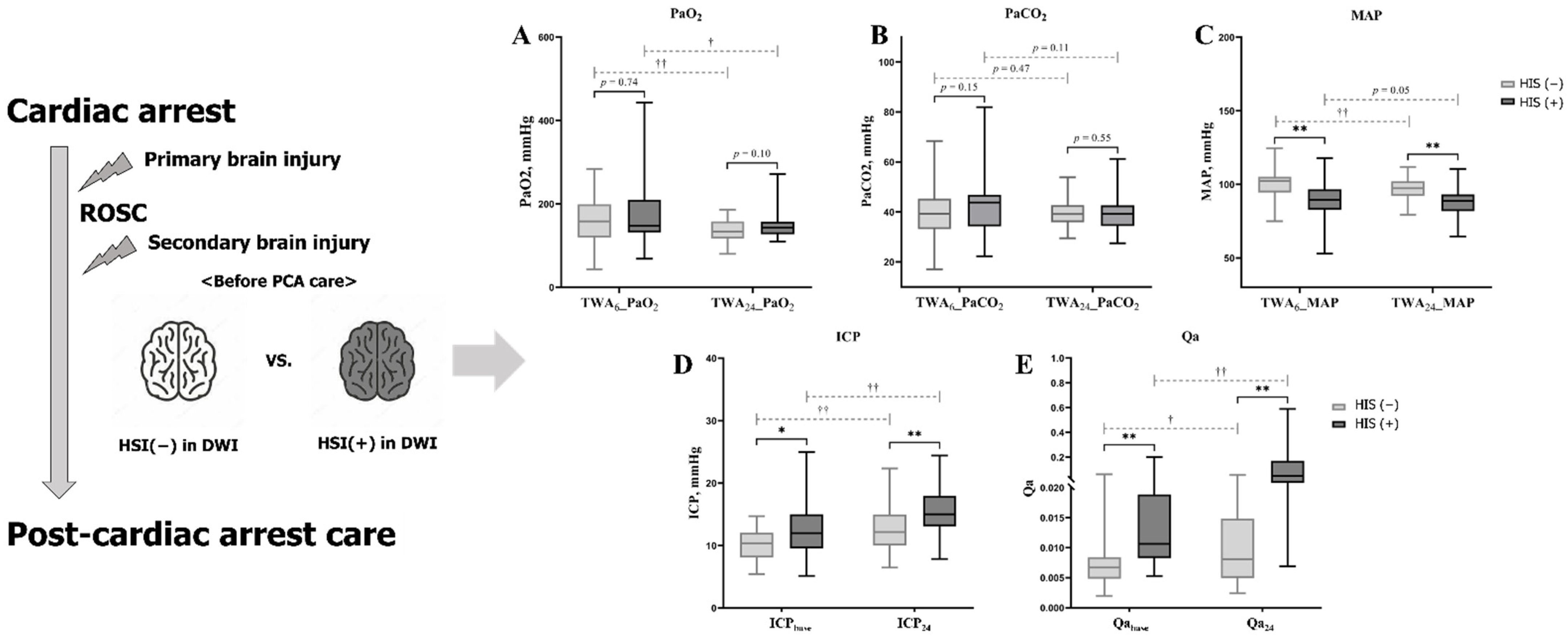
3.2. Characteristics and PFs Associated with HSI on DWI
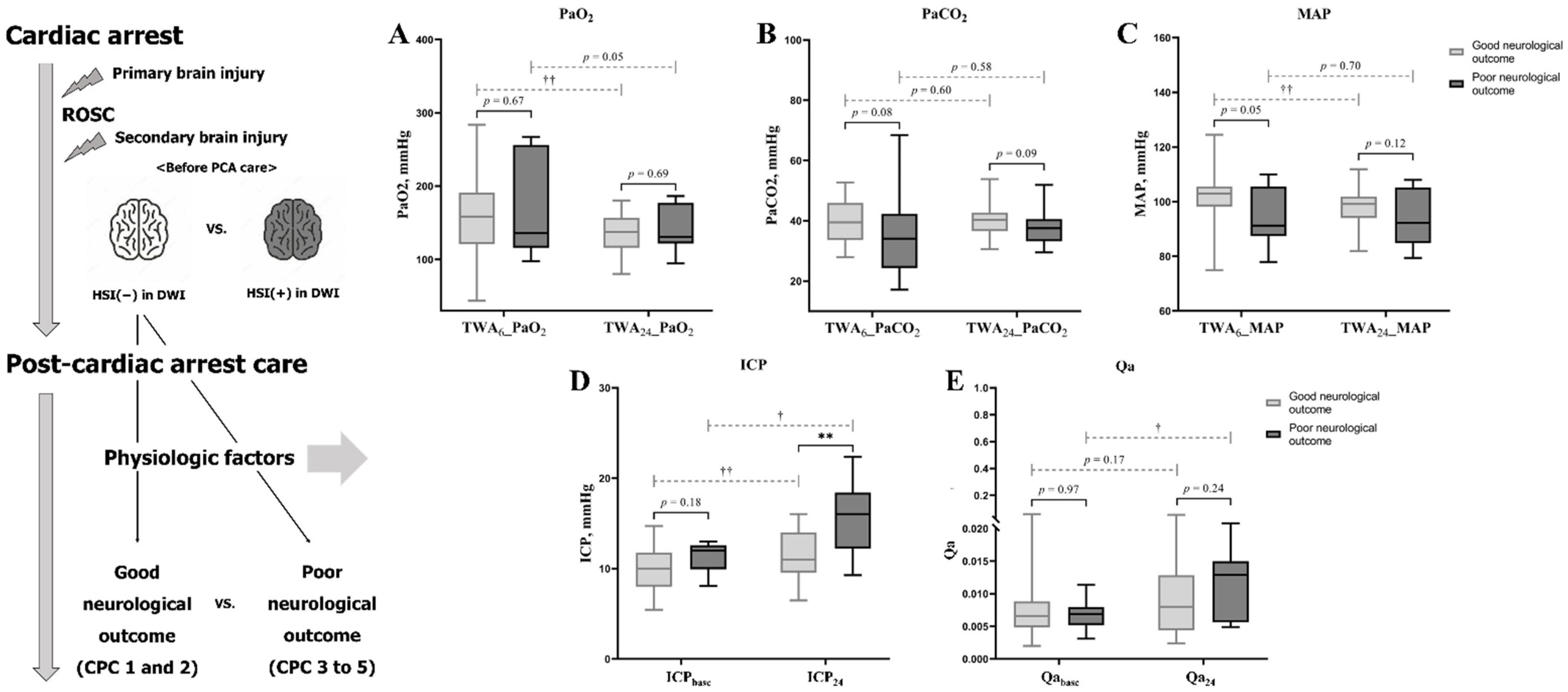
3.3. Characteristics and PFs Associated with Neurological Outcome within the Non-HSI Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nolan, J.P.; Berg, R.A.; Callaway, C.W.; Morrison, L.J.; Nadkarni, V.; Perkins, G.D.; Sandroni, C.; Skrifvars, M.B.; Soar, J.; Sunde, K.; et al. The present and future of cardiac arrest care: International experts reach out to caregivers and healthcare authorities. Intensive Care Med. 2018, 44, 823–832. [Google Scholar] [CrossRef] [Green Version]
- Sandroni, C.; Combes, A.; Nolan, J.P. Focus on post-resuscitation care. Intensive Care Med. 2019, 45, 1283–1287. [Google Scholar] [CrossRef] [Green Version]
- Sekhon, M.S.; Ainslie, P.N.; Griesdale, D.E. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: A “two-hit” model. Crit. Care 2017, 21, 90. [Google Scholar] [CrossRef] [Green Version]
- Nutma, S.; le Feber, J.; Hofmeijer, J. Neuroprotective Treatment of Postanoxic Encephalopathy: A Review of Clinical Evidence. Front. Neurol. 2021, 12, 614698. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.; Bose, G. Clinical neuroprotection and secondary neuronal injury mechanisms. Anaesth. Intensive Care Med. 2020, 21, 293–297. [Google Scholar] [CrossRef]
- Fuller, Z.L.; Faro, J.W.; Callaway, C.W.; Coppler, P.J.; Elmer, J. Recovery among post-arrest patients with mild-to-moderate cerebral edema. Resuscitation 2021, 162, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Cristia, C.; Ho, M.-L.; Levy, S.; Andersen, L.W.; Perman, S.M.; Giberson, T.; Salciccioli, J.D.; Saindon, B.Z.; Cocchi, M.N.; Donnino, M.W. The association between a quantitative computed tomography (CT) measurement of cerebral edema and outcomes in post-cardiac arrest: A validation study. Resuscitation 2014, 85, 1348–1353. [Google Scholar] [CrossRef] [Green Version]
- Metter, R.B.; Rittenberger, J.C.; Guyette, F.X.; Callaway, C.W. Association between a quantitative CT scan measure of brain edema and outcome after cardiac arrest. Resuscitation 2011, 82, 1180–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Ameloot, K.; De Deyne, C.; Eertmans, W.; Ferdinande, B.; Dupont, M.; Palmers, P.J.; Petit, T.; Nuyens, P.; Maeremans, J.; Vundelinckx, J.; et al. Early goal-directed haemodynamic optimization of cerebral oxygenation in comatose survivors after cardiac arrest: The neuroprotect post-cardiac arrest trial. Eur. Heart J. 2019, 40, 1804–1814. [Google Scholar] [CrossRef]
- Jakkula, P.; Reinikainen, M.; Hastbacka, J.; Loisa, P.; Tiainen, M.; Pettila, V.; Toppila, J.; Lahde, M.; Backlund, M.; Okkonen, M.; et al. Targeting two different levels of both arterial carbon dioxide and arterial oxygen after cardiac arrest and resuscitation: A randomised pilot trial. Intensive Care Med. 2018, 44, 2112–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grand, J.; Meyer, A.S.; Kjaergaard, J.; Wiberg, S.; Thomsen, J.H.; Frydland, M.; Ostrowski, S.R.; Johansson, P.I.; Hassager, C. A randomised double-blind pilot trial comparing a mean arterial pressure target of 65 mmHg versus 72 mmHg after out-of-hospital cardiac arrest. Eur. Heart J. Acute Cardiovasc. Care 2020, 9 (Suppl. 4), S100–S109. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.H.; Park, J.S.; Lee, J.H.; Kim, H.; Kim, S.C.; Park, K.H.; Yi, K.S.; Kim, S.M.; Youn, C.S.; Kim, Y.-M.; et al. Comparison of brain computed tomography and diffusion-weighted magnetic resonance imaging to predict early neurologic outcome before target temperature management comatose cardiac arrest survivors. Resuscitation 2017, 118, 21–26. [Google Scholar] [CrossRef]
- Park, J.S.; In, Y.N.; You, Y.H.; Min, J.H.; Ahn, H.J.; Yoo, I.S.; Kim, S.W.; Lee, J.W.; Ryu, S.; Jeong, W.J.; et al. Ultra-early neurologic outcome prediction of out-of-hospital cardiac arrest survivors using combined diffusion-weighted imaging findings and quantitative analysis of apparent diffusion coefficient. Resuscitation 2020, 148, 39–48. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Park, J.; Min, J.; Yoo, I.; Jeong, W.; Cho, Y.; Ryu, S.; Lee, J.; Kim, S.; Cho, S.; et al. Relationship between time related serum albumin concentration, optic nerve sheath diameter, cerebrospinal fluid pressure, and neurological prognosis in cardiac arrest survivors. Resuscitation 2018, 131, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Callaway, C.W.; Donnino, M.W.; Fink, E.L.; Geocadin, R.G.; Golan, E.; Kern, K.B.; Leary, M.; Meurer, W.J.; Peberdy, M.A.; Thompson, T.M.; et al. Part 8: Post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015, 132 (Suppl. 2), S465–S482. [Google Scholar] [CrossRef] [Green Version]
- Nolan, J.P.; Sandroni, C.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: Post-resuscitation care. Intensive Care Med. 2021, 47, 369–421. [Google Scholar] [CrossRef]
- Deisenhammer, F.; Bartos, A.; Egg, R.; Gilhus, N.E.; Giovannoni, G.; Rauer, S.; Sellebjerg, F. Guidelines on routine cerebrospinal fluid analysis: Report from an EFNS task force. Eur. J. Neurol. 2006, 13, 913–922. [Google Scholar] [CrossRef]
- Park, J.S.; You, Y.; Min, J.H.; Yoo, I.; Jeong, W.; Cho, Y.; Ryu, S.; Lee, J.; Kim, S.W.; Cho, S.U.; et al. Study on the timing of severe blood-brain barrier disruption using cerebrospinal fluid-serum albumin quotient in post cardiac arrest patients treated with targeted temperature management. Resuscitation 2019, 135, 118–123. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, S.W.; Kim, H.; Min, J.H.; Kang, J.H.; Yi, K.S.; Park, K.H.; Lee, B.K. Efficacy of diffusion-weighted magnetic resonance imaging performed before therapeutic hypothermia in predicting clinical outcome in comatose cardiopulmonary arrest survivors. Resuscitation 2015, 88, 132–137. [Google Scholar] [CrossRef]
- Gutierrez, L.G.; Rovira, A.; Portela, L.A.; Leite Cda, C.; Lucato, L.T. CT and MR in non-neonatal hypoxic-ischemic encephalopathy: Radiological findings with pathophysiological correlations. Neuroradiology 2010, 52, 949–976. [Google Scholar] [CrossRef] [PubMed]
- Hayman, E.G.; Patel, A.P.; Kimberly, W.T.; Sheth, K.N.; Simard, J.M. Cerebral edema after cardiopulmonary resuscitation: A therapeutic target following cardiac arrest? Neurocrit. Care 2018, 28, 276–287. [Google Scholar] [CrossRef]
- Eastwood, G.M.; Schneider, A.G.; Suzuki, S.; Peck, L.; Young, H.; Tanaka, A.; Mårtensson, J.; Warrillow, S.; McGuinness, S.; Parke, R.; et al. Targeted therapeutic mild hypercapnia after cardiac arrest: A phase II multi-centre randomised controlled trial (the CCC trial). Resuscitation 2016, 104, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Jakkula, P.; Pettilä, V.; Skrifvars, M.B.; Hästbacka, J.; Loisa, P.; Tiainen, M.; Wilkman, E.; Toppila, J.; Koskue, T.; Bendel, S.; et al. Targeting low-normal or high-normal mean arterial pressure after cardiac arrest and resuscitation: A randomised pilot trial. Intensive Care Med. 2018, 44, 2091–2101. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Li, Z.; Zhang, S.; Wu, L.; Li, Y.; Shi, G.; Zhou, J. Mild hypercapnia improves brain tissue oxygen tension but not diffusion limitation in asphyxial cardiac arrest: An experimental study in pigs. BMC Anesthesiol. 2020, 20, 252. [Google Scholar] [CrossRef] [PubMed]
- Babini, G.; Ristagno, G.; Boccardo, A.; De Giorgio, D.; De Maglie, M.; Affatato, R.; Ceriani, S.; Zani, D.; Novelli, D.; Staszewsky, L.; et al. Effect of mild hypercapnia on outcome and histological injury in a porcine post cardiac arrest model. Resuscitation 2019, 135, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Pilcher, J.; Weatherall, M.; Shirtcliffe, P.; Bellomo, R.; Young, P.; Beasley, R. The effect of hyperoxia following cardiac arrest: A systematic review and meta-analysis of animal trials. Resuscitation 2012, 83, 417–422. [Google Scholar] [CrossRef]
- Sekhon, M.S.; Griesdale, D.E. Individualized perfusion targets in hypoxic ischemic brain injury after cardiac arrest. Crit. Care 2017, 21, 259. [Google Scholar] [CrossRef] [Green Version]
- Sekhon, M.S.; Ainslie, P.N.; Menon, D.K.; Thiara, S.S.; Cardim, D.; Gupta, A.K.; Hoiland, R.L.; Gooderham, P.; Griesdale, D.E. Brain hypoxia secondary to diffusion limitation in hypoxic ischemic brain injury postcardiac arrest. Crit. Care Med. 2020, 48, 378–384. [Google Scholar] [CrossRef]
- Magliocca, A.; Rezoagli, E.; Zani, D.; Manfredi, M.; De Giorgio, D.; Olivari, D.; Fumagalli, F.; Langer, T.; Avalli, L.; Grasselli, G.; et al. Cardiopulmonary Resuscitation-associated Lung Edema (CRALE): A translational Study. Am. J. Respir. Crit. Care Med. 2021, 203, 447–457. [Google Scholar] [CrossRef]
- Takahashi, M.; Macdonald, R.L. Vascular aspects of neuroprotection. Neurol. Res. 2004, 26, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, C.; Yuan, W.; Wu, J.; Li, J.; Li, Z.; Zhao, Y. Mild hypothermia alleviates brain oedema and blood-brain barrier disruption by attenuating tight junction and adherens junction breakdown in a swine model of cardiopulmonary resuscitation. PLoS ONE 2017, 12, e0174596. [Google Scholar] [CrossRef] [Green Version]
- Fergusson, N.A.; Hoiland, R.L.; Thiara, S.; Foster, D.; Gooderham, P.; Rikhraj, K.; Grunau, B.; Christenson, J.; Ainslie, P.N.; Griesdale, D.E.G.; et al. Goal-directed care using invasive neuromonitoring versus standard of care after cardiac arrest: A matched cohort study. Crit. Care Med. 2021, 49, 1333–1346. [Google Scholar] [CrossRef]
- Naito, H.; Isotani, E.; Callaway, C.W.; Hagioka, S.; Morimoto, N. Intracranial Pressure Increases During Rewarming Period After Mild Therapeutic Hypothermia in Postcardiac Arrest Patients. Ther. Hypothermia Temp. Manag. 2016, 6, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Youn, C.S.; Callaway, C.W.; Rittenberger, J.C. Combination of initial neurologic examination, quantitative brain imaging and electroencephalography to predict outcome after cardiac arrest. Resuscitation 2017, 110, 120–125. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, K.G.; Fischbein, N.; Mlynash, M.; Kemp, S.; Bammer, R.; Eyngorn, I.; Tong, J.; Moseley, M.; Venkatasubramanian, C.; Caulfield, A.F.; et al. Prognostic value of diffusion-weighted MRI for post-cardiac arrest coma. Neurology 2020, 94, e1684–e1692. [Google Scholar] [CrossRef]
- Hoekema, D.; Schmidt, R.H.; Ross, I. Lumbar drainage for subarachnoid hemorrhage: Technical considerations and safety analysis. Neurocrit. Care 2007, 7, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Tuettenberg, J.; Czabanka, M.; Horn, P.; Woitzik, J.; Barth, M.; Thome, C.; Vajkoczy, P.; Schmiedek, P.; Muench, E. Clinical evaluation of the safety and efficacy of lumbar cerebrospinal fluid drainage for the treatment of refractory increased intracranial pressure. J. Neurosurg. 2009, 110, 1200–1208. [Google Scholar] [CrossRef] [Green Version]
| Total Number of Patients, n = 97 | HSI Group n = 46 | Non-HSI Group, n = 51 | p-Value | |
|---|---|---|---|---|
| Age, years | 57 (16–87) | 58 (16–87) | 57 (18–83) | 0.43 |
| Male | 69 (71.1) | 32 (69.6) | 37 (72.5) | 0.51 |
| Pre-existing illnesses | ||||
| CAD | 7 (7.2) | 2 (4.3) | 5 (9.8) | 0.44 |
| Hypertension | 32 (33.0) | 17 (37.0) | 15 (29.4) | 0.52 |
| Diabetes mellitus | 29 (29.9) | 15 (32.6) | 14 (27.5) | 0.66 |
| Ischemic stroke | 5 (5.2) | 3 (6.5) | 2 (3.9) | 0.67 |
| Pulmonary disease | 8 (8.2) | 3 (6.5) | 5 (9.8) | 0.72 |
| Renal disease | 14 (14.4) | 7 (15.2) | 7 (13.7) | 1.00 |
| Liver disease | 4 (4.1) | 1 (2.2) | 3 (5.9) | 0.62 |
| Cardiac arrest characteristics | ||||
| Witnessed | 63 (64.9) | 26 (56.5) | 37 (72.5) | 0.01 |
| Bystander CPR | 68 (70.1) | 28 (60.9) | 40 (78.4) | <0.01 |
| Shockable rhythm | 27 (27.8) | 3 (6.5) | 24 (47.1) | <0.01 |
| Cardiac etiology | 35 (38.1) | 9 (19.6) | 26 (51.0) | <0.01 |
| No flow time, min | 2.0 (0.0–365.0) | 7.0 (0.0–365.0) | 5.0 (0.0–330.0) | <0.01 |
| Low flow time, min | 19.0 (2.0–58.0) | 30.0 (4.0–58.0) | 10.0 (2.0–30.0) | <0.01 |
| SOFA score | 10 (6–17) | 11 (6–16) | 9 (6–17) | 0.10 |
| GWR before TTM | 1.21 (0.97–1.56) | 1.17 (0.97–1.35) | 1.25 (0.98–1.56) | <0.01 |
| Serum NSE, ng/mL | ||||
| at baseline | 32.7 (8.5–300.0) | 56.0 (13.8–300.0) | 25.4 (8.5–68.7) | <0.01 |
| at 24 h from ROSC | 35.8 (13.2–300.0) | 115.0 (18.2–300.0) | 26.5 (13.2–156.7) | <0.01 |
| Induction time to 33 °C, hours | 6.0 (2.0–19.0) | 5.9 (2.0–19.0) | 6.1 (2.6–12.8) | 0.92 |
| Time to measure initial ICP, hours | 4.6 (1.2–19.5) | 4.7 (2.3–19.5) | 4.5 (1.2–11.6) | 0.55 |
| Time to DWI from ROSC, hours | 3.0 (0.9–9.0) | 3.0 (0.9–7.8) | 3.0 (0.9–9.0) | 0.85 |
| PFs | ||||
| TWA, mmHg | ||||
| Number of performed ABG for 24 h after ROSC | 12 (4–19) | 12 (4–18) | 12 (4–19) | 0.76 |
| TWA6_PaO2 | 149.95 (43.50–442.88) | 147.13 (48.48–442.88) | 158.23 (43.50–283.78) | 0.74 |
| TWA24_PaO2 | 141.09 (80.20–271.45) | 143.40 (109.73–271.45) | 133.71 (80.20–186.36) | 0.10 |
| TWA6_PaCO2 | 40.78 (17.11–87.94) | 43.82 (22.31–81.94) | 39.22 (17.11–68.39) | 0.13 |
| TWA24_PaCO2 | 39.16 (27.52–61.25) | 39.21 (27.52–61.25) | 39.13 (29.49–53.83) | 0.55 |
| TWA6_MAP | 95.93 (52.85–124.50) | 89.51 (52.85–117.76) | 102.37 (74.97–124.50) | <0.01 |
| TWA24_MAP | 92.96 (64.68–111.89) | 88.79 (64.68–110.58) | 97.34 (79.41–111.89) | <0.01 |
| ICP, mmHg | ||||
| ICPbase | 11.0 (5.2–25.0) | 12.0 (5.2–25.0) | 10.4 (5.4–14.7) | 0.02 |
| ICP24 | 14.0 (6.5–24.4) | 15.0 (7.9–24.4) | 12.2 (6.5–22.4) | <0.01 |
| Qa | ||||
| Qabase | 0.0083 (0.0020–0.2000) | 0.0107 (0.0053–0.2000) | 0.0067 (0.0020–0.0613) | <0.01 |
| Qa24 | 0.0161 (0.0024–0.5893) | 0.0484 (0.0069–0.5893) | 0.0081 (0.0024–0.0556) | <0.01 |
| Total Number of Patients, n = 51 | Good Neurological Outcome, n = 40 | Poor Neurological Outcome, n = 11 | p-Value | |
|---|---|---|---|---|
| Age, years | 57 (18–83) | 58 (18–83) | 42 (19–76) | 0.34 |
| Male | 37 (72.5) | 30 (75.0) | 7 (63.6) | 0.47 |
| Pre-existing illnesses | ||||
| CAD | 5 (9.8) | 5 (12.5) | 3 (27.3) | 0.57 |
| Hypertension | 15 (29.4) | 12 (30.0) | 3 (27.3) | 1.00 |
| Diabetes mellitus | 14 (27.5) | 11 (27.5) | 1 (9.1) | 1.00 |
| Ischemic stroke | 2 (3.9) | 1 (2.5) | 1 (9.1) | 0.39 |
| Pulmonary disease | 5 (9.8) | 4 (10.0) | 1 (9.1) | 1.00 |
| Renal disease | 7 (13.7) | 7 (17.5) | 0 | 0.32 |
| Liver disease | 3 (5.9) | 1 (2.5) | 2 (18.2) | 0.11 |
| Cardiac arrest characteristics | ||||
| Witnessed | 37 (72.5) | 32 (80.0) | 5 (45.5) | 0.05 |
| Bystander CPR | 40 (78.4) | 34 (85.0) | 6 (54.5) | 0.04 |
| Shockable rhythm | 24 (47.1) | 21 (52.5) | 3 (27.3) | 0.18 |
| Cardiac etiology | 26 (51.0) | 22 (55.0) | 4 (36.4) | 0.32 |
| No flow time, min | 5.0 (0.0–330) | 0.0 (0.0–33.0) | 2.0 (0.0–20.0) | 0.36 |
| Low flow time, min | 10.0 (2.0–30.0) | 10.0 (2.0–30.0) | 14.0 (2.0–30.0) | 0.44 |
| SOFA score | 9 (6–17) | 9 (6–17) | 9 (7–15) | 0.50 |
| GWR before TTM | 1.25 (0.98–1.56) | 1.25 (1.10–1.56) | 1.21 (0.98–1.40) | 0.12 |
| Serum NSE, ng/mL | ||||
| at baseline | 25.4 (8.5–68.7) | 25.7 (8.5–54.8) | 24.3 (16.1–68.7) | 0.99 |
| at 24 h from ROSC | 26.5 (13.2–156.7) | 26.4 (13.2–85.3) | 26.6 (14.6–156.7) | 0.97 |
| Induction time to 33 °C, hours | 6.1 (2.6–12.8) | 5.6 (2.6–12.8) | 6.4 (5.5–8.8) | 0.10 |
| Time to measure initial ICP, hours | 4.5 (1.2–11.6) | 4.2 (1.2–11.6) | 4.9 (3.0–7.5) | 0.30 |
| DWI time from ROSC, hours | 3.0 (0.9–9.0) | 2.7 (0.9–5.8) | 3.7 (1.3–9.0) | 0.27 |
| PFs | ||||
| TWA, mmHg | ||||
| Number of performed ABG for 24 h after ROSC | 12 (4–19) | 11.5 (4–19) | 13 (8–15) | 0.08 |
| TWA6_PaO2 | 158.23 (43.50–283.78) | 158.41 (43.50–283.78) | 135.75 (97.65–267.25) | 0.67 |
| TWA24_PaO2 | 133.71 (80.20–186.36) | 137.31 (80.20–180.39) | 130.83 (94.53–186.36) | 0.69 |
| TWA6_PaCO2 | 39.22 (17.11–68.39) | 39.48 (27.90–52.74) | 34.07 (17.11–68.39) | 0.08 |
| TWA24_PaCO2 | 39.13 (29.49–53.83) | 40.65 (30.57–53.83) | 37.54 (29.49–51.88) | 0.09 |
| TWA6_MAP | 102.37 (74.97–124.50) | 102.94 (74.97–124.50) | 99.15 (81.97–111.89) | 0.05 |
| TWA24_MAP | 97.34 (79.41–111.89) | 91.22 (77.94–109.92) | 92.20 (79.41–107.96) | 0.12 |
| ICP, mmHg | ||||
| ICPbase | 10.4 (5.4–14.7) | 10.0 (5.4–14.7) | 12.0 (8.1–13.0) | 0.18 |
| ICP24 | 12.2 (6.5–22.4) | 11.0 (6.5–16.0) | 16.0 (9.3–22.4) | <0.01 |
| Qa | ||||
| Qabase | 0.0067 (0.0020–0.0613) | 0.0066 (0.0020–0.0613) | 0.0069 (0.0031–0.0114) | 0.97 |
| Qa24 | 0.0081 (0.0024–0.0556) | 0.0080 (0.0024–0.0556) | 0.0129 (0.0049–0.0294) | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, C.; Jeong, W.; Park, J.S.; You, Y.; Min, J.H.; Cho, Y.C.; Ahn, H.J.; In, Y.N.; Lee, I.H. Different Stratification of Physiological Factors Affecting Cerebral Perfusion Pressure in Hypoxic-Ischemic Brain Injury after Cardiac Arrest According to Visible or Non-Visible Primary Brain Injury: A Retrospective Observational Study. J. Clin. Med. 2021, 10, 5385. https://doi.org/10.3390/jcm10225385
Kang C, Jeong W, Park JS, You Y, Min JH, Cho YC, Ahn HJ, In YN, Lee IH. Different Stratification of Physiological Factors Affecting Cerebral Perfusion Pressure in Hypoxic-Ischemic Brain Injury after Cardiac Arrest According to Visible or Non-Visible Primary Brain Injury: A Retrospective Observational Study. Journal of Clinical Medicine. 2021; 10(22):5385. https://doi.org/10.3390/jcm10225385
Chicago/Turabian StyleKang, Changshin, Wonjoon Jeong, Jung Soo Park, Yeonho You, Jin Hong Min, Yong Chul Cho, Hong Joon Ahn, Yong Nam In, and In Ho Lee. 2021. "Different Stratification of Physiological Factors Affecting Cerebral Perfusion Pressure in Hypoxic-Ischemic Brain Injury after Cardiac Arrest According to Visible or Non-Visible Primary Brain Injury: A Retrospective Observational Study" Journal of Clinical Medicine 10, no. 22: 5385. https://doi.org/10.3390/jcm10225385
APA StyleKang, C., Jeong, W., Park, J. S., You, Y., Min, J. H., Cho, Y. C., Ahn, H. J., In, Y. N., & Lee, I. H. (2021). Different Stratification of Physiological Factors Affecting Cerebral Perfusion Pressure in Hypoxic-Ischemic Brain Injury after Cardiac Arrest According to Visible or Non-Visible Primary Brain Injury: A Retrospective Observational Study. Journal of Clinical Medicine, 10(22), 5385. https://doi.org/10.3390/jcm10225385






