What Motivates Patients with COPD to Be Physically Active? A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Data Collection
2.3.1. Motives to Be Physically Active
2.3.2. Physical Activity
2.3.3. Clinical Variables
2.4. Data Analysis
3. Results
3.1. Participants
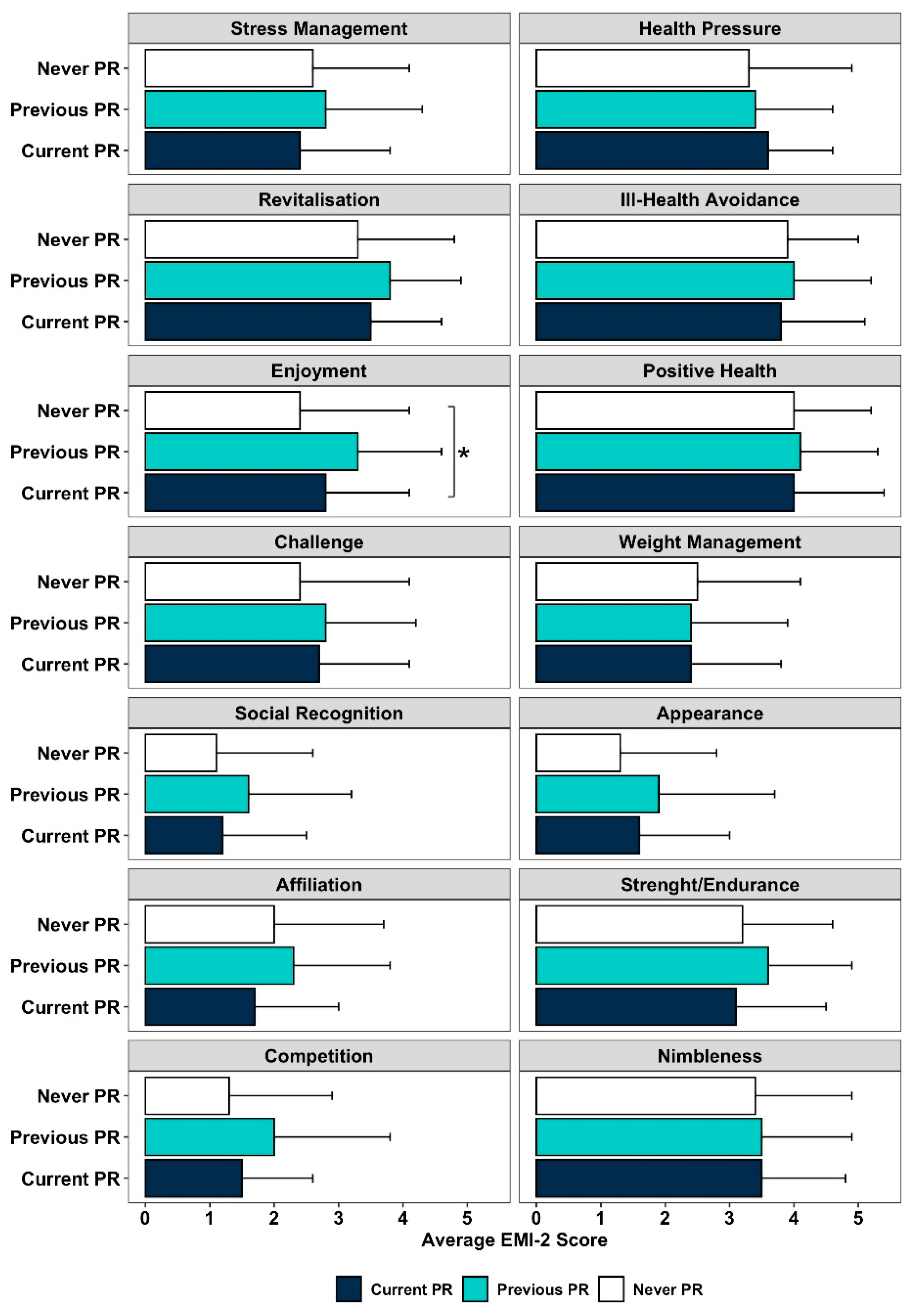
3.2. Motives to Be Physically Active and PR Participation
3.3. Motives to Be Physically Active and Physical Activity
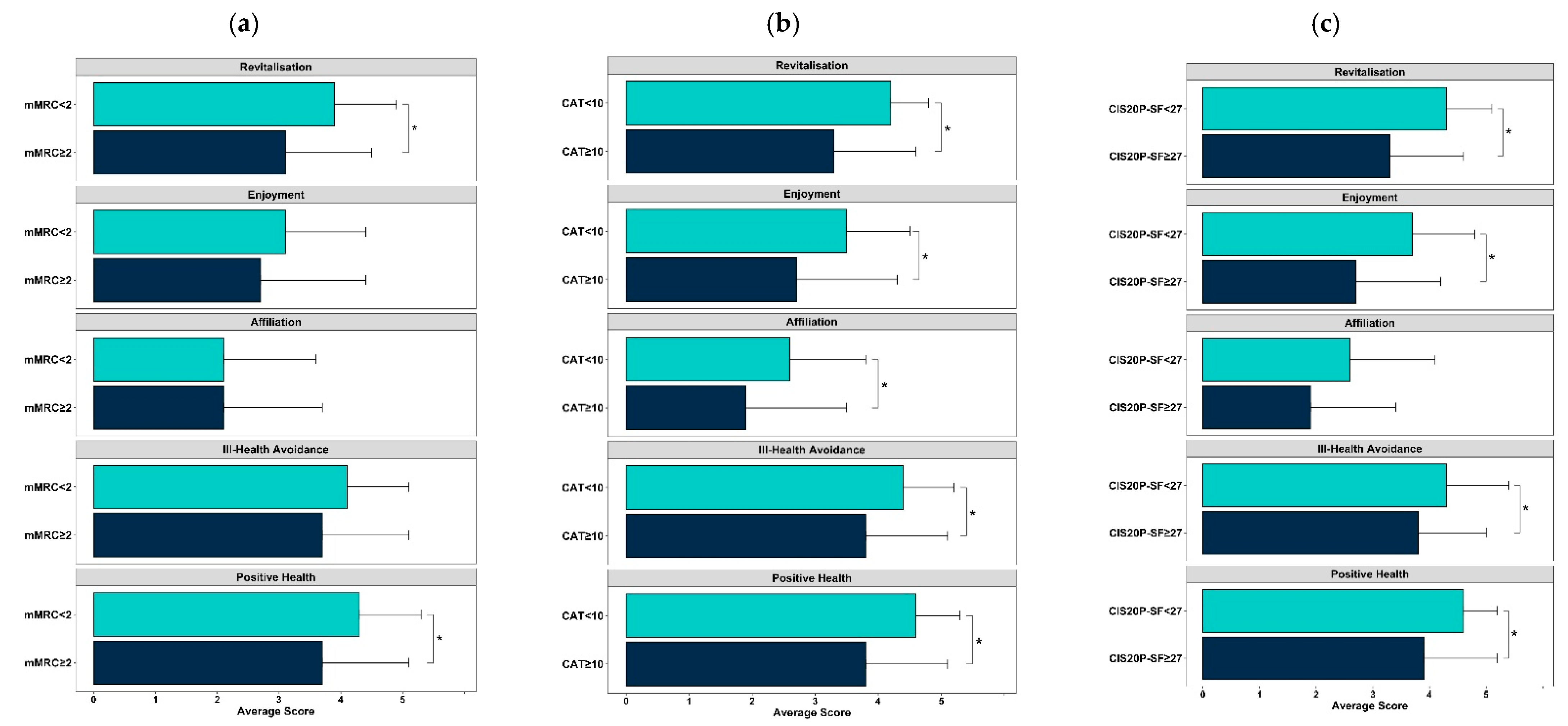
3.4. Motives to Be Physically Active and Clinical Characteristics
4. Discussion
4.1. Implications for Pulmonary Rehabilitation and Future Research
4.2. Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watz, H.; Pitta, F.; Rochester, C.L.; Aymerich, J.G.; ZuWallack, R.; Troosters, T.; Vaes, A.W.; Puhan, M.; Jehn, M.; Polkey, M.I.; et al. An official European Respiratory Society statement on physical activity in COPD. Eur. Respir. J. 2014, 44, 1521–1537. [Google Scholar] [CrossRef]
- Sritharan, S.S.; Østergaard, E.B.; Callesen, J.; Elkjaer, M.; Sand, L.; Hilberg, O.; Skaarup, S.H.; Løkke, A. Barriers toward Physical Activity in COPD: A Quantitative Cross-Sectional, Questionnaire-Based Study. COPD J. Chronic Obstr. Pulm. Dis. 2021, 18, 272–280. [Google Scholar] [CrossRef]
- Kosteli, M.-C.; Heneghan, N.R.; Roskell, C.; Williams, S.; Adab, P.; Dickens, A.P.; Enocson, A.; Fitzmaurice, D.A.; Jolly, K.; Jordan, R.; et al. Barriers and enablers of physical activity engagement for patients with COPD in primary care. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1019–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altenburg, W.A.; Bossenbroek, L.; de Greef, M.H.; Kerstjens, H.A.; Hacken, N.H.T.; Wempe, J.B. Functional and psychological variables both affect daily physical activity in COPD: A structural equations model. Respir. Med. 2013, 107, 1740–1747. [Google Scholar] [CrossRef] [Green Version]
- Thorpe, O.; Johnston, K.; Kumar, S. Barriers and Enablers to Physical Activity Participation in Patients with COPD: A systematic review. J. Cardiopulm. Rehabil. Prev. 2012, 32, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.M.; Deci, E.L. Intrinsic and Extrinsic Motivations: Classic Definitions and New Directions. Contemp. Educ. Psychol. 2000, 25, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Fortier, M.S.; Duda, J.L.; Guerin, E.; Teixeira, P.J. Promoting physical activity: Development and testing of self-determination theory-based interventions. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Patrick, H.; Williams, G.C. Self-determination theory: Its application to health behavior and complementarity with motivational interviewing. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, J.T.; Carraça, E.V.; Markland, D.; Silva, M.N.; Ryan, R.M. Exercise, physical activity, and self-determination theory: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Markland, D.; Ingledew, D.K. The measurement of exercise motives: Factorial validity and invariance across gender of a revised Exercise Motivations Inventory. Br. J. Health Psychol. 1997, 2, 361–376. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C.; et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef]
- Spruit, M.A.; Pitta, F.; McAuley, E.; ZuWallack, R.L.; Nici, L. Pulmonary Rehabilitation and Physical Activity in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015, 192, 924–933. [Google Scholar] [CrossRef]
- Burge, A.T.; Cox, N.S.; Abramson, M.J.; Holland, A.E. Interventions for promoting physical activity in people with chronic obstructive pulmonary disease (COPD). Cochrane Database Syst. Rev. 2020, 4, CD012626. [Google Scholar] [CrossRef]
- Cho, H.-L.; Tung, H.-H.; Lin, M.-S.; Hsu, W.-C.; Lee, C.-P. Self-determined motivation and exercise behaviour in COPD patients. Int. J. Nurs. Pract. 2017, 23, e12530. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabe, K.F. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis Management and Prevention of COPD. GOLD executive summary. Am. J. Respir. Crit. Care Med. 2007, 176, 532–555. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Alves, J.; Lourenço, A. Tradução e adaptação do Questionário de Motivação para o Exercício (Exercise Motivation Inventory 2—EMI2). Desporto Investig. Ciência 2003, 2, 3–11. [Google Scholar]
- Dacey, M.; Baltzell, A.; Zaichkowsky, L. Older Adults’ Intrinsic and Extrinsic Motivation toward Physical Activity. Am. J. Health Behav. 2008, 32, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Ednie, A.; Stibor, M. Influence and interpretation of intrinsic and extrinsic exercise motives. J. Hum. Sport Exerc. 2017, 12, 414–425. [Google Scholar] [CrossRef] [Green Version]
- Ingledew, D.; Markland, D.; Ferguson, E. Three Levels of Exercise Motivation. Appl. Psychol. Health Well-Being 2009, 1, 336–355. [Google Scholar] [CrossRef]
- Ingledew, D.K.; Markland, D.; Medley, A.R. Exercise motives and stages of change. J. Health Psychol. 1998, 3, 477–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quindry, J.C.; Yount, D.; O’Bryant, H.; Rudisill, M.E. Exercise Engagement Is Differentially Motivated by Age-Dependent Factors. Am. J. Health Behav. 2011, 35, 334–345. [Google Scholar] [CrossRef]
- Thow, M.; Rafferty, D.; Kelly, H. Exercise motives of long-term phase IV cardiac rehabilitation participants. Physiotherapy 2008, 94, 281–285. [Google Scholar] [CrossRef]
- Prochaska, J.; Velicer, W. The transtheoretical model. Am. J. Health Promot. 1997, 12, 6–7. [Google Scholar] [CrossRef]
- Moutão, J.M. Motivação para a Prática de Exercício Físico: Estudo dos Motivos para a Prática de Actividades de Fitness em Ginásios. Master Thesis, University of Trás-os-Montes and Alto Douro, Vila Real, Portugal, 2005. [Google Scholar]
- Rabinovich, R.A.; Louvaris, Z.; Raste, Y.; Langer, D.; Van Remoortel, H.; Giavedoni, S.; Burtin, C.; Regueiro, E.M.; Vogiatzis, I.; Hopkinson, N.; et al. Validity of physical activity monitors during daily life in patients with COPD. Eur. Respir. J. 2013, 42, 1205–1215. [Google Scholar] [CrossRef] [Green Version]
- Van Remoortel, H.; Raste, Y.; Louvaris, Z.; Giavedoni, S.; Burtin, C.; Langer, D.; Wilson, F.; Rabinovich, R.; Vogiatzis, I.; Hopkinson, N.S.; et al. Validity of Six Activity Monitors in Chronic Obstructive Pulmonary Disease: A Comparison with Indirect Calorimetry. PLoS ONE 2012, 7, e39198. [Google Scholar]
- Demeyer, H.; Mohan, D.; Burtin, C.; Vaes, A.; Heasley, M.; Bowler, R.P.; Casaburi, R.; Cooper, C.B.; Corriol-Rohou, S.; Frei, A.; et al. Objectively Measured Physical Activity in Patients with COPD: Recommendations from an International Task Force on Physical Activity. Chronic. Obstr. Pulm. Dis. 2021, in press. [Google Scholar] [CrossRef]
- Freedson, S.; Melanson, E.; Sirard, J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med. Sci. Sports Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Craig, C.L.; Thyfault, J.; Spence, J.C. A step-defined sedentary lifestyle index: <5000 steps/day. Appl. Physiol. Nutr. Metab. 2013, 38, 100–114. [Google Scholar] [CrossRef]
- Hajiro, T.; Nishimura, K.; Tsukino, M.; Ikeda, A.; Koyama, H.; & Izumi, T. Analysis of clinical methods used to evaluate dyspnea in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 158, 1185–1189. [Google Scholar] [CrossRef]
- Jones, P.; Harding, G.; Wiklund, I.; Berry, P.; Leidy, N. Improving the process and outcome of care in COPD: Development of a standardised assessment tool. Prim. Care Respir. J. 2009, 18, 208–215. [Google Scholar] [CrossRef]
- Cordeiro, A.P.S. Adaptação Portuguesa do Questionário Checklist of Individual Strength (CIS20-P): Análise das Propriedades Psicométricas. Master Thesis, Instituto Superior de Psicologia Aplicada (ISPA)—Instituto Universitário, Lisbon, Portugal, 2012. [Google Scholar]
- Vercoulen, J.H.; Swanink, C.M.; Fennis, J.F.; Galama, J.M.; van der Meer, J.W.; Bleijenberg, G. Dimensional assessment of chronic fatigue syndrome. J. Psychosom. Res. 1994, 38, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Vercoulen, J.H.; Alberts, M.; Bleijenberg, G. De Checklist Individual Strength (CIS). Gedragstherapie 1999, 32, 131–136. [Google Scholar]
- Rebelo, P.; Oliveira, A.; Andrade, L.; Valente, C.; Marques, A. Minimal Clinically Important Differences for Patient-Reported Outcome Measures of Fatigue in Patients with COPD Following Pulmonary Rehabilitation. Chest 2020, 158, 550–561. [Google Scholar] [CrossRef]
- Peters, J.B.; Heijdra, Y.F.; Daudey, L.; Boer, L.; Molema, J.; Dekhuijzen, P.R.; Schermer, T.R.; Vercoulen, J.H. Course of normal and abnormal fatigue in patients with Chronic Obstructive Pulmonary Disease, and its relationship with domains of health status. Patient Educ. Couns. 2011, 85, 281–285. [Google Scholar] [CrossRef]
- Van Herck, M.; Antons, J.; Vercoulen, J.H.; Goërtz, Y.M.J.; Ebadi, Z.; Burtin, C.; Janssen, D.J.A.; Thong, M.S.Y.; Otker, J.; Coors, A.; et al. Pulmonary Rehabilitation Reduces Subjective Fatigue in COPD: A Responder Analysis. J. Clin. Med. 2019, 8, 1264. [Google Scholar] [CrossRef] [Green Version]
- Goërtz, Y.M.J.; Spruit, M.A.; Hul, A.V.T.; Peters, J.; Van Herck, M.; Nakken, N.; Djamin, R.S.; Burtin, C.; Thong, M.S.Y.; Coors, A.; et al. Fatigue is highly prevalent in patients with COPD and correlates poorly with the degree of airflow limitation. Ther. Adv. Respir. Dis. 2019, 13, 1753466619878128. [Google Scholar] [CrossRef]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Marques, A.; Rebelo, P.; Paixão, C.; Almeida, S.; Jácome, C.; Cruz, J.; Oliveira, A. Enhancing the assessment of cardiorespiratory fitness using field tests. Physiotherapy 2020, 109, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Cote, C.G.; Casanova, C.; Marin, J.M.; Lopez, M.V.; Pinto-Plata, V.; De Oca, M.M.; Dordelly, L.J.; Nekach, H.; Celli, B.R. Validation and comparison of reference equations for the 6-min walk distance test. Eur. Respir. J. 2008, 31, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Polkey, M.I.; Celli, B.; Edwards, L.; Watkins, M.L.; Pinto-Plata, V.; Vestbo, J.; Calverley, P.M.; Tal-Singer, R.; Agusti, A.; et al. Predicting outcomes from 6-minute walk distance in chronic obstructive pulmonary disease. J. Am. Med. Dir. Assoc. 2012, 13, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Wrobel, J. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2014, 9, 871–888. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Karloh, M.; Matias, T.S.; Mayer, A.F. The COVID-19 Pandemic Confronts the Motivation Fallacy within Pulmonary Rehabilitation Programs. J. Chronic Obstr. Pulm. Dis. 2020, 17, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Scheermesser, M.; Reicherzer, L.; Beyer, S.; Gisi, D.; Rezek, S.; Hess, T.; Wirz, M.; Osthoff, A.-K.R. The Influence of Pulmonary Rehabilitation and Counselling on Perceptions of Physical Activity in Individuals with COPD—A Qualitative Study. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 2337–2350. [Google Scholar] [CrossRef]
- Danilack, V.A.; Weston, N.A.; Richardson, C.; Mori, D.L.; Moy, M.L. Reasons persons with COPD do not walk and relationship with daily step count. COPD J. Chronic Obstr. Pulm. Dis. 2014, 11, 290–299. [Google Scholar] [CrossRef]
- Bauman, A.E.; Reis, R.S.; Sallis, J.F.; Wells, J.C.; Loos, R.J.F.; Martin, B.W.; for the Lancet Physical Activity Series Working Group. Correlates of physical activity: Why are some people physically active and others not? Lancet 2012, 380, 258–271. [Google Scholar] [CrossRef]
- Gimeno-Santos, E.; Frei, A.; Steurer-Stey, C.; de Batlle, J.; Rabinovich, R.A.; Raste, Y.; Hopkinson, N.S.; Polkey, M.I.; Van Remoortel, H.; Troosters, T.; et al. Determinants and outcomes of physical activity in patients with COPD: A systematic review. Thorax 2014, 69, 731–739. [Google Scholar] [CrossRef] [Green Version]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Prinsen, C.A.C.; Mokkink, L.B.; Bouter, L.M.; Alonso, J.; Patrick, D.L.; De Vet, H.C.W.; Terwee, C.B. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual. Life Res. 2018, 27, 1147–1157. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, F.; Moutão, J.; Teixeira, D.; Cid, L.; Monteiro, D. Examining exercise motives between gender, age and activity: A first-order scale analysis and measurement invariance. Curr. Psychol. 2019, 38, 1–14. [Google Scholar] [CrossRef]
- Rochester, C.L. Patient assessment and selection for pulmonary rehabilitation. Respirology 2019, 24, 844–853. [Google Scholar] [CrossRef] [PubMed]
| Dimensions | Factors | Items |
|---|---|---|
| Psychological Motives | Stress Management a | 6–20–34–46 |
| Revitalisation a | 3–17–31 | |
| Enjoyment a | 9–23–37–48 | |
| Challenge a | 14–28–42–51 | |
| Interpersonal Motives | Social Recognition b | 5–19–33–45 |
| Affiliation a | 10–24–38–49 | |
| Competition c | 12–26–40–50 | |
| Health Motives | Health Pressure b | 11–25–39 |
| Ill Health Avoidance b | 2–16–30 | |
| Positive Health c | 7–21–35 | |
| Body-Related Motives | Weight Management b | 1–15–29–43 |
| Appearance b | 4–18–32–44 | |
| Fitness Motives | Strength/Endurance c | 8–22–36–47 |
| Nimbleness a | 13–27–41 |
| Total Sample (n = 92) | Never PR (n = 28) | Previous PR (n = 47) | Current PR (n = 17) | p-Value | |
|---|---|---|---|---|---|
| Age, mean (SD) | 67.4 (8.1) | 65.5 (8.7) | 68.3 (6.4) | 68.0 (11.2) | 0.353 a |
| Sex, n (%) | |||||
| Female | 16 (17.4) | 9 (32.1) | 7 (14.9) | - | - d |
| Male | 76 (82.6) | 19 (67.9) | 40 (85.1) | 17 (100.0) | |
| Smoking status, n (%) | |||||
| Current smoker | 20 (21.7) | 9 (32.1) | 9 (19.1) | 2 (11.8) | - d |
| Former smoker | 64 (69.6) | 17 (60.7) | 33 (70.2) | 14 (82.4) | |
| Never smoker | 8 (8.7) | 2 (7.1) | 5 (10.6) | 1 (5.9) | |
| BMI (kg/m2), mean (SD) | 26.2 (4.7) | 26.9 (4.1) | 25.1 (4.9) | 28.0 (4.4) | 0.055 c |
| FEV1 (% predicted), mean (SD) | 48.3 (18.9) | 61.4 (17.0) | 43.3 (16.3) | 41.2 (19.2) | <0.001 * a,e |
| GOLD 1–4, n (%) | |||||
| GOLD 1 | 4 (4.4) | 3 (11.1) | - | 1 (5.9) | - d |
| GOLD 2 | 35 (38.5) | 17 (63.0) | 14 (29.8) | 4 (23.5) | |
| GOLD 3 | 36 (39.6) | 6 (22.2) | 24 (51.1) | 6 (35.3) | |
| GOLD 4 | 16 (17.4) | 1 (3.7) | 9 (19.1) | 6 (35.3) | |
| GOLD ABCD, n (%) | |||||
| A | 40 (45.5) | 16 (59.3) | 19 (41.3) | 5 (33.3) | 0.718 b |
| B | 16 (18.2) | 4 (14.8) | 9 (19.6) | 3 (20.0) | |
| C | 15 (17.0) | 4 (14.8) | 8 (17.4) | 3 (20.0) | |
| D | 17 (19.3) | 3 (11.1) | 10 (21.7) | 4 (26.7) | |
| Comorbidities, n (%) | |||||
| 0 to 1 | 17 (18.5) | 5 (17.9) | 10 (21.3) | 2 (11.8) | 0.684 |
| ≥2 | 75 (81.5) | 23 (82.1) | 37 (78.7) | 15 (88.2) | |
| CAT, mean (SD) | 14.8 (8.6) | 12.8 (11.1) | 15.3 (7.7) | 16.5 (6.0) | 0.045 * c |
| mMRC, median (Q1to Q3) | 1 (1 to 2) | 1 (1 to 2) | 2 (1 to 2) | 2 (1 to 2.5) | 0.009 * c,e |
| CIS20-SF, n (%) | |||||
| Normal fatigue | 26 (29.5) | 10 (38.5) | 14 (31.1) | 2 (11.8) | 0.127 b |
| Mild fatigue | 25 (28.4) | 5 (19.2) | 16 (35.6) | 4 (23.5) | |
| Severe fatigue | 37 (42.0) | 11 (42.3) | 15 (33.3) | 11 (64.7) | |
| 6MWD (m), mean (SD) | 421.8 (95.7) | 446.8 (68.6) | 414.0 (96.1) | 400.8 (126.0) | 0.223 a |
| 6MWD% predicted, mean (SD) | 85.3 (19.1) | 88.9 (11.7) | 85.0 (20.9) | 80.1 (23.1) | 0.326 a |
| PA, mean (SD) | |||||
| MVPA (min/day) | 29.3 (25.1) | 35.7 (32.7) | 24.3 (19.2) | 32.6 (24.2) | 0.409 c |
| No. Steps | 5178.9 (3168.3) | 6217.5 (4030.0) | 4560.1 (2457.2) | 5203.6 (3089.1) | 0.338 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimenta, S.; Silva, C.G.; Flora, S.; Hipólito, N.; Burtin, C.; Oliveira, A.; Morais, N.; Brites-Pereira, M.; Carreira, B.P.; Januário, F.; et al. What Motivates Patients with COPD to Be Physically Active? A Cross-Sectional Study. J. Clin. Med. 2021, 10, 5631. https://doi.org/10.3390/jcm10235631
Pimenta S, Silva CG, Flora S, Hipólito N, Burtin C, Oliveira A, Morais N, Brites-Pereira M, Carreira BP, Januário F, et al. What Motivates Patients with COPD to Be Physically Active? A Cross-Sectional Study. Journal of Clinical Medicine. 2021; 10(23):5631. https://doi.org/10.3390/jcm10235631
Chicago/Turabian StylePimenta, Sara, Cândida G. Silva, Sofia Flora, Nádia Hipólito, Chris Burtin, Ana Oliveira, Nuno Morais, Marcelo Brites-Pereira, Bruno P. Carreira, Filipa Januário, and et al. 2021. "What Motivates Patients with COPD to Be Physically Active? A Cross-Sectional Study" Journal of Clinical Medicine 10, no. 23: 5631. https://doi.org/10.3390/jcm10235631
APA StylePimenta, S., Silva, C. G., Flora, S., Hipólito, N., Burtin, C., Oliveira, A., Morais, N., Brites-Pereira, M., Carreira, B. P., Januário, F., Andrade, L., Martins, V., Rodrigues, F., Brooks, D., Marques, A., & Cruz, J. (2021). What Motivates Patients with COPD to Be Physically Active? A Cross-Sectional Study. Journal of Clinical Medicine, 10(23), 5631. https://doi.org/10.3390/jcm10235631







