Is Central Sensitisation the Missing Link of Persisting Symptoms after COVID-19 Infection?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Demographic Statistics
3.2. Symptoms of Central Sensitisation, Functional Status and Health-Related Quality of Life
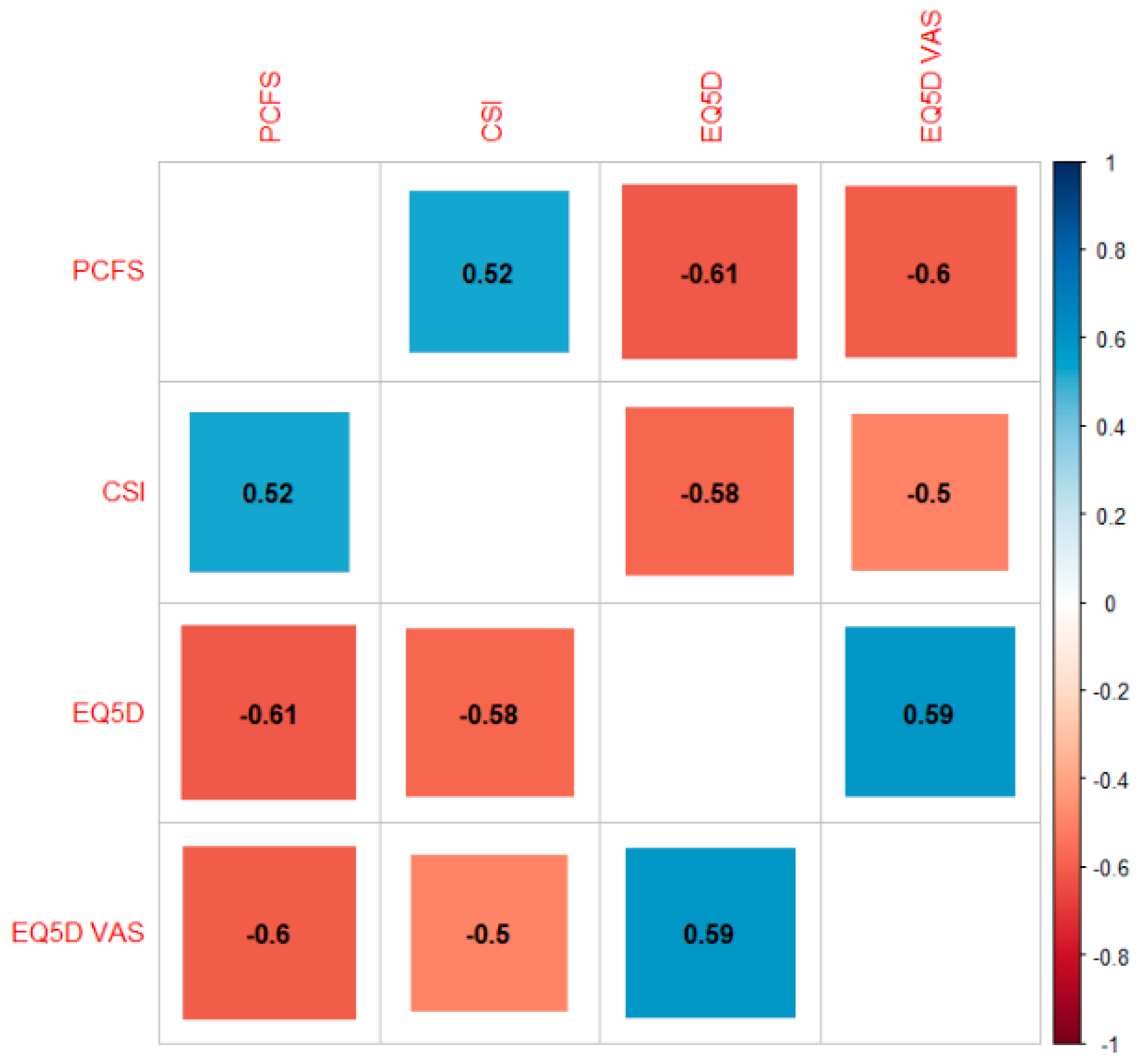
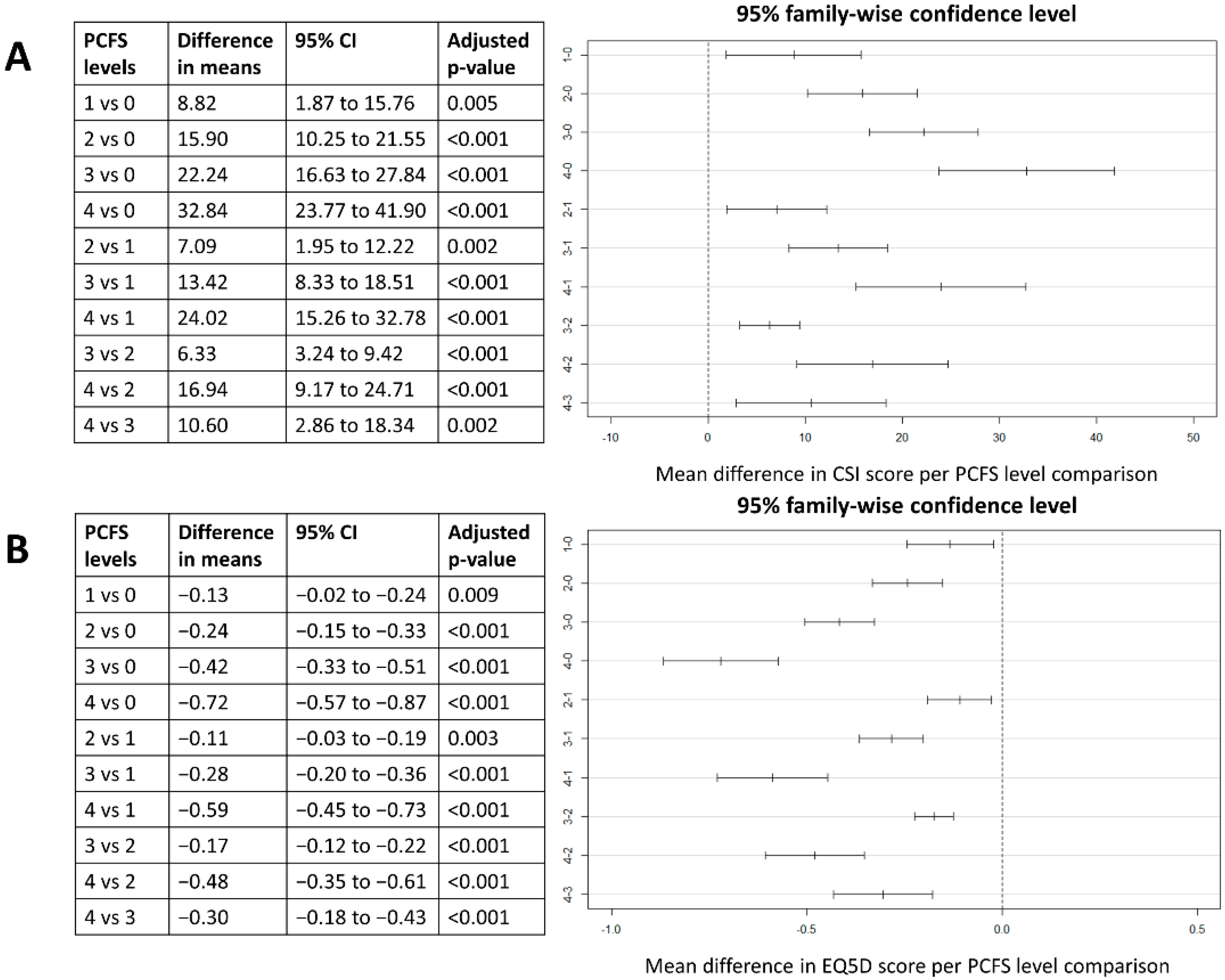
3.3. Association between Symptoms of Central Sensitisation, Functional Status and Health-Related Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eliezer, M.; Eloit, C.; Hautefort, C. Olfactory Loss of Function as a Possible Symptom of COVID-19—Reply. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 874–875. [Google Scholar] [CrossRef]
- Joob, B.; Wiwanitkit, V. Arthralgia as an initial presentation of COVID-19: Observation. Rheumatol. Int. 2020, 40, 823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Shen, D.; Zhou, H.; Liu, J.; Chen, S. Guillain-Barre syndrome associated with SARS-CoV-2 infection: Causality or coincidence? Lancet Neurol. 2020, 19, 383–384. [Google Scholar] [CrossRef]
- Sun, P.; Lu, X.; Xu, C.; Sun, W.; Pan, B. Understanding of COVID-19 based on current evidence. J. Med. Virol. 2020, 92, 548–551. [Google Scholar] [CrossRef]
- Abdullahi, A.; Candan, S.A.; Abba, M.A.; Bello, A.H.; Alshehri, M.A.; Afamefuna Victor, E.; Umar, N.A.; Kundakci, B. Neurological and Musculoskeletal Features of COVID-19: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 687. [Google Scholar] [CrossRef] [PubMed]
- Pfortmueller, C.A.; Spinetti, T.; Urman, R.D.; Luedi, M.M.; Schefold, J.C. COVID-19-associated acute respiratory distress syndrome (CARDS): Current knowledge on pathophysiology and ICU treatment—A narrative review. Best Pract. Res. Clin. Anaesthesiol. 2021, 35, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef]
- Bansal, M. Cardiovascular disease and COVID-19. Diabetes Metab. Syndr. 2020, 14, 247–250. [Google Scholar] [CrossRef]
- Cheung, K.S.; Hung, I.F.N.; Chan, P.P.Y.; Lung, K.C.; Tso, E.; Liu, R.; Ng, Y.Y.; Chu, M.Y.; Chung, T.W.H.; Tam, A.R.; et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples from a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology 2020, 159, 81–95. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, W.; Wang, D.; Mao, L.; Jin, H.; Li, Y.; Hong, C.; Chen, S.; Chang, J.; He, Q.; et al. Clinical time course of COVID-19, its neurological manifestation and some thoughts on its management. Stroke Vasc. Neurol. 2020, 5, 177–179. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [Green Version]
- The, L. Understanding long COVID: A modern medical challenge. Lancet 2021, 398, 725. [Google Scholar] [CrossRef]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Nijs, J.; Lahousse, A.; Kapreli, E.; Bilika, P.; Saracoglu, I.; Malfliet, A.; Coppieters, I.; De Baets, L.; Leysen, L.; Roose, E.; et al. Nociplastic Pain Criteria or Recognition of Central Sensitization? Pain Phenotyping in the Past, Present and Future. J. Clin. Med. 2021, 10, 3203. [Google Scholar] [CrossRef]
- Neblett, R.; Cohen, H.; Choi, Y.; Hartzell, M.M.; Williams, M.; Mayer, T.G.; Gatchel, R.J. The Central Sensitization Inventory (CSI): Establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J. Pain Off. J. Am. Pain Soc. 2013, 14, 438–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candan, S.A.; Elibol, N.; Abdullahi, A. Consideration of prevention and management of long-term consequences of post-acute respiratory distress syndrome in patients with COVID-19. Physiother. Theory Pract. 2020, 36, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Carfi, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients after Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Aaron, L.A.; Buchwald, D. A review of the evidence for overlap among unexplained clinical conditions. Ann. Intern. Med. 2001, 134, 868–881. [Google Scholar] [CrossRef]
- Mayer, T.G.; Neblett, R.; Cohen, H.; Howard, K.J.; Choi, Y.H.; Williams, M.J.; Perez, Y.; Gatchel, R.J. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012, 12, 276–285. [Google Scholar] [CrossRef] [Green Version]
- Kregel, J.; Vuijk, P.J.; Descheemaeker, F.; Keizer, D.; van der Noord, R.; Nijs, J.; Cagnie, B.; Meeus, M.; van Wilgen, P. The Dutch Central Sensitization Inventory (CSI): Factor Analysis, Discriminative Power, and Test-Retest Reliability. Clin. J. Pain 2016, 32, 624–630. [Google Scholar] [CrossRef]
- Cuesta-Vargas, A.I.; Neblett, R.; Nijs, J.; Chiarotto, A.; Kregel, J.; van Wilgen, C.P.; Pitance, L.; Knezevic, A.; Gatchel, R.J.; Mayer, T.G.; et al. Establishing Central Sensitization-Related Symptom Severity Subgroups: A Multicountry Study Using the Central Sensitization Inventory. Pain Med. 2020, 21, 2430–2440. [Google Scholar] [CrossRef]
- Corsi, G.; Nava, S.; Barco, S. A novel tool to monitor the individual functional status after COVID-19: The Post-COVID-19 Functional Status (PCFS) scale. G. Ital. Cardiol. 2020, 21, 757. [Google Scholar] [CrossRef]
- Klok, F.A.; Boon, G.; Barco, S.; Endres, M.; Geelhoed, J.J.M.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The Post-COVID-19 Functional Status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020, 56, 2001494. [Google Scholar] [CrossRef]
- Rabin, R.; de Charro, F. EQ-5D: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef] [PubMed]
- The EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- EuroQol Group. Self-Reported Population Health: An International Perspective Based on EQ-5D; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef] [Green Version]
- Aydede, M.; Shriver, A. Recently introduced definition of “nociplastic pain” by the International Association for the Study of Pain needs better formulation. Pain 2018, 159, 1176–1177. [Google Scholar] [CrossRef] [PubMed]
- Fillingim, R.B.; Loeser, J.D.; Baron, R.; Edwards, R.R. Assessment of Chronic Pain: Domains, Methods, and Mechanisms. J. Pain Off. J. Am. Pain Soc. 2016, 17, T10–T20. [Google Scholar] [CrossRef] [Green Version]
- Kosek, E.; Clauw, D.; Nijs, J.; Baron, R.; Gilron, I.; Harris, R.E.; Mico, J.A.; Rice, A.S.; Sterling, M. Chronic nociplastic pain affecting the musculoskeletal system: Clinical criteria and grading system. Pain 2021, 162, 2629–2634. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, I.; De Pauw, R.; Kregel, J.; Malfliet, A.; Goubert, D.; Lenoir, D.; Cagnie, B.; Meeus, M. Differences between Women with Traumatic and Idiopathic Chronic Neck Pain and Women without Neck Pain: Interrelationships among Disability, Cognitive Deficits, and Central Sensitization. Phys. Ther. 2017, 97, 338–353. [Google Scholar] [CrossRef]
- Staud, R. Abnormal endogenous pain modulation is a shared characteristic of many chronic pain conditions. Expert Rev. Neurother. 2012, 12, 577–585. [Google Scholar] [CrossRef]
- Kennedy, D.L.; Kemp, H.I.; Ridout, D.; Yarnitsky, D.; Rice, A.S. Reliability of conditioned pain modulation: A systematic review. Pain 2016, 157, 2410–2419. [Google Scholar] [CrossRef]
- Soon, B.; Vicenzino, B.; Schmid, A.B.; Coppieters, M.W. Facilitatory and inhibitory pain mechanisms are altered in patients with carpal tunnel syndrome. PLoS ONE 2017, 12, e0183252. [Google Scholar] [CrossRef]
- Imamura, M.; Chen, J.; Matsubayashi, S.R.; Targino, R.A.; Alfieri, F.M.; Bueno, D.K.; Hsing, W.T. Changes in pressure pain threshold in patients with chronic nonspecific low back pain. Spine 2013, 38, 2098–2107. [Google Scholar] [CrossRef]
- Goubert, D.; Danneels, L.; Graven-Nielsen, T.; Descheemaeker, F.; Meeus, M. Differences in Pain Processing between Patients with Chronic Low Back Pain, Recurrent Low Back Pain, and Fibromyalgia. Pain Physician 2017, 20, 307–318. [Google Scholar]
- Rasa, S.; Nora-Krukle, Z.; Henning, N.; Eliassen, E.; Shikova, E.; Harrer, T.; Scheibenbogen, C.; Murovska, M.; Prusty, B.K.; European Network on ME/CFS (EUROMENE). Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2018, 16, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straus, S.E.; Tosato, G.; Armstrong, G.; Lawley, T.; Preble, O.T.; Henle, W.; Davey, R.; Pearson, G.; Epstein, J.; Brus, I.; et al. Persisting illness and fatigue in adults with evidence of Epstein-Barr virus infection. Ann. Intern. Med. 1985, 102, 7–16. [Google Scholar] [CrossRef]
- Holmes, G.P.; Kaplan, J.E.; Stewart, J.A.; Hunt, B.; Pinsky, P.F.; Schonberger, L.B. A cluster of patients with a chronic mononucleosis-like syndrome. Is Epstein-Barr virus the cause? JAMA 1987, 257, 2297–2302. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.J. Detection of RNA sequences in cultures of a stealth virus isolated from the cerebrospinal fluid of a health care worker with chronic fatigue syndrome. Case report. Pathobiology 1997, 65, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, D.; Cheney, P.R.; Peterson, D.L.; Henry, B.; Wormsley, S.B.; Geiger, A.; Ablashi, D.V.; Salahuddin, S.Z.; Saxinger, C.; Biddle, R.; et al. A chronic illness characterized by fatigue, neurologic and immunologic disorders, and active human herpesvirus type 6 infection. Ann. Intern. Med. 1992, 116, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, S.; Kuratsune, H.; Yamaguchi, K.; Kitani, T.; Yamanishi, K. Prevalence of human herpesvirus 6 variants A and B in patients with chronic fatigue syndrome. Microbiol. Immunol. 1994, 38, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Ablashi, D.V.; Eastman, H.B.; Owen, C.B.; Roman, M.M.; Friedman, J.; Zabriskie, J.B.; Peterson, D.L.; Pearson, G.R.; Whitman, J.E. Frequent HHV-6 reactivation in multiple sclerosis (MS) and chronic fatigue syndrome (CFS) patients. J. Clin. Virol. 2000, 16, 179–191. [Google Scholar] [CrossRef]
- Nasralla, M.; Haier, J.; Nicolson, G.L. Multiple mycoplasmal infections detected in blood of patients with chronic fatigue syndrome and/or fibromyalgia syndrome. Eur. J. Clin. Microbiol. Infect. Dis. 1999, 18, 859–865. [Google Scholar] [CrossRef]
- Song, C.-Y.; Xu, J.; He, J.-Q.; Lu, Y.-Q. Immune dysfunction following COVID-19, especially in severe patients. Sci. Rep. 2020, 10, 15838. [Google Scholar] [CrossRef]
- Rendeiro, A.F.; Casano, J.; Vorkas, C.K.; Singh, H.; Morales, A.; DeSimone, R.A.; Ellsworth, G.B.; Soave, R.; Kapadia, S.N.; Saito, K.; et al. Profiling of immune dysfunction in COVID-19 patients allows early prediction of disease progression. Life Sci. Alliance 2021, 4, e202000955. [Google Scholar] [CrossRef]
- Li, T.; Chen, X.; Zhang, C.; Zhang, Y.; Yao, W. An update on reactive astrocytes in chronic pain. J. Neuroinflamm. 2019, 16, 140. [Google Scholar] [CrossRef]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Soung, A.; Sissoko, C.; Nordvig, A.; Canoll, P.; Mariani, M.; Jiang, X.; Bricker, T.; Goldman, J.; Rosoklija, G.; et al. COVID-19 induces neuroinflammation and loss of hippocampal neurogenesis. Res. Sq. 2021, 3, 1031824. [Google Scholar] [CrossRef]
- Chowdhury, B.; Sharma, A.; Satarker, S.; Mudgal, J.; Nampoothiri, M. Dialogue between Neuroinflammation and Neurodegenerative Diseases in COVID-19. J. Environ. Pathol. Toxicol. Oncol. 2021, 40, 37–49. [Google Scholar] [CrossRef]
- Machado, F.V.C.; Meys, R.; Delbressine, J.M.; Vaes, A.W.; Goertz, Y.M.J.; van Herck, M.; Houben-Wilke, S.; Boon, G.; Barco, S.; Burtin, C.; et al. Construct validity of the Post-COVID-19 Functional Status Scale in adult subjects with COVID-19. Health Qual. Life Outcomes 2021, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Carreon, L.Y.; Bratcher, K.R.; Das, N.; Nienhuis, J.B.; Glassman, S.D. Estimating EQ-5D values from the Oswestry Disability Index and numeric rating scales for back and leg pain. Spine 2014, 39, 678–682. [Google Scholar] [CrossRef]
- Pant, P.; Joshi, A.; Basnet, B.; Shrestha, B.M.; Bista, N.R.; Bam, N.; Das, S.K. Prevalence of Functional Limitation in COVID-19 Recovered Patients Using the Post COVID-19 Functional Status Scale. JNMA J. Nepal Med. Assoc. 2021, 59, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef] [PubMed]
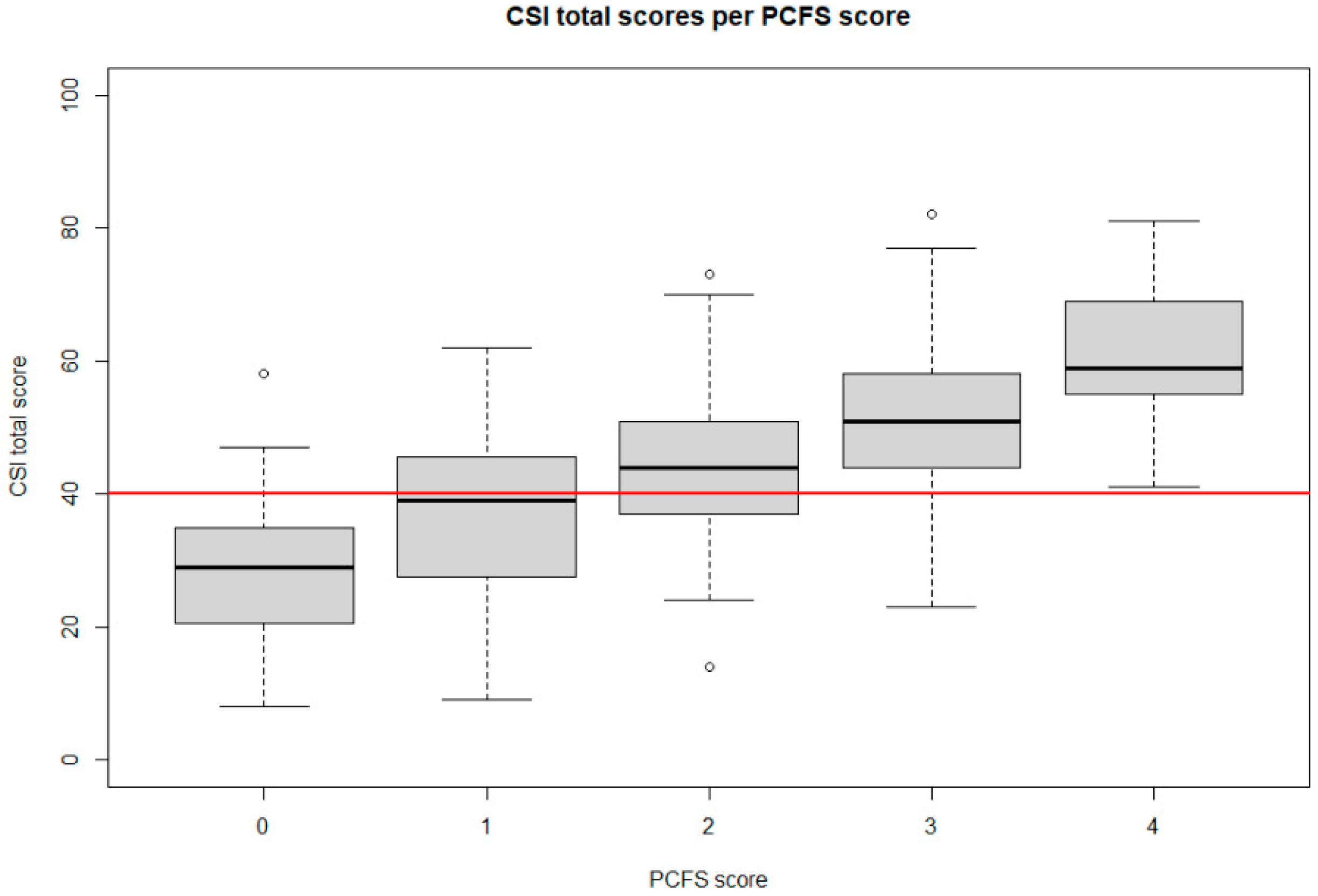
| Variable | Level | Mean CSI Score | Mean EQ5D | Mean EQ5D Vas |
|---|---|---|---|---|
| Sample | 45.9 (SD 13.1) (N = 491) | 0.57 (SD 0.23) (N = 547) | 56.6 (SD 18.2) (N = 537) | |
| Sex | Male | 41.2 (SD 13.8) (N = 68) | 0.60 (SD 0.23) (N = 75) | 61.8 (SD 18.6) (N = 74) |
| Female | 46.6 (SD 12.8) (N = 423) | 0.56 (SD 0.23) (N = 472) | 55.8 (SD 18.1) (N = 463) | |
| COVID-19 DIAGNOSIS | Confirmatory | 45.5 (SD 13.2) (N = 390) | 0.57 (SD 0.24) (N = 433) | 56.6 (SD 17.9) (N = 112) |
| Presumptive | 47.4 (SD 12.5) (N = 101) | 0.57 (SD 0.22) (N = 114) | 57.0 (SD 19.5) (N = 425) | |
| PCFS | Score 0 | 28.5 (SD 11.8) (N = 35) | 0.87 (SD 0.15) (N = 37) | 78.4 (SD 15.8) (N = 37) |
| Score 1 | 37.3 (SD 13.1) (N = 44) | 0.73 (SD 0.12) (N = 46) | 72.3 (SD 12.7) (N = 46) | |
| Score 2 | 44.4 (SD 10.8) (N = 187) | 0.63 (SD 0.15) (N = 188) | 60.7 (SD 13.1) (N = 188) | |
| Score 3 | 50.7 (SD 11.1) (N = 208) | 0.45 (SD 0.22) (N = 216) | 47.3 (SD 15.2) (N = 216) | |
| Score 4 | 61.3 (SD 10.9) (N = 17) | 0.15 (SD 0.11) (N = 17) | 34.1 (SD 14.5) (N = 17) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goudman, L.; De Smedt, A.; Noppen, M.; Moens, M. Is Central Sensitisation the Missing Link of Persisting Symptoms after COVID-19 Infection? J. Clin. Med. 2021, 10, 5594. https://doi.org/10.3390/jcm10235594
Goudman L, De Smedt A, Noppen M, Moens M. Is Central Sensitisation the Missing Link of Persisting Symptoms after COVID-19 Infection? Journal of Clinical Medicine. 2021; 10(23):5594. https://doi.org/10.3390/jcm10235594
Chicago/Turabian StyleGoudman, Lisa, Ann De Smedt, Marc Noppen, and Maarten Moens. 2021. "Is Central Sensitisation the Missing Link of Persisting Symptoms after COVID-19 Infection?" Journal of Clinical Medicine 10, no. 23: 5594. https://doi.org/10.3390/jcm10235594
APA StyleGoudman, L., De Smedt, A., Noppen, M., & Moens, M. (2021). Is Central Sensitisation the Missing Link of Persisting Symptoms after COVID-19 Infection? Journal of Clinical Medicine, 10(23), 5594. https://doi.org/10.3390/jcm10235594







