Geometric Reproducibility of Three-Dimensional Oral Implant Planning Based on Magnetic Resonance Imaging and Cone-Beam Computed Tomography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Imaging Data
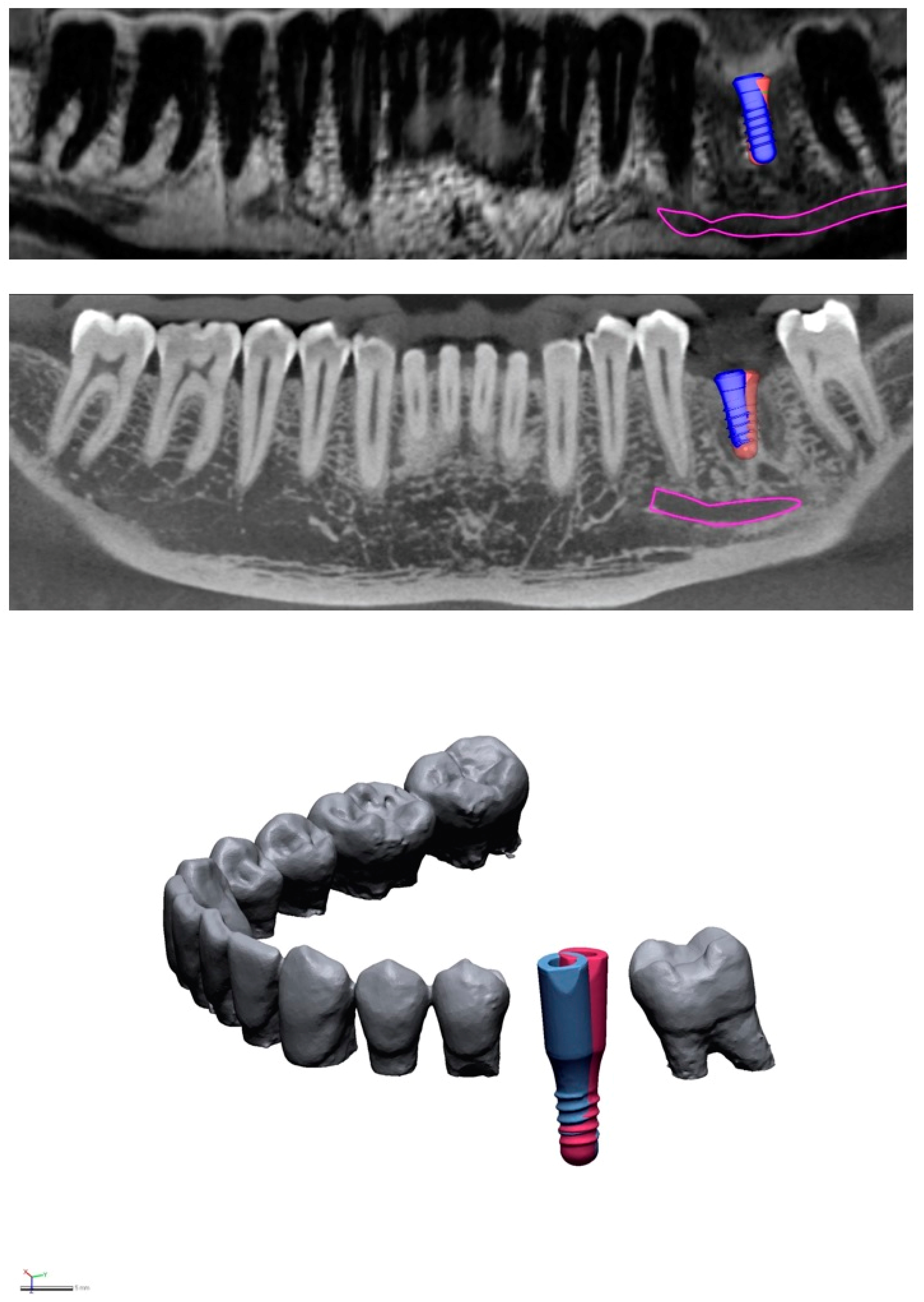
2.3. Virtual Implant Positioning
2.4. Geometric Deviation Analyses
3. Results
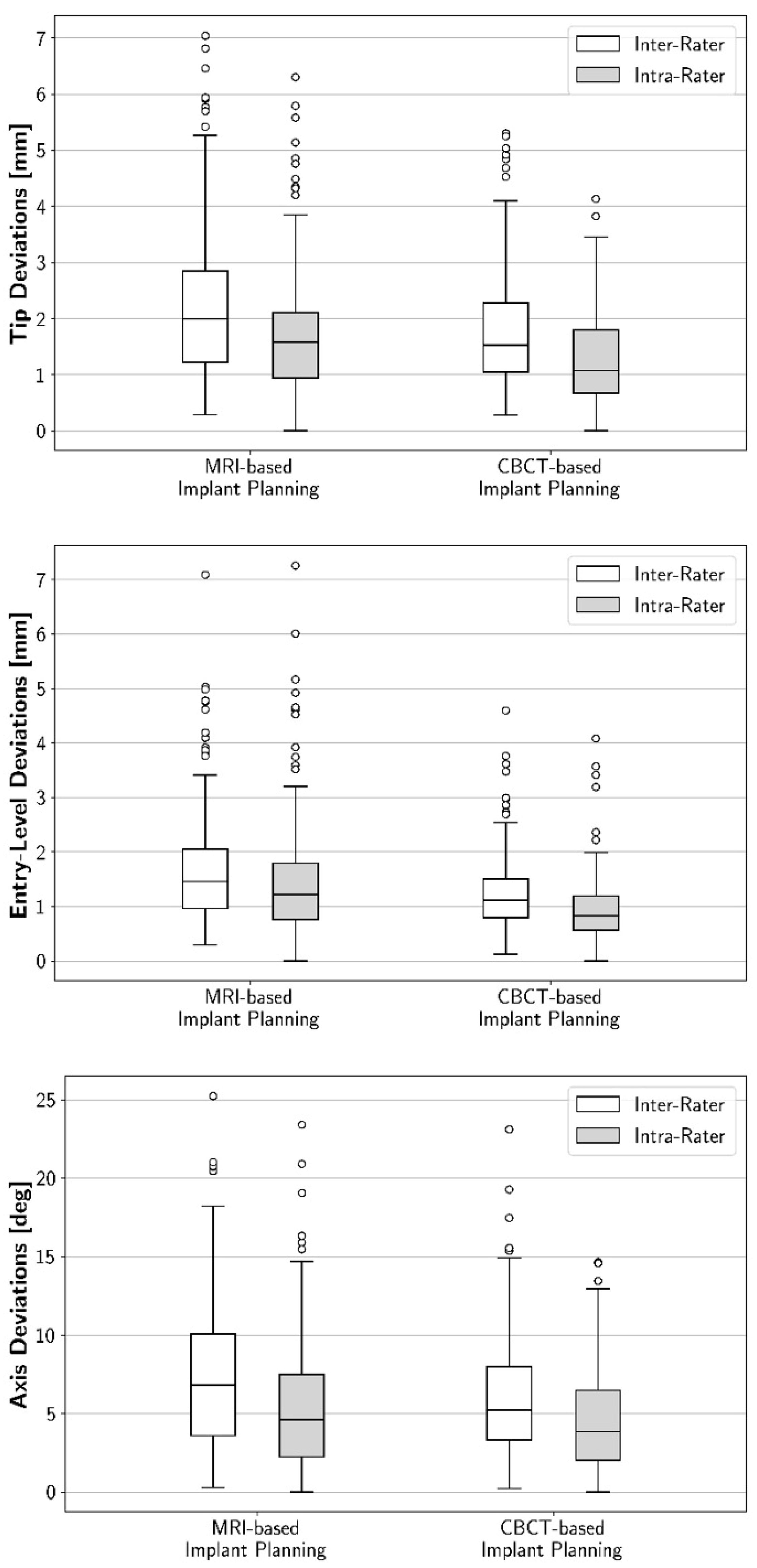
3.1. Intra-Rater Reproducibility: Geometric Deviations between First and Second 3D Implant Plans
3.2. Inter-Rater Reproducibility: Geometric Deviations between the Raters’ 3D Implant Plans
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guentsch, A.; Sukhtankar, L.; An, H.; Luepke, P.G. Precision and trueness of implant placement with and without static surgical guides: An in vitro study. J. Prosthet, Dent. 2020, 126, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Tahmaseb, A.; Wismeijer, D.; Coucke, W.; Derksen, W. Computer technology applications in surgical implant dentistry: A systematic review. Int. J. Oral. Maxillofac. Implant. 2014, 29, 25–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnutenhaus, S.; Edelmann, C.; Rudolph, H.; Dreyhaupt, J.; Luthardt, R.G. 3D accuracy of implant positions in template-guided implant placement as a function of the remaining teeth and the surgical procedure: A retrospective study. Clin. Oral Investig. 2018, 22, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.; Marquardt, P.; Zwahlen, M.; Jung, R.E. A systematic review on the accuracy and the clinical outcome of computer-guided template-based implant dentistry. Clin. Oral. Implant. Res. 2009, 20 (Suppl. 4), 73–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, R.E.; Schneider, D.; Ganeles, J.; Wismeijer, D.; Zwahlen, M.; Hämmerle, C.H.; Tahmaseb, A. Computer technology applications in surgical implant dentistry: A systematic review. Int. J. Oral. Maxillofac. Implant. 2009, 24, 92–109. [Google Scholar]
- Cassetta, M.; Giansanti, M.; Di Mambro, A.; Calasso, S.; Barbato, E. Accuracy of two stereolithographic surgical templates: A retrospective study. Clin. Implant. Dent. Relat. Res. 2013, 15, 448–459. [Google Scholar] [CrossRef]
- Arisan, V.; Karabuda, Z.C.; Ozdemir, T. Accuracy of two stereolithographic guide systems for computer-aided implant placement: A computed tomography-based clinical comparative study. J. Periodontol. 2010, 81, 43–51. [Google Scholar] [CrossRef]
- Flügge, T.; Ludwig, U.; Hovener, J.B.; Kohal, R.; Wismeijer, D.; Nelson, K. Virtual implant planning and fully guided implant surgery using magnetic resonance imaging-Proof of principle. Clin. Oral. Implant. Res. 2020, 31, 575–583. [Google Scholar] [CrossRef] [Green Version]
- Hilgenfeld, T.; Juerchott, A.; Jende, J.M.E.; Rammelsberg, P.; Heiland, S.; Bendszus, M.; Schwindling, F.S. Use of dental MRI for radiation-free guided dental implant planning: A prospective, in vivo study of accuracy and reliability. Eur. Radiol. 2020, 30, 6392–6401. [Google Scholar] [CrossRef]
- Flügge, T.; Ludwig, U.; Winter, G.; Amrein, P.; Kernen, F.; Nelson, K. Fully guided implant surgery using Magnetic Resonance Imaging—An in vitro study on accuracy in human mandibles. Clin. Oral. Implant. Res. 2020, 31, 737–746. [Google Scholar] [CrossRef]
- Probst, F.A.; Schweiger, J.; Stumbaum, M.J.; Karampinos, D.; Burian, E.; Probst, M. Magnetic resonance imaging based computer-guided dental implant surgery-A clinical pilot study. Clin. Implant. Dent. Relat. Res. 2020, 22, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Mercado, F.; Mukaddam, K.; Filippi, A.; Bieri, O.P.; Lambrecht, T.J.; Kühl, S. Fully Digitally Guided Implant Surgery Based on Magnetic Resonance Imaging. Int. J. Oral. Maxillofac. Implant. 2019, 34, 529–534. [Google Scholar] [CrossRef]
- Hilgenfeld, T.; Prager, M.; Schwindling, F.S.; Nittka, M.; Rammelsberg, P.; Bendszus, M.; Heiland, S.; Juerchott, A. MSVAT-SPACE-STIR and SEMAC-STIR for Reduction of Metallic Artifacts in 3T Head and Neck MRI. Am. J. Neuroradiol. 2018, 39, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Hilgenfeld, T.; Prager, M.; Heil, A.; Schwindling, F.S.; Nittka, M.; Grodzki, D.; Rammelsberg, P.; Bendszus, M.; Heiland, S. PETRA, MSVAT-SPACE and SEMAC sequences for metal artefact reduction in dental MR imaging. Eur. Radiol. 2017, 27, 5104–5112. [Google Scholar] [CrossRef] [PubMed]
- Wismeijer, D.; Joda, T.; Flügge, T.; Fokas, G.; Tahmaseb, A.; Bechelli, D.; Bohner, L.; Bornstein, M.; Burgoyne, A.; Caram, S.; et al. Group 5 ITI Consensus Report: Digital technologies. Clin. Oral. Implant. Res. 2018, 29 (Suppl. 16), 436–442. [Google Scholar] [CrossRef]
- Luangchana, P.; Pornprasertsuk-Damrongsri, S.; Kiattavorncharoen, S.; Jirajariyavej, B. Accuracy of linear measurements using cone beam computed tomography and panoramic radiography in dental implant treatment planning. Int. J. Oral. Maxillofac. Implant. 2015, 30, 1287–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Ekrish, A.A.; Ekram, M. A comparative study of the accuracy and reliability of multidetector computed tomography and cone beam computed tomography in the assessment of dental implant site dimensions. Dentomaxillofac. Radiol. 2011, 40, 67–75. [Google Scholar] [CrossRef]
- Peterson, A.G.; Wang, M.; Gonzalez, S.; Covell, D.A., Jr.; Katancik, J.; Sehgal, H.S. An In Vivo and Cone Beam Computed Tomography Investigation of the Accuracy in Measuring Alveolar Bone Height and Detecting Dehiscence and Fenestration Defects. Int. J. Oral. Maxillofac. Implant. 2018, 33, 1296–1304. [Google Scholar] [CrossRef]
- Elsyad, M.A.; Eltowery, S.M.; Gebreel, A.A. Peri-implant strain around mesially inclined two-implant-retained mandibular overdentures with Locator attachments. J. Oral. Sci. 2017, 59, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Elsyad, M.A.; Setta, F.A.; Khirallah, A.S. Strains around distally inclined implants retaining mandibular overdentures with Locator attachments: An in vitro study. J. Adv. Prosthodont. 2016, 8, 116–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, F.; Hata, Y.; Komatsu, S.; Ramos, T.C.; Fukuda, H. Finite element analysis of the influence of implant inclination, loading position, and load direction on stress distribution. Odontology 2003, 91, 31–36. [Google Scholar] [CrossRef]
- Sinjari, B.; D’Addazio, G.; Traini, T.; Varvara, G.; Scarano, A.; Murmura, G.; Caputi, S.A. A 10-year retrospective comparative human study on screw-retained versus cemented dental implant abutments. J. Biol. Regul. Homeost. Agents 2019, 33, 787–797. [Google Scholar]
- Sinibaldi, R.; Conti, A.; Sinjari, B.; Spadone, S.; Pecci, R.; Palombo, M.; Komlev, V.S.; Ortore, M.G.; Tromba, G.; Capuani, S.; et al. Multimodal-3D imaging based on muMRI and muCT techniques bridges the gap with histology in visualization of the bone regeneration process. J. Tissue Eng. Regen. Med. 2018, 12, 750–761. [Google Scholar] [CrossRef]
- Buser, D.; Martin, W.; Belser, U.C. Optimizing esthetics for implant restorations in the anterior maxilla: Anatomic and surgical considerations. Int. J. Oral. Maxillofac. Implant. 2004, 19, 43–61. [Google Scholar]
- Schnutenhaus, S.; Edelmann, C.; Rudolph, H.; Luthardt, R.G. Retrospective study to determine the accuracy of template-guided implant placement using a novel nonradiologic evaluation method. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2016, 121, e72–e79. [Google Scholar] [CrossRef]
- Schwindling, F.S.; Juerchott, A.; Boehm, S.; Rues, S.; Kronsteiner, D.; Heiland, S.; Bendszus, M.; Rammelsberg, P.; Hilgenfeld, T. Three-dimensional accuracy of partially guided implant surgery based on dental magnetic resonance imaging. Clin. Oral. Implant. Res. 2021, 32, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.; Lange, S. Multiple test procedures other than Bonferroni’s deserve wider use. BMJ 1999, 318, 600–601. [Google Scholar] [CrossRef] [PubMed]
- Kühl, S.; Zürcher, S.; Mahid, T.; Muller-Gerbl, M.; Filippi, A.; Cattin, P. Accuracy of full guided vs. half-guided implant surgery. Clin. Oral. Implant. Res. 2013, 24, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Bover-Ramos, F.; Vina-Almunia, J.; Cervera-Ballester, J.; Penarrocha-Diago, M.; Garcia-Mira, B. Accuracy of Implant Placement with Computer-Guided Surgery: A Systematic Review and Meta-Analysis Comparing Cadaver, Clinical, and In Vitro Studies. Int. J. Oral. Maxillofac. Implant. 2018, 33, 101–115. [Google Scholar] [CrossRef]
- Younes, F.; Cosyn, J.; De Bruyckere, T.; Cleymaet, R.; Bouckaert, E.; Eghbali, A. A randomized controlled study on the accuracy of free-handed, pilot-drill guided and fully guided implant surgery in partially edentulous patients. J. Clin. Periodontol. 2018, 45, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Vercruyssen, M.; Cox, C.; Coucke, W.; Naert, I.; Jacobs, R.; Quirynen, M. A randomized clinical trial comparing guided implant surgery (bone- or mucosa-supported) with mental navigation or the use of a pilot-drill template. J. Clin. Periodontol. 2014, 41, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Derksen, W.; Wismeijer, D.; Flugge, T.; Hassan, B.; Tahmaseb, A. The accuracy of computer-guided implant surgery with tooth-supported, digitally designed drill guides based on CBCT and intraoral scanning. A prospective cohort study. Clin. Oral. Implant. Res. 2019, 30, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Rater | Mean Difference between Modalities | 95% Confidence Interval | p-Value |
|---|---|---|---|---|
| Tip [mm] | Mean | 0.5 | (0.3, 0.7) | * |
| 1 | 0.6 | (0.1, 1) | 0.015 | |
| 2 | 0.4 | (0, 0.9) | 0.061 | |
| 3 | 0.6 | (0.3, 1) | 0.002 | |
| 4 | 0.3 | (0, 0.7) | 0.090 | |
| Entry-level [mm] | Mean | 0.5 | (0.3, 0.7) | * |
| 1 | 0.6 | (0.3, 1) | 0.002 | |
| 2 | 0.7 | (0.3, 1.2) | 0.003 | |
| 3 | 0.6 | (0.2, 1) | 0.002 | |
| 4 | 0.2 | (−0.2, 0.6) | 0.283 | |
| Axis [°] | Mean | 1 | (0.2, 1.8) | * |
| 1 | 1.4 | (−0.3, 3.2) | 0.109 | |
| 2 | 0.2 | (−1.8, 2.2) | 0.826 | |
| 3 | 0.8 | (−0.3, 1.9) | 0.135 | |
| 4 | 1.5 | (0.1, 2.9) | 0.037 |
| Parameter | Rater Combination | Mean Difference between Modalities | 95% Confidence Interval | p-Value (Bonferroni-Corrected) |
|---|---|---|---|---|
| Tip [mm] | Mean | 0.5 | (0.3, 0.7) | * |
| 1 vs. 2 | 0.4 | (−0.1, 1) | 0.033 | |
| 1 vs. 3 | 0.2 | (−0.3, 0.7) | 0.084 | |
| 1 vs. 4 | 0.6 | (0.1, 1.1) | <0.001 | |
| 2 vs. 3 | 0.2 | (−0.2, 0.6) | 0.399 | |
| 2 vs. 4 | 0.6 | (0.1, 1) | 0.669 | |
| 3 vs. 4 | 1.0 | (0.5, 1.5) | 1 | |
| Entry-level [mm] | Mean | 0.4 | (0.3, 0.6) | * |
| 1 vs. 2 | 0.5 | (0, 0.9) | 0.030 | |
| 1 vs. 3 | 0.3 | (−0.1, 0.7) | 0.156 | |
| 1 vs. 4 | 0.4 | (0, 0.8) | <0.001 | |
| 2 vs. 3 | 0.4 | (0.1, 0.6) | 0.147 | |
| 2 vs. 4 | 0.5 | (0.1, 0.8) | 0.060 | |
| 3 vs. 4 | 0.7 | (0.3, 1) | 0.399 | |
| Axis [°] | Mean | 1.4 | (0.7, 2.2) | * |
| 1 vs. 2 | 1.2 | (−0.8, 3.2) | 0.102 | |
| 1 vs. 3 | 1.0 | (−0.6, 2.6) | 0.012 | |
| 1 vs. 4 | 2.8 | (1, 4.6) | 0.039 | |
| 2 vs. 3 | −0.1 | (−2.1, 1.8) | 0.720 | |
| 2 vs. 4 | 1.7 | (0.1, 3.3) | 1 | |
| 3 vs. 4 | 2.2 | (0.5, 3.9) | 0.675 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwindling, F.S.; Boehm, S.; Herpel, C.; Kronsteiner, D.; Vogel, L.; Juerchott, A.; Heiland, S.; Bendszus, M.; Rammelsberg, P.; Hilgenfeld, T. Geometric Reproducibility of Three-Dimensional Oral Implant Planning Based on Magnetic Resonance Imaging and Cone-Beam Computed Tomography. J. Clin. Med. 2021, 10, 5546. https://doi.org/10.3390/jcm10235546
Schwindling FS, Boehm S, Herpel C, Kronsteiner D, Vogel L, Juerchott A, Heiland S, Bendszus M, Rammelsberg P, Hilgenfeld T. Geometric Reproducibility of Three-Dimensional Oral Implant Planning Based on Magnetic Resonance Imaging and Cone-Beam Computed Tomography. Journal of Clinical Medicine. 2021; 10(23):5546. https://doi.org/10.3390/jcm10235546
Chicago/Turabian StyleSchwindling, Franz Sebastian, Sophia Boehm, Christopher Herpel, Dorothea Kronsteiner, Lorenz Vogel, Alexander Juerchott, Sabine Heiland, Martin Bendszus, Peter Rammelsberg, and Tim Hilgenfeld. 2021. "Geometric Reproducibility of Three-Dimensional Oral Implant Planning Based on Magnetic Resonance Imaging and Cone-Beam Computed Tomography" Journal of Clinical Medicine 10, no. 23: 5546. https://doi.org/10.3390/jcm10235546
APA StyleSchwindling, F. S., Boehm, S., Herpel, C., Kronsteiner, D., Vogel, L., Juerchott, A., Heiland, S., Bendszus, M., Rammelsberg, P., & Hilgenfeld, T. (2021). Geometric Reproducibility of Three-Dimensional Oral Implant Planning Based on Magnetic Resonance Imaging and Cone-Beam Computed Tomography. Journal of Clinical Medicine, 10(23), 5546. https://doi.org/10.3390/jcm10235546







