Self-Reported Adverse Events of COVID-19 Vaccines in Polish Healthcare Workers and Medical Students. Cross-Sectional Study and Pooled Analysis of CoVaST Project Results in Central Europe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Instrument
2.4. Ethics
2.5. Analysis
2.6. Pooled Analysis of Results of CoVaST Project Studies Conducted in Central Europe
3. Results
3.1. Demographic Characteristics
3.2. Medical Anamnesis
3.3. COVID-19-Related Anamnesis
3.4. Local Side Effects
3.5. Systemic SRAEs
3.6. Orofacial and Skin-Related SRAEs
3.7. Over the Counter, Alleviating Drugs Taken after Vaccination
3.8. COVID-19 Vaccines SRAEss by Gender
3.9. COVID-19 Vaccines SRAEs by Age
3.10. Predictors of SRAEs of COVID-19 Vaccines
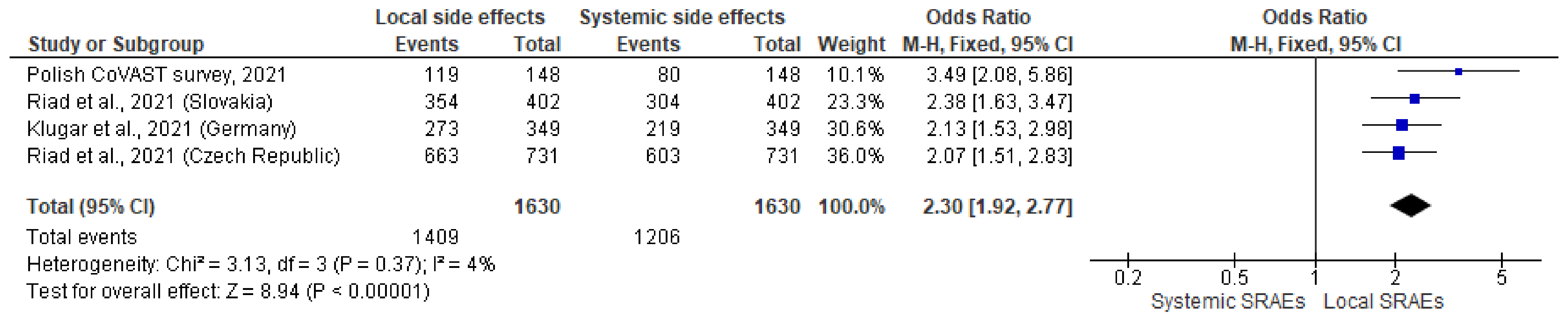
3.11. Pooled Analysis Results from CoVAST Studies Conducted in Czech Republic, Slovakia, Germany, and Poland
4. Discussion
4.1. Strengths and Limitations
4.2. Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khunti, K.; Kamal, A.; Pareek, M.; Griffiths, A. Should vaccination for healthcare workers be mandatory? J. R. Soc. Med. 2021, 114, 235–236. [Google Scholar] [CrossRef]
- World Health Organization. Strategic Advisory Group of Experts on Immunization. WHO SAGE Roadmap for Prioritizing uses of COVID-19 Vaccines in the Context of Limited Supply. November 2020. Available online: https://www.who.int/publications/i/item/who-sage-roadmap-for-prioritizing-uses-of-covid-19-vaccines-in-the-context-of-limited-supply (accessed on 30 October 2021).
- Johnson, S.B.; Butcher, F. Doctors during the COVID-19 pandemic: What are their duties and what is owed to them? J. Med. Ethics 2021, 47, 12–15. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N. Engl. J. Med. 2021, 385, 1393. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Poland. Available online: https://covid19.who.int/region/euro/country/pl (accessed on 10 September 2021).
- European Medicines Agency (EMA). EMA Recommends First COVID-19 Vaccine for Authorisation in the EU. Available online: https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu (accessed on 15 September 2021).
- European Medicines Agency (EMA). Comirnaty. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/21fizer21ty (accessed on 15 August 2021).
- European Medicines Agency (EMA). EMA Recommends COVID-19 Vaccine Moderna for Authorisation in the EU. Available online: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-moderna-authorisation-eu (accessed on 15 August 2021).
- European Medicines Agency (EMA). EMA Recommends COVID-19 Vaccine AstraZeneca for Authorisation in the EU. Available online: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-astrazeneca-authorisation-eu (accessed on 15 August 2021).
- COVID-19 Vaccine Tracker. Available online: https://covid19.trackvaccines.org/country/21fizer (accessed on 20 September 2021).
- Serwis Rzeczypospolitej Polskiej. The Report of Vaccination against COVID-19. Available online: https://www.gov.pl/web/szczepimysie/raport-szczepien-przeciwko-covid-19 (accessed on 15 September 2021).
- Serwis Rzeczypospolitej Polskiej. The National Vaccination Program. Available online: https://www.gov.pl/web/szczepimysie/narodowy-program-szczepien-przeciw-covid-19 (accessed on 15 September 2021).
- Nour, S.; Plourde, G. Pharmacoepidemiology and Pharmacovigilance: Synergistic Tools to Better Investigate Drug Safety; Academic Press: Cambridge, MA, USA, 2018; pp. 1–224. [Google Scholar]
- Medicines & Healthcare Products Regulatory Agency (MHRA). Medicines and Healthcare Products Regulatory Agency (MHRA) Report of the Commission on Human Medicines Expert Working Group on COVID-19 Vaccine Safety Surveillance. Available online: https://www.gov.uk/government/publications/report-of-the-commission-on-human-medicines-expert-working-group-on-covid-19-vaccine-safety-surveillance/report-of-the-commission-on-human-medicines-expert-working-group-on-covid-19-vaccine-safety-surveillance (accessed on 12 September 2021).
- Serwis Rzeczypospolitej Polskiej. The Adverse Post-Vaccination Events (Poland). Available online: https://www.gov.pl/web/szczepimysie/niepozadane-odczyny-poszczepienne (accessed on 12 September 2021).
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. Lancet Infect. Dis 2021. [Google Scholar] [CrossRef]
- Riad, A.; Pokorná, A.; Attia, S.; Klugarová, J.; Koščík, M.; Klugar, M. Prevalence of COVID-19 Vaccine Side Effects among Healthcare Workers in the Czech Republic. J. Clin. Med. 2021, 10, 1428. [Google Scholar] [CrossRef] [PubMed]
- Jęśkowiak, I.; Wiatrak, B.; Grosman-Dziewiszek, P.; Szeląg, A. The Incidence and Severity of Post-Vaccination Reactions after Vaccination against COVID-19. Vaccines 2021, 9, 502. [Google Scholar] [CrossRef]
- Riad, A.; Hocková, B.; Kantorová, L.; Slávik, R.; Spurná, L.; Stebel, A.; Havriľak, M.; Klugar, M. Side Effects of mRNA-Based COVID-19 Vaccine: Nationwide Phase IV Study among Healthcare Workers in Slovakia. Pharmaceuticals 2021, 14, 873. [Google Scholar] [CrossRef]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet. Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- Kadali, R.A.K.; Janagama, R.; Peruru, S.; Gajula, V.; Madathala, R.R.; Chennaiahgari, N.; Malayala, S.V. Adverse effects of COVID-19 mRNA-1273 vaccine: A randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J. Med. Virol. 2021, 93, 4420–4429. [Google Scholar] [CrossRef] [PubMed]
- Kadali, R.A.K.; Janagama, R.; Peruru, S.; Malayala, S.V. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int. J. Infect. Dis. 2021, 106, 376–381. [Google Scholar] [CrossRef]
- Almufty, H.B.; Mohammed, S.A.; Abdullah, A.M.; Merza, M.A. Potential adverse effects of COVID19 vaccines among Iraqi population; a comparison between the three available vaccines in Iraq; a retrospective cross-sectional study. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102207. [Google Scholar] [CrossRef]
- Vita, E.D.; Sbrana, F.; Quattrone, F.; Pino, B.D.; Megaro, M.; Lombardi, R.; Prontera, C.; Passino, C.; Petrillo, M. Adverse events and humoral response after two doses of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) mRNA vaccine in the hospital personnel of a cardiopulmonary tertiary-care center. Infect. Control. Hosp. Epidemiol. 2021, 1–3. [Google Scholar] [CrossRef]
- Abu-Hammad, O.; Alduraidi, H.; Abu-Hammad, S.; Alnazzawi, A.; Babkair, H.; Abu-Hammad, A.; Nourwali, I.; Qasem, F.; Dar-Odeh, N. Side Effects Reported by Jordanian Healthcare Workers Who Received COVID-19 Vaccines. Vaccines 2021, 9, 577. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, S.; Borg, M.; Agius, S.; Souness, J.; Brincat, A.; Grech, V. Adverse reactions to Pfizer-BioNTech vaccination of healthcare workers at Malta’s state hospital. Int. J. Clin. Pract. 2021, 75, e14605. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; Harakeh, S.; Badr-Eldin, S.M.; Bagher, A.M.; Eid, B.G.; Almukadi, H.S.; Alghamdi, B.S.; Alahmadi, A.A.; Hassan, N.A.; Sindi, N.; et al. Minor to Moderate Side Effects of Pfizer-BioNTech COVID-19 Vaccine among Saudi Residents: A Retrospective Cross-Sectional Study. Int. J. Gen. Med. 2021, 14, 1389–1401. [Google Scholar] [CrossRef]
- Klugar, M.; Riad, A.; Mekhemar, M.; Conrad, J.; Buchbender, M.; Howaldt, H.-P.; Attia, S. Side Effects of mRNA-Based and Viral Vector-Based COVID-19 Vaccines among German Healthcare Workers. Biology 2021, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- Centres for Diseases Control and Prevention (CDC). Reactions and Adverse Events of the Pfizer-BioNTech COVID-19 Vaccine. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/fizer/reactogenicity.html (accessed on 17 September 2021).
- Babicki, M.; Mastalerz-Migas, A. Attitudes toward Vaccination against COVID-19 in Poland. A Longitudinal Study Performed before and Two Months after the Commencement of the Population Vaccination Programme in Poland. Vaccines 2021, 13, 503. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, S.; Hitzenbichler, F.; Huppertz, G.; Zeman, F.; Koller, M.; Schmidt, B.; Plentz, A.; Bauswein, M.; Mohr, A.; Salzberger, B. Brief report: Attitudes towards Covid-19 vaccination among hospital employees in a tertiary care university hospital in Germany in December 2020. Infection 2021, 1, 1–5. [Google Scholar] [CrossRef]
- Szmyd, B.; Karuga, F.F.; Bartoszek, A.; Staniecka, K.; Siwecka, N.; Bartoszek, A.; Błaszczyk, M.; Radek, M. Attitude and Behaviors towards SARS-CoV-2 Vaccination among Healthcare Workers: A Cross-Sectional Study from Poland. Vaccines 2021, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.; Biddle, N.; Gray, M.; Sollis, K. COVID-19 vaccine hesitancy and resistance: Correlates in a nationally representative longitudinal survey of the Australian population. PLoS ONE 2021, 16, e0248892. [Google Scholar] [CrossRef]
- Salali, G.D.; Uysal, M.S. COVID-19 vaccine hesitancy is associated with beliefs on the origin of the novel coronavirus in the UK and Turkey. Psychol. Med. 2020, 19, 1–3. [Google Scholar] [CrossRef]
- Aw, J.; Seng, J.J.B.; Seah, S.S.Y.; Low, L.L. COVID-19 Vaccine Hesitancy-A Scoping Review of Literature in High-Income Countries. Vaccines 2021, 13, 900. [Google Scholar] [CrossRef]
- Momentive Inc. SurveyMonkey: The World’s Most Popular Free Online Survey Tool. Available online: https://www.surveymonkey.com (accessed on 15 July 2021).
- Klugar, M.; Riad, A. COVID-19 Vaccines Safety Tracking (CoVaST). ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04834869 (accessed on 15 July 2021).
- Portal Gov.pl. Pytania i Odpowiedzi. Szczepienie Przeciwko COVID-19. Available online: https://www.gov.pl/web/szczepimysie/pytania-i-odpowiedzi (accessed on 15 July 2021).
- Riad, A.; Schünemann, H.; Attia, S.; Peričić, T.P.; Žuljević, M.F.; Jürisson, M.; Kalda, R.; Lang, K.; Morankar, S.; Yesuf, E.A.; et al. COVID-19 Vaccines Safety Tracking (CoVaST): Protocol of a Multi-Center Prospective Cohort Study for Active Surveillance of COVID-19 Vaccines’ Side Effects. Int. J. Environ. Res. Public Health 2021, 18, 7859. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Pokorná, A.; Mekhemar, M.; Conrad, J.; Klugarová, J.; Koščík, M.; Klugar, M.; Attia, S. Safety of ChAdOx1 nCoV-19 Vaccine: Independent Evidence from Two EU States. Vaccines 2021, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Sağıroğlu, D.; Üstün, B.; Pokorná, A.; Klugarová, J.; Attia, S.; Klugar, M. Prevalence and Risk Factors of CoronaVac Side Effects: An Independent Cross-Sectional Study among Healthcare Workers in Turkey. J. Clin. Med. 2021, 10, 2629. [Google Scholar] [CrossRef]
- Centres for Diseases Control and Prevention (CDC). Pfizer-BioNTech COVID-19 Vaccine Overview and Safety (Also Known as COMIRNATY). Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Pfizer-BioNTech.html (accessed on 14 November 2020).
- Centres for Diseases Control and Prevention (CDC). Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: Moderna COVID-19 Vaccine. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html (accessed on 2 April 2021).
- European Medicines Agency (EMA). COVID-19 Vaccine AstraZeneca. Available online: https://www.ema.europa.eu/en/documents/product-information/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-product-information_en.pdf (accessed on 15 July 2021).
- World Health Organization (WHO). Process of Translation and Adaptation of Instruments. Available online: https://www.who.int/substance_abuse/research_tools/translation/en/ (accessed on 25 December 2020).
- WMA. World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. UroToday Int. J. 2009, 2. [Google Scholar] [CrossRef] [Green Version]
- Proton Technologies AG. General Data Protection Regulation (GDPR) Compliance Guidelines. HORIZON 2020—Project REP-791727-1. Available online: https://gdpr.eu/ (accessed on 1 May 2021).
- Mathioudakis, A.G.; Ghrew, M.; Ustianowski, A.; Ahmad, S.; Borrow, R.; Papavasileiou, L.P.; Petrakis, D.; Bakerly, N.D. Self-Reported Real-World Safety and Reactogenicity of COVID-19 Vaccines: A Vaccine Recipient Survey. Life 2021, 17, 249. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ostropolets, A.; Makadia, R.; Shoaibi, A.; Rao, G.; Sena, A.G.; Martinez-Hernandez, E.; Delmestri, A.; Verhamme, K.; Rijnbeek, P.R.; et al. Characterising the background incidence rates of adverse events of special interest for covid-19 vaccines in eight countries: Multinational network cohort study. BMJ 2021, 373, n1435. [Google Scholar] [CrossRef]
- Alhazmi, A.; Alamer, E.; Daws, D.; Hakami, M.; Darraj, M.; Abdelwahab, S.; Maghfuri, A.; Algaissi, A. Evaluation of Side Effects Associated with COVID-19 Vaccines in Saudi Arabia. Vaccines 2021, 18, 674. [Google Scholar] [CrossRef]
- Resta, C.D.; Ferrari, D.; Viganò, M.; Moro, M.; Sabetta, E.; Minerva, M.; Ambrosio, A.; Locatelli, M.; Tomaiuolo, R. The Gender Impact Assessment among Healthcare Workers in the SARS-CoV-2 Vaccination—An Analysis of Serological Response and Side Effects. Vaccines 2021, 9, 522. [Google Scholar] [CrossRef]
- Luxi, N.; Giovanazzi, A.; Capuano, A.; Crisafulli, S.; Cutroneo, P.M.; Fantini, M.P.; Ferrajolo, C.; Moretti, U.; Poluzzi, E.; Raschi, E.; et al. Ilmiovaccino COVID19 collaborating group. COVID-19 Vaccination in Pregnancy, Paediatrics, Immunocompromised Patients, and Persons with History of Allergy or Prior SARS-CoV-2 Infection: Overview of Current Recommendations and Pre- and Post-Marketing Evidence for Vaccine Efficacy and Safety. Drug. Saf. 2021, 5, 1–23. [Google Scholar]
- Negahdaripour, M.; Shafiekhani, M.; Moezzi, S.M.; Amiri, S.; Rasekh, S.; Bagheri, A.; Mosaddeghi, P.; Vazin, A. Administration of COVID-19 vaccines in immunocompromised patients. Int. Immunopharmacol. 2021, 99, 108021. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef]
- Bettini, E.; Locci, M. SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond. Vaccines 2021, 9, 147. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, S.M.; O’Shea, K.J.; Wedlock, P.T.; Strych, U.; Fergusons, M.C.; Bottazzi, M.E.; Randall, S.L.; Siegmund, S.S.; Cox, S.N.; Hotez, P.J.; et al. The Benefits of Vaccinating with the First Available COVID-19 Coronavirus Vaccine. Am. J. Prev. Med. 2021, 60, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Zdziarski, K.; Landowski, M.; Zabielska, P.; Karakiewicz, B. Subjective Feelings of Polish Doctors after Receiving the COVID-19 Vaccine. Int. J. Environ. Res. Public Health. 2021, 10, 6291. [Google Scholar] [CrossRef]
- Freeman, D.; Loe, B.S.; Chadwick, A.; Vaccari, C.; Waite, F.; Rosebrock, L.; Jenner, L.; Petit, A.; Lewandowsky, S.; Vanderslott, S.; et al. COVID-19 vaccine hesitancy in the UK: The Oxford coronavirus explanations, attitudes, and narratives survey (Oceans) II. Psychol. Med. 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pataka, A.; Kotoulas, S.; Stefanidou, E.; Grigoriou, I.; Tzinas, A.; Tsiouprou, I.; Zarogoulidis, P.; Courcoutsakis, N.; Argyropoulou, P. Acceptability of Healthcare Professionals to Get Vaccinated against COVID-19 Two Weeks before Initiation of National Vaccination. Medicina 2021, 57, 611. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 28, NEJMoa2109072. [Google Scholar] [CrossRef]
- Li, M.; Luo, Y.; Watson, R.; Zheng, Y.; Ren, J.; Tang, J.; Chen, Y. Healthcare workers’ (HCWs) attitudes and related factors towards COVID-19 vaccination: A rapid systematic review. Postgrad. Med. J. 2021, 30. [Google Scholar] [CrossRef] [PubMed]
- Di Gennaro, F.; Murri, R.; Segala, F.V.; Cerruti, L.; Abdulle, A.; Saracino, A.; Bavaro, D.F.; Fantoni, M. Attitudes towards Anti-SARS-CoV2 Vaccination among Healthcare Workers: Results from a National Survey in Italy. Viruses 2021, 26, 371. [Google Scholar] [CrossRef] [PubMed]
- Kateeb, E.; Danadneh, M.; Pokorná, A.; Klugarová, J.; Abdulqader, H.; Klugar, M.; Riad, A. Predictors of Willingness to Receive COVID-19 Vaccine: Cross-Sectional Study of Palestinian Dental Students. Vaccines 2021, 9, 954. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Huang, Y.; Abdulqader, H.; Morgado, M.; Domnori, S.; Koščík, M.; Mendes, J.J.; Klugar, M.; Kateeb, E.; IADS-SCORE. Universal Predictors of Dental Students’ Attitudes towards COVID-19 Vaccination: Machine Learning-Based Approach. Vaccines 2021, 9, 1158. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.H.; Schaub, J.P.; Nagata, J.M.; Park, M.J.; Brindis, C.D.; Irwin, C.E., Jr. Young Adult Perspectives on COVID-19 Vaccinations. J. Adolesc. Health 2021, 69, 511–514. [Google Scholar] [CrossRef]
- Riad, A.; Abdulqader, H.; Morgado, M.; Domnori, S.; Koščík, M.; Mendes, J.J.; Klugar, M.; Kateeb, E.; on behalf of IADS-SCORE. Global Prevalence and Drivers of Dental Students’ COVID-19 Vaccine Hesitancy. Vaccines 2021, 9, 566. [Google Scholar] [CrossRef]
- Baumeister, A.; Chakraverty, D.; Aldin, A.; Seven, Ü.S.; Skoetz, N.; Kalbe, E.; Woopen, C. “The system has to be health literate, too”—Perspectives among healthcare professionals on health literacy in transcultural treatment settings. BMC Health Serv. Res. 2021, 21, 716. [Google Scholar] [CrossRef]
- Lo Re, V.; Klungel, O.H.; Chan, K.A.; Panozzo, C.A.; Zhou, W.; Winterstein, A.G. Global covid-19 vaccine rollout and safety surveillance—How to keep pace. BMJ 2021, 373, 1416. [Google Scholar] [CrossRef]

| Variable | Outcome | mRNA-Based (n = 187) | Viral Vector-Based (n = 60) | Total (n = 247) |
|---|---|---|---|---|
| Gender | Female | 148 (79.1%) | 49 (81.7%) | 197 (79.8%) |
| Male | 39 (20.9%) | 11 (18.3%) | 50 (20.2%) | |
| Prefer not to disclose | 0 (0%) | 0 (0%) | 0 (0%) | |
| Age | ≤29 years-old | 100 (53.5%) | 52 (86.7%) | 152 (61.5%) |
| >29 years-old | 87 (46.5%) | 8 (13.3%) | 95 (38.5%) | |
| Profession | Physician | 30 (16%) | 1 (1.7%) | 31 (12.6%) |
| Dentist | 27 (14.4%) | 7 (11.7%) | 34 (13.8%) | |
| Nurse | 9 (4.8%) | 1 (1.7%) | 10 (4%) | |
| Midwife | 10 (5.3%) | 0 (0%) | 10 (4%) | |
| Laboratory Worker | 12 (6.4%) | 2 (3.3%) | 14 (5.7%) | |
| Physiotherapist | 2 (1.1%) | 1 (1.7%) | 3 (1.2%) | |
| Pharmacist | 3 (1.6%) | 0 (0%) | 3 (1.2%) | |
| Psychologist | 1 (0.5%) | 1 (1.7%) | 2 (0.8%) | |
| Student | 76 (50.6%) | 42 (70%) | 118 (47.8%) | |
| Dietician | 3 (1.6%) | 1 (1.7%) | 4 (1.6%) | |
| Other | 14 (7.5%) | 4 (6.7%) | 18 (7.3%) | |
| Region | Wielkopolskie | 0 (0%) | 2 (3.3%) | 2 (0.8%) |
| Kujawsko-Pomorskie | 1 (0.5%) | 0 (0%) | 1 (0.4%) | |
| Małopolskie | 10 (5.3%) | 14 (23.3%) | 24 (9.7%) | |
| Łódzkie | 4 (2.1%) | 0 (0%) | 4 (1.6%) | |
| Dolnośląskie | 6 (3.2%) | 2 (3.3%) | 8 (3.2%) | |
| Lubelskie | 0 (0%) | 1 (1.7%) | 1 (0.4%) | |
| Lubuskie | 2 (1.1%) | 0 (0%) | 2 (0.8%) | |
| Mazowieckie | 3 (1.6%) | 1 (1.7%) | 4 (1.6%) | |
| Opolskie | 2 (1.1%) | 1 (1.7%) | 3 (1.2%) | |
| Podlaskie | 1 (0.5%) | 0 (0%) | 1 (0.4%) | |
| Pomorskie | 0 (0%) | 2 (3.3%) | 2 (0.8%) | |
| Śląskie | 146 (78.1%) | 35 (58.3%) | 181 (73.3%) | |
| Podkarpackie | 7 (3.7%) | 1(1.7%) | 8 (3.2%) | |
| Swiętokrzystkie | 3 (1.6%) | 0 (0%) | 3 (1.2%) | |
| Warmińko-mazurskie | 2 (1.1%) | 0 (0%) | 2 (0.8%) | |
| Zachodnio-pomorskie | 0 (0%) | 1 (1.7%) | 1 (0.4%) |
| Variable | Outcome | mRNA-Based (n = 187) | Viral Vector-Based (n = 60) | Total (n = 247) | p |
|---|---|---|---|---|---|
| Chronic Illnesses | Asthma | 4 (2.1%) | 0 (0%) | 4 (1.6%) | 0.575 * |
| Blood Disease | 1 (0.5%) | 2 (3.3%) | 3 (1.2%) | 0.147 * | |
| Cancer | 0 (0%) | 0 (0%) | 0 (0%) | N/A | |
| Chronic Hypertension | 14 (7.5%) | 0 (0%) | 14 (5.7%) | 0.025 * | |
| COPD | 6 (3.2%) | 0 (0%) | 6 (2.4%) | 0.341 * | |
| Diabetes Mellitus I | 0 (0%) | 0 (0%) | 0 (0%) | N/A | |
| Diabetes Mellitus II | 1 (0.5%) | 0 (0%) | 0 (0%) | 1.000 * | |
| Psychological Distress | 10 (5.3%) | 1 (1.7%) | 11 (4.5%) | 0.304 * | |
| Rheumatoid Arthritis | 2 (1.1%) | 0 (0%) | 2 (0.8%) | 1.000 * | |
| Thyroid Disease | 21 (11.2%) | 11 (18.3%) | 32 (13%) | 0.154 | |
| Other | 5 (2.7%) | 1 (1.7%) | 6 (2.4%) | 1.000 * | |
| Total | 50 (26.7%) | 14 (23.3%) | 64 (25.9%) | 0.600 | |
| Medical Treatments | Antibiotics | 1 (0.5%) | 0 (0%) | 0 (0%) | 1.000 * |
| Anticoagulants | 2 (1.1%) | 1 (1.7%) | 3 (1.2%) | 1.000 * | |
| Antidepressants | 11 (5.9%) | 3 (5%) | 14 (5.7%) | 1.000 * | |
| Antidiabetics | 3 (1.6%) | 0 (0%) | 3 (1.2%) | 1.000 * | |
| Antihistamines | 12 (6.4%) | 4 (6.7%) | 16 (6.5%) | 1.000 * | |
| Antihypertensives | 12 (6.4%) | 0 (0%) | 12 (4.9%) | 0.043 * | |
| Cholesterol-lowering | 4 (2.1%) | 1 (1.7%) | 5 (2%) | 1.000 * | |
| Contraceptives | 29 (15.5%) | 14 (23.3%) | 43 (17.4%) | 0.164 | |
| Corticosteroids | 3 (1.6%) | 0 (0%) | 3 (1.2%) | 1.000 * | |
| Thyroid Hormone | 21 (11.2%) | 10 (16.7%) | 31 (21.6%) | 0.269 | |
| Other | 15 (8%) | 3 (5%) | 18 (7.3%) | 0.574 * | |
| Total | 79 (42.2%) | 27 (45%) | 106 (42.9%) | 0.708 |
| Variable | Outcome | mRNA-Based (n = 187) | Viral Vector-Based (n = 60) | Total (n = 247) | p |
|---|---|---|---|---|---|
| Doses | One | 11 (5.9%) | 45 (75%) | 56 (22.7%) | <0.001 |
| Two | 176 (94.1%) | 15 (25%) | 191 (77.3%) | <0.001 | |
| Infection | Before 1st Dose | 2 (1.1%) | 3 (5%) | 5 (2%) | 0.094 * |
| Before 2nd Dose | 1 (0.5%) | 0 (0%) | 1 (0.4%) | 1.000 * |
| Variable | Outcome | mRNA-Based (n = 187) | Viral Vector-Based (n = 60) | Total (n = 247) | p |
|---|---|---|---|---|---|
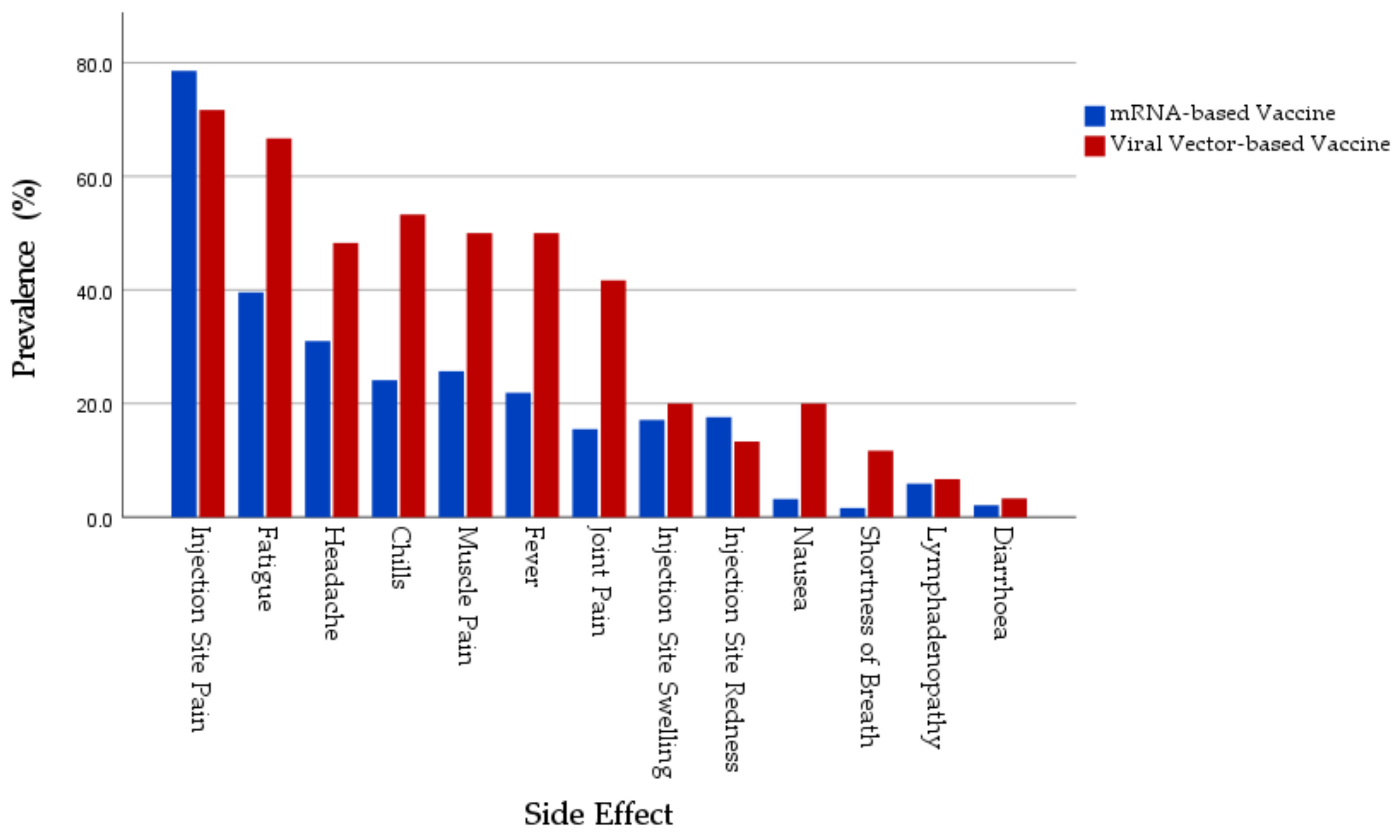
| Local SE Prevalence | Pain at injection site | 147 (78.6%) | 43 (71.7%) | 190 (76.9%) | 0.267 |
| Swelling at injection site | 32 (17.1%) | 12 (20%) | 44 (17.8%) | 0.611 | |
| Redness at injection site | 33 (17.6%) | 8 (13.3%) | 41 (16.6%) | 0.435 | |
| Total | 152 (81.3%) | 43 (71.7%) | 195 (78.9%) | 0.112 | |
| Local SE Onset | Only After 1st Dose | 44 (29.1%) | 37 (88.1%) | 81 (42%) | <0.001 |
| Only After 2nd Dose | 10 (6.6%) | 0 (0%) | 10 (5.2%) | 0.124 * | |
| After Both Doses | 97 (64.2%) | 5 (11.9%) | 102 (52.8%) | <0.001 | |
| Local SE Duration | 1 Day | 61 (40.4%) | 7 (16.7%) | 68 (35.2%) | 0.002 |
| 3 Days | 70 (46.4%) | 22 (52.4%) | 92 (47.7%) | 0.915 | |
| 5 Days | 14 (9.3%) | 4 (9.5%) | 18 (9.3%) | 1.000 * | |
| 1 Week | 2 (1.3%) | 7 (16.7%) | 9 (4.7%) | 0.001 * | |
| >1 Week | 4 (2.6%) | 2 (4.8%) | 6 (3.1%) | 0.635 * |
| Variable | Outcome | mRNA-Based (n = 187) | Viral Vector-Based (n = 60) | Total (n = 247) | p |
|---|---|---|---|---|---|
| Systemic SE Prevalence | Fatigue | 74 (39.6%) | 40 (66.7%) | 114 (46.2%) | <0.001 |
| Headache | 58 (31%) | 35 (58.3%) | 93 (37.7%) | <0.001 | |
| Muscle Pain | 48 (25.7%) | 30 (50%) | 78 (31.6%) | <0.001 | |
| Joint Pain | 29 (15.5%) | 25 (41.7%) | 54 (21.9%) | <0.001 | |
| Fever | 41 (21.9%) | 30 (50%) | 71 (28.7%) | <0.001 | |
| Chills | 45 (24.1%) | 32 (53.3%) | 77 (31.2%) | <0.001 | |
| Nausea | 6 (3.2%) | 12 (20%) | 18 (7.3%) | <0.001 * | |
| Diarrhoea | 4 (2.1%) | 2 (3.3%) | 6 (2.4%) | 0.635 * | |
| Lymphadenopathy | 11 (5.9%) | 4 (6.7%) | 15 (6.1%) | 0.763 * | |
| Shortness of Breath | 3 (1.6%) | 7 (11.7%) | 10 (4%) | 0.002 * | |
| Anaphylaxis | 0 (0%) | 0 (0%) | 0 (0%) | N/A | |
| Total | 104 (55.6%) | 46 (76.7%) | 150 (60.7%) | 0.004 | |
| Systemic SE Onset | Only After 1st Dose | 16 (15.7%) | 40 (93%) | 56 (38.6%) | <0.001 |
| Only After 2nd Dose | 46 (45.1%) | 0 (0%) | 46 (31.7%) | <0.001 | |
| After Both Doses | 40 (39.2%) | 3 (7%) | 43 (29.7%) | <0.001 | |
| Systemic SE Duration | 1 Day | 48 (47.1%) | 17 (39.5%) | 65 (44.8%) | 0.683 |
| 3 Days | 39 (38.2%) | 19 (44.2%) | 58 (40%) | 0.086 | |
| 5 Days | 3 (2.9%) | 1 (2.3%) | 4 (2.8%) | 1.000 * | |
| 1 Week | 4 (3.9%) | 1 (2.3%) | 5 (3.4%) | 1.000 * | |
| >1 Week | 3 (2.9%) | 4 (9.3%) | 7 (4.8%) | 0.061 * | |
| >1 Month | 5 (4.9%) | 1 (2.3%) | 6 (4.1%) | 1.000 * |
| Variable | Outcome | mRNA-Based (n = 187) | Viral Vector-Based (n = 60) | Total (n = 247) | p |
|---|---|---|---|---|---|
| Orofacial SE Prevalence | Oral Paraesthesia | 4 (2.1%) | 1 (1.7%) | 5 (2%) | 1.000 * |
| Dysgeusia | 3 (1.6%) | 3 (5%) | 6 (2.4%) | 0.156 * | |
| Oral Ulcers | 1 (0.5%) | 0 (0%) | 1 (0.4%) | 1.000 * | |
| Xerostomia | 1 (0.5%) | 1 (1.7%) | 2 (0.8%) | 0.428 * | |
| Facial Nerve Palsy | 0 (0%) | 0 (0%) | 0 (0%) | N/A | |
| Skin-related SE Prevalence | Skin Rash | 3 (1.6%) | 3 (5%) | 6 (2.4%) | 0.156 * |
| Skin Eruptions | 1 (0.5%) | 2 (3.3%) | 3 (1.2%) | 0.147 * | |
| Total | 10 (5.3%) | 8 (13.3%) | 18 (7.3%) | 0.048 * |
| Drug | mRNA-Based (n = 187) | Viral Vector-Based (n = 60) | Total (n = 247) | p |
|---|---|---|---|---|
| Paracetamol | 35 (18.7%) | 28 (46.7%) | 63 (25.5%) | <0.001 |
| Pyralgin | 6 (3.2%) | 3 (5%) | 9 (3.6%) | 0.457 * |
| Ibuprofen | 18 (9.6%) | 7 (11.7%) | 25 (10.1%) | 0.648 |
| Total | 61 (32.6%) | 34 (56.7%) | 95 (38.5%) | 0.001 |
| Variable | Outcome | mRNA-Based Vaccine | Viral Vector-Based Vaccine | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Female (n = 148) | Male (n = 39) | p | Female (n = 49) | Male (n = 11) | p | Female (n = 197) | Male (n = 50) | p | ||
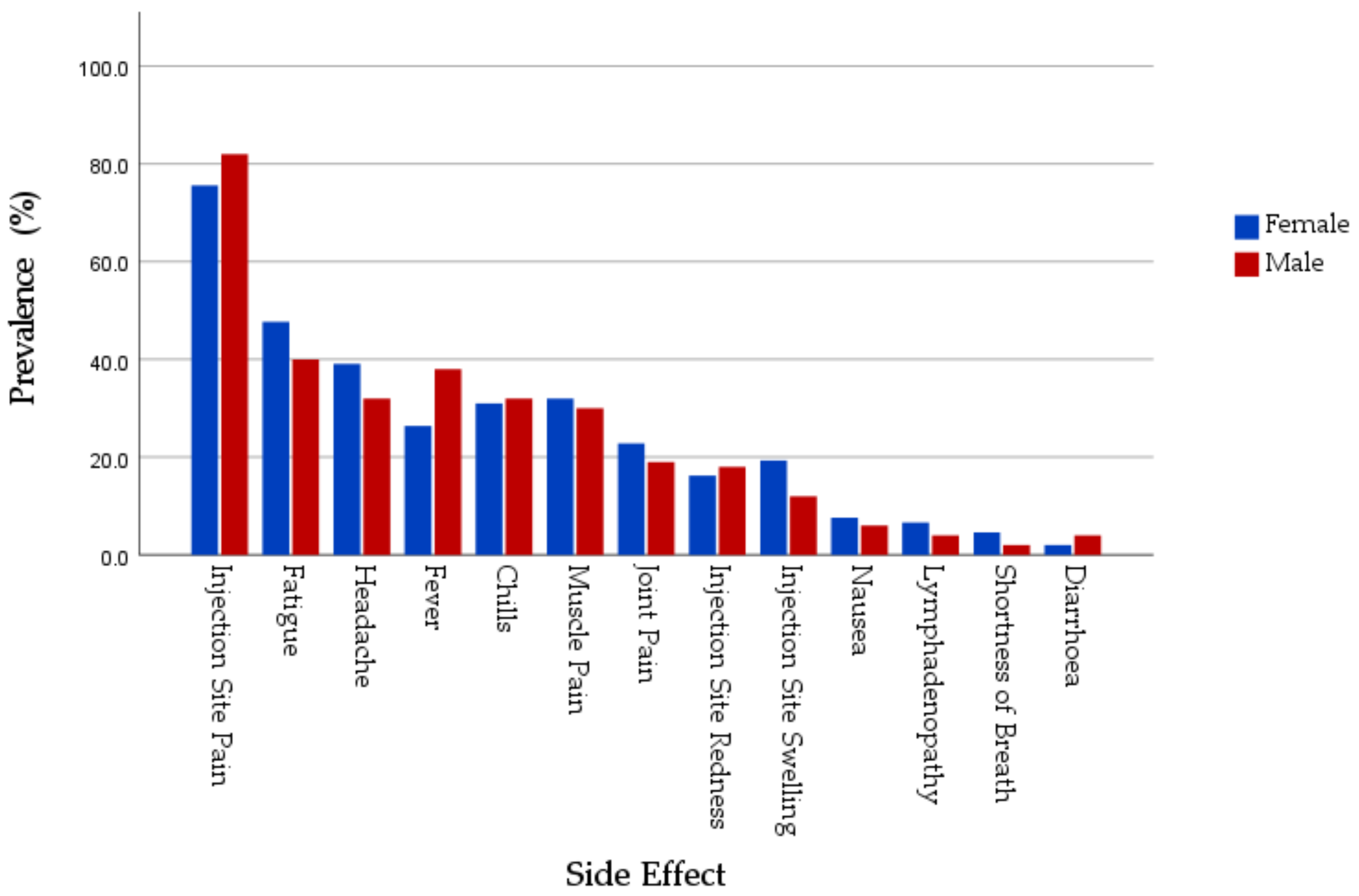
| Local SE Prevalence | Injection Site Pain | 114 (77%) | 33 (84.6%) | 0.304 | 35 (71.4%) | 8 (72.7%) | 1.000 * | 149 (75.6%) | 41 (82%) | 0.340 |
| Injection Site Swelling | 30 (20.3%) | 2 (5.1%) | 0.026 | 8 (16.3%) | 4 (36.4%) | 0.206 * | 38 (19.3%) | 6 (12%) | 0.229 | |
| Injection Site Redness | 27 (18.2%) | 6 (15.4%) | 0.677 | 5 (10.2%) | 3 (27.3%) | 0.154 * | 32 (16.2%) | 9 (18%) | 0.766 | |
| Total | 119 (80.4%) | 33 (84.6%) | 0.549 | 35 (71.4%) | 8 (72.7%) | 1.000 * | 154 (78.2%) | 41 (82%) | 0.553 | |
| Local SE Onset | Only After 1st Dose | 34 (28.6%) | 10 (31.3%) | 0.727 | 29 (85.3%) | 8 (100%) | 0.506 * | 63 (41.2%) | 18 (45%) | 0.589 |
| Only After 2nd Dose | 9 (7.6%) | 1 (3.1%) | 0.691 * | 0 (0%) | 0 (0%) | N/A | 9 (5.9%) | 1 (2.5%) | 0.692 * | |
| After Both Doses | 76 (63.9%) | 21 (65.6%) | 0.781 | 5 (14.7%) | 0 (0%) | 0.573 * | 81 (52.9%) | 21 (52.5%) | 0.910 | |
| Systemic SE Prevalence | Fatigue | 60 (40.5%) | 14 (35.9%) | 0.598 | 34 (69.4%) | 6 (54.5%) | 0.481 * | 94 (47.7%) | 20 (40%) | 0.328 |
| Headache | 48 (32.4%) | 10 (25.6%) | 0.415 | 29 (59.2%) | 6 (54.5%) | 1.000 * | 77 (39.1%) | 16 (32%) | 0.356 | |
| Muscle Pain | 39 (26.4%) | 9 (23.1%) | 0.677 | 24 (49%) | 6 (54.5%) | 0.739 | 63 (32%) | 15 (30%) | 0.788 | |
| Joint Pain | 23 (15.5%) | 6 (15.4%) | 0.981 | 22 (44.9%) | 3 (27.3%) | 0.332 * | 45 (22.8%) | 9 (18%) | 0.459 | |
| Fever | 28 (18.9%) | 13 (33.3%) | 0.053 | 24 (49%) | 6 (54.5%) | 0.739 | 52 (26.4%) | 19 (38%) | 0.105 | |
| Chills | 24 (23%) | 11 (28.2%) | 0.496 | 27 (55.1%) | 5 (45.5%) | 0.562 | 61 (31%) | 16 (32%) | 0.888 | |
| Nausea | 4 (2.7%) | 2 (5.1%) | 0.606 * | 11 (22.4%) | 1 (9.1%) | 0.435 * | 15 (7.6%) | 3 (6%) | 1.000 * | |
| Diarrhoea | 3 (2%) | 1 (2.6%) | 1.000 * | 1 (2%) | 1 (9.1%) | 0.336 * | 4 (2%) | 2 (4%) | 0.351 * | |
| Lymphadenopathy | 9 (6.1%) | 2 (5.1%) | 1.000 * | 4 (8.2%) | 0 (0%) | 1.000 * | 13 (6.6%) | 2 (4%) | 0.742 * | |
| Shortness of Breath | 3 (2%) | 0 (0%) | 1.000 * | 6 (12.2%) | 1 (9.1%) | 1.000 * | 9 (4.6%) | 1 (2%) | 0.692 * | |
| Anaphylaxis | 0 (0%) | 0 (0%) | N/A | 0 (0%) | 0 (0%) | N/A | 0 (0%) | 0 (0%) | N/A | |
| Total | 80 (54.1%) | 24 (61.5%) | 0.403 | 40 (81.6%) | 6 (54.5%) | 0.107 * | 120 (60.9%) | 30 (60%) | 0.906 | |
| Systemic SE Onset | Only After 1st Dose | 12 (15.4%) | 4 (16.7%) | 0.747 | 35 (92.1%) | 5 (100%) | 0.155 * | 47 (40.5%) | 9 (31%) | 0.377 |
| Only After 2nd Dose | 34 (43.6%) | 12 (50%) | 0.315 | 0 (0%) | 0 (0%) | N/A | 34 (29.3%) | 12 (41.4%) | 0.274 | |
| After Both Doses | 32 (41%) | 8 (33.3%) | 0.881 | 3 (7.9%) | 0 (0%) | 1.000 * | 35 (30.2%) | 8 (27.6%) | 0.769 | |
| Orofacial SE Prevalence | Oral Paraesthesia | 4 (2.7%) | 0 (0%) | 0.581 * | 1 (2%) | 0 (0%) | 1.000 * | 5 (2.5%) | 0 (0%) | 0.586 * |
| Taste Disturbance | 2 (1.4%) | 1 (2.6%) | 0.506 * | 2 (4.1%) | 1 (9.1%) | 0.462 * | 4 (2%) | 2 (4%) | 0.351 * | |
| Oral Ulcers | 1 (0.7%) | 0 (0%) | 1.000 * | 0 (0%) | 0 (0%) | N/A | 1 (0.5%) | 0 (0%) | 1.000 * | |
| Xerostomia | 1 (0.7%) | 0 (0%) | 1.000 * | 0 (0%) | 1 (9.1%) | 0.183 * | 1 (0.5%) | 1 (2%) | 0.365 * | |
| Facial Nerve Palsy | 0 (0%) | 0 (0%) | N/A | 0 (0%) | 0 (0%) | N/A | 0 (0%) | 0 (0%) | N/A | |
| Skin-related SE Prevalence | Skin Rash | 3 (2%) | 0 (0%) | 1.000 * | 3 (6.1%) | 0 (0%) | 1.000 * | 6 (3%) | 0 (0%) | 0.604 * |
| Skin Eruptions | 1 (0.7%) | 0 (0%) | 1.000 * | 2 (4.1%) | 0 (0%) | 1.000 * | 3 (1.5%) | 0 (0%) | 1.000 * | |
| Total | 9 (6.1%) | 1 (2.6%) | 0.691 * | 6 (12.2%) | 2 (18.2%) | 0.631 * | 15 (7.6%) | 3 (6%) | 1.000 * | |
| Palliative Drugs | Paracetamol | 30 (20.3%) | 5 (12.8%) | 0.289 | 24 (49%) | 4 (36.4%) | 0.448 | 54 (27.4%) | 9 (18%) | 0.173 |
| Pyralgina | 5 (3.4%) | 1 (2.6%) | 1.000 * | 3 (6.1%) | 0 (0%) | 1.000 * | 8 (4.1%) | 1 (2%) | 0.691 * | |
| Ibuprofen | 16 (10.8%) | 2 (5.1%) | 0.373 * | 6 (12.2%) | 1 (9.1%) | 1.000 * | 22 (11.2%) | 3 (6%) | 0.279 | |
| Total | 51 (34.5%) | 10 (25.6%) | 0.296 | 29 (59.2%) | 5 (45.5%) | 0.507 * | 80 (40.6%) | 15 (30%) | 0.168 | |
| Variable | Outcome | mRNA-Based Vaccine | Viral Vector-Based Vaccine | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤29 yo (n = 100) | >29 yo (n = 87) | p | ≤29 yo (n = 52) | >29 yo (n = 8) | p | ≤29 yo (n = 152) | >29 yo (n = 92) | p | ||
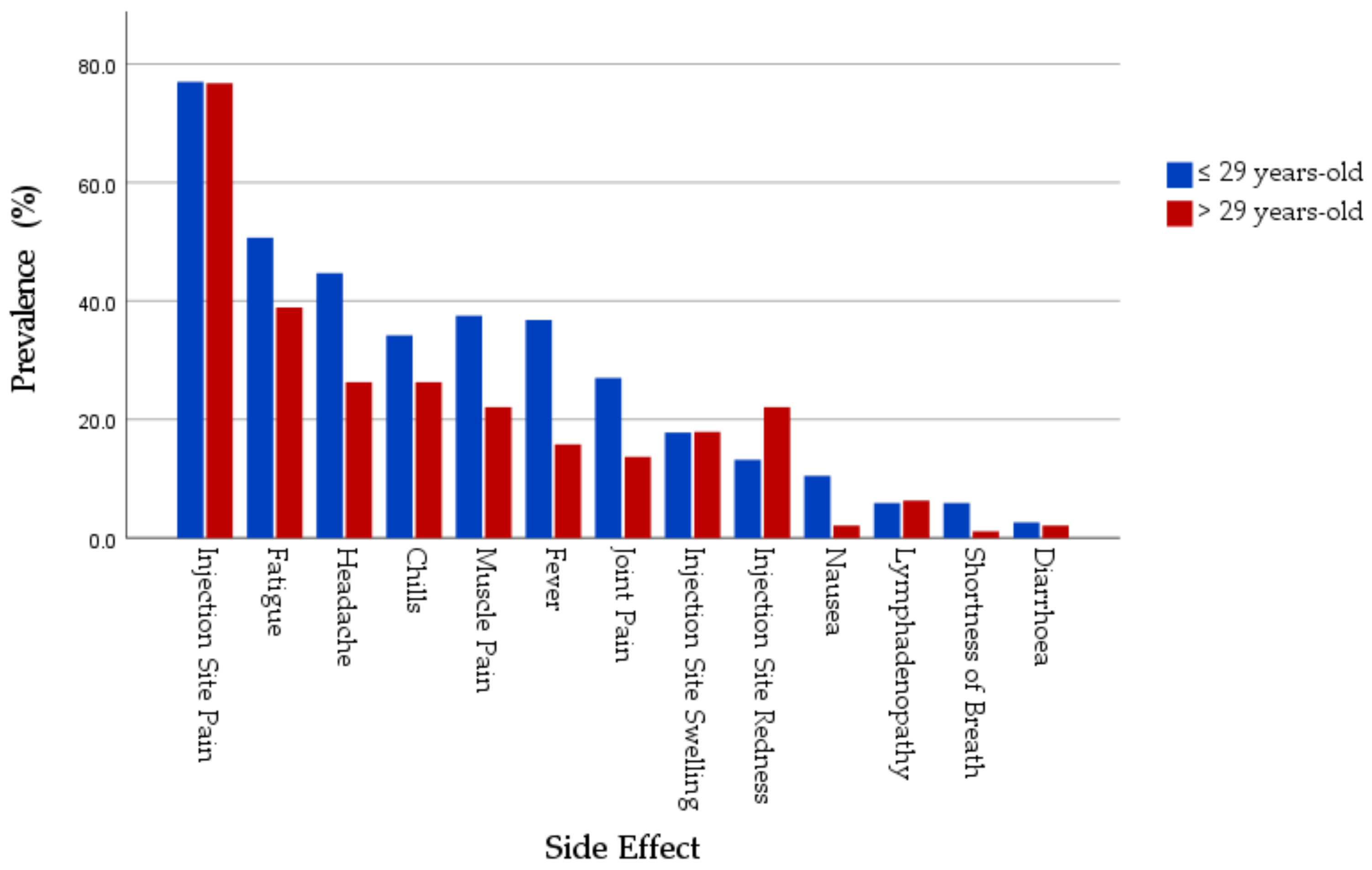
| Local SE Prevalence | Injection Site Pain | 80 (80%) | 67 (77%) | 0.619 | 37 (71.2%) | 6 (75%) | 1.000 * | 117 (77%) | 73 (76.8%) | 0.981 |
| Injection Site Swelling | 18 (18%) | 14 (16.1%) | 0.730 | 9 (17.3%) | 3 (37.5%) | 0.191 * | 27 (17.8%) | 17 (17.9%) | 0.979 | |
| Injection Site Redness | 15 (15%) | 18 (20.7%) | 0.309 | 5 (9.6%) | 3 (37.5%) | 0.065 * | 20 (13.2%) | 21 (22.1%) | 0.066 | |
| Total | 83 (83%) | 69 (79.3%) | 0.519 | 37 (71.2%) | 6 (75%) | 1.000 * | 120 (78.9%) | 75 (78.9%) | 1.000 | |
| Local SE Onset | Only After 1st Dose | 20 (24.4%) | 24 (34.8%) | 0.223 | 31 (86.1%) | 6 (100%) | 0.698 * | 51 (43.2%) | 30 (40%) | 0.748 |
| Only After 2nd Dose | 3 (3.7%) | 7 (10.1%) | 0.192 * | 0 (0%) | 0 (0%) | N/A | 3 (2.5%) | 7 (9.3%) | 0.048 * | |
| After Both Doses | 59 (72%) | 38 (55.1%) | 0.036 | 5 (13.9%) | 0 (0%) | 1.000 * | 64 (54.2%) | 38 (50.7%) | 0.744 | |
| Systemic SE Prevalence | Fatigue | 41 (41%) | 33 (37.9%) | 0.669 | 36 (69.2%) | 4 (50%) | 0.422 * | 77 (50.7%) | 37 (38.9%) | 0.072 |
| Headache | 37 (37%) | 21 (24.1%) | 0.058 | 31 (59.6%) | 4 (50%) | 0.708 * | 68 (44.7%) | 25 (26.3%) | 0.004 | |
| Muscle Pain | 29 (29%) | 19 (21.8%) | 0.263 | 28 (53.8%) | 2 (25%) | 0.254 * | 57 (37.5%) | 21 (22.1%) | 0.011 | |
| Joint Pain | 17 (17%) | 12 (13.8%) | 0.546 | 24 (46.2%) | 1 (12.5%) | 0.123 * | 41 (27%) | 13 (13.7%) | 0.014 | |
| Fever | 27 (27%) | 14 (16.1%) | 0.072 | 29 (55.8%) | 1 (12.5%) | 0.052 * | 56 (36.8%) | 15 (15.8%) | <0.001 | |
| Chills | 24 (24%) | 21 (24.1%) | 0.982 | 28 (53.8%) | 4 (50%) | 1.000 * | 52 (34.2%) | 25 (26.3%) | 0.193 | |
| Nausea | 4 (4%) | 2 (2.3%) | 0.687 * | 12 (23.1%) | 0 (0%) | 0.338 * | 16 (10.5%) | 2 (2.1%) | 0.013 | |
| Diarrhoea | 2 (2%) | 2 (2.3%) | 1.000 * | 2 (3.8%) | 0 (0%) | 1.000 * | 4 (2.6%) | 2 (2.1%) | 1.000 * | |
| Lymphadenopathy | 5 (5%) | 6 (6.9%) | 0.582 | 4 (7.7%) | 0 (0%) | 1.000 * | 9 (5.9%) | 6 (6.3%) | 0.899 | |
| Shortness of Breath | 2 (2%) | 1 (1.1%) | 1.000 * | 7 (13.5%) | 0 (0%) | 0.578 * | 9 (5.9%) | 1 (1.1%) | 0.094 * | |
| Anaphylaxis | 0 (0%) | 0 (0%) | N/A | 0 (0%) | 0 (0%) | N/A | 0 (0%) | 0 (0%) | N/A | |
| Total | 59 (59%) | 45 (51.7%) | 0.318 | 41 (78.8%) | 5 (62.5%) | 0.374 * | 100 (65.8%) | 50 (52.6%) | 0.039 | |
| Systemic SE Onset | Only After 1st Dose | 13 (22.4%) | 3 (6.8%) | 0.020 | 37 (94.9%) | 3 (75%) | 0.103 * | 50 (51.5%) | 6 (12.5%) | <0.001 |
| Only After 2nd Dose | 23 (39.7%) | 23 (52.3%) | 0.586 | 0 (0%) | 0 (0%) | N/A | 23 (23.7%) | 23 (47.9%) | 0.075 | |
| After Both Doses | 22 (37.9%) | 18 (40.9%) | 0.827 | 2 (5.1%) | 1 (25%) | 0.354 * | 24 (24.7%) | 19 (39.6%) | 0.396 | |
| Orofacial SE Prevalence | Oral Paraesthesia | 2 (2%) | 2 (2.3%) | 1.000 * | 0 (0%) | 1 (12.5%) | 0.133 * | 2 (1.3%) | 3 (3.2%) | 0.376 * |
| Taste Disturbance | 2 (2%) | 1 (1.1%) | 1.000 * | 3 (5.8%) | 0 (0%) | 1.000 * | 5 (3.3%) | 1 (1.1%) | 0.411 * | |
| Oral Ulcers | 0 (0%) | 1 (1.1%) | 0.465 * | 0 (0%) | 0 (0%) | N/A | 0 (0%) | 1 (1.1%) | 0.385 * | |
| Xerostomia | 1 (1%) | 0 (0%) | 1.000 * | 1 (1.9%) | 0 (0%) | 1.000 * | 2 (1.3%) | 0 (0%) | 0.525 * | |
| Facial Nerve Palsy | 0 (0%) | 0 (0%) | N/A | 0 (0%) | 0 (0%) | N/A | 0 (0%) | 0 (0%) | N/A | |
| Skin-related SE Prevalence | Skin Rash | 3 (3%) | 0 (0%) | 0.250 * | 3 (5.8%) | 0 (0%) | 1.000 * | 6 (3.9%) | 0 (0%) | 0.085 * |
| Skin Eruptions | 1 (1%) | 0 (0%) | 1.000 * | 2 (3.8%) | 0 (0%) | 1.000 * | 3 (2%) | 0 (0%) | 0.287 * | |
| Total | 8 (8%) | 2 (2.3%) | 0.108 * | 7 (13.5%) | 1 (12.5%) | 1.000 * | 15 (9.9%) | 3 (3.2%) | 0.048 | |
| Palliative Drugs | Paracetamol | 21 (21%) | 14 (16.1%) | 0.391 | 24 (46.2%) | 4 (50%) | 1.000 * | 45 (29.6%) | 18 (18.9%) | 0.062 |
| Pyralgina | 3 (3%) | 3 (3.4%) | 1.000 * | 3 (5.8%) | 0 (0%) | 1.000 * | 6 (3.9%) | 3 (3.2%) | 1.000 * | |
| Ibuprofen | 9 (9%) | 9 (10.3%) | 0.756 | 7 (13.5%) | 0 (0%) | 0.578 * | 16 (10.5%) | 9 (9.5%) | 0.790 | |
| Total | 34 (34%) | 27 (31%) | 0.666 | 30 (57.7%) | 4 (50%) | 0.717 * | 64 (42.1%) | 31 (32.6%) | 0.137 | |
| mRNA-Based Vaccine | Viral Vector-Based Vaccine | Total | ||||
|---|---|---|---|---|---|---|
| Predictor | Local Side EffectsOR (CI 95%) | Systemic Side Effects OR (CI 95%) | Local Side Effects OR (CI 95%) | Systemic Side Effects OR (CI 95%) | Local Side Effects OR (CI 95%) | Systemic Side Effects OR (CI 95%) |
| Gender: Female | 0.746 (0.286–1.948) | 0.735 (0.357–1.513) | 0.938 (0.217–4.055) | 3.704 (0.923–14.866) | 0.786 (0.354–1.744) | 1.039 (0.551–1.959) |
| Age: ≤29 years-old | 1.274 (0.610–2.658) | 1.343 (0.752–2.397) | 0.822 (0.149–4.542) | 2.236 (0.461–10.841) | 1.000 (0.533–1.875) | 1.731 (1.025–2.923) |
| Chronic Illness: Yes | 1.067 (0.461–2.468) | 1.022 (0.532–1.961) | 2.903 (0.575–14.654) | 2.118 (0.413–10.865) | 1.391 (0.666–2.906) | 1.106 (0.615–1.988) |
| Medication: Yes | 1.299 (0.609–2.769) | 1.099 (0.613–1.972) | 3.737 (1.049–13.319) | 1.650 (0.479–5.684) | 1.729 (0.908–3.292) | 1.200 (0.715–2.015) |
| Doses: One | 2.394 (0.296–19.347) | 0.648 (0.191–2.203) | 1.375 (0.390–4.850) | 1.273 (0.332–4.875) | 0.849 (0.416–1.732) | 1.841 (0.964–3.515) |
| Palliative Drug: Yes | 4.650 (1.561–13.856) | 18.200 (6.811–48.636) | 12.056 (2.932–49.569) | 33.000 (3.911–278.471) | 5.287 (2.272–12.306) | 22.128 (9.105–53.779) |
| CoVasST Study (N) | SRAEs (Total %) Local/Systemic | mRNA Vaccine | Vector-Based Vaccine | mRNA | Vector-Based | One Dose (%) Two Doses (%) | ||
|---|---|---|---|---|---|---|---|---|
| Local (%) | Systemic (%) | Local (%) | Systemic (%) | Female: Local/Systemic (%) Male: Local/Systemic (%) | Female: Local/Systemic (%) Male: Local/Systemic (%) | |||
| Czech Republic (877) | 91.79 82.90 | 91.7 | 82.90 | - | - | 90.70/82.49 95.29/74.12 | - - | 6.4 93.6 |
| Slovakia (522) | 85.82 70.50 | 85.82 | 70.50 | - | - | 88.06/75.62 78.33/53.33 | - - | n/s |
| Germany (474) | n/s | 78.27 | 60.97 | 70.40 | 87.20 | 78.22/62.75 77.87/55.74 | 77.38/91.67 56.10/78.05 | 28.4 71.6 |
| Poland (247) | n/s | 81.3 | 55.6 | 71.7 | 76.7 | 80.4/54.1 84.6/61.5 | 71.4/81.6 72.7/54.5 | 22.7 77.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziedzic, A.; Riad, A.; Attia, S.; Klugar, M.; Tanasiewicz, M. Self-Reported Adverse Events of COVID-19 Vaccines in Polish Healthcare Workers and Medical Students. Cross-Sectional Study and Pooled Analysis of CoVaST Project Results in Central Europe. J. Clin. Med. 2021, 10, 5338. https://doi.org/10.3390/jcm10225338
Dziedzic A, Riad A, Attia S, Klugar M, Tanasiewicz M. Self-Reported Adverse Events of COVID-19 Vaccines in Polish Healthcare Workers and Medical Students. Cross-Sectional Study and Pooled Analysis of CoVaST Project Results in Central Europe. Journal of Clinical Medicine. 2021; 10(22):5338. https://doi.org/10.3390/jcm10225338
Chicago/Turabian StyleDziedzic, Arkadiusz, Abanoub Riad, Sameh Attia, Miloslav Klugar, and Marta Tanasiewicz. 2021. "Self-Reported Adverse Events of COVID-19 Vaccines in Polish Healthcare Workers and Medical Students. Cross-Sectional Study and Pooled Analysis of CoVaST Project Results in Central Europe" Journal of Clinical Medicine 10, no. 22: 5338. https://doi.org/10.3390/jcm10225338
APA StyleDziedzic, A., Riad, A., Attia, S., Klugar, M., & Tanasiewicz, M. (2021). Self-Reported Adverse Events of COVID-19 Vaccines in Polish Healthcare Workers and Medical Students. Cross-Sectional Study and Pooled Analysis of CoVaST Project Results in Central Europe. Journal of Clinical Medicine, 10(22), 5338. https://doi.org/10.3390/jcm10225338










