Are Aortic Root and Ascending Aorta Diameters Measured by the Pediatric versus the Adult American Society of Echocardiography Guidelines Interchangeable?
Abstract
:1. Introduction
2. Materials and Methods
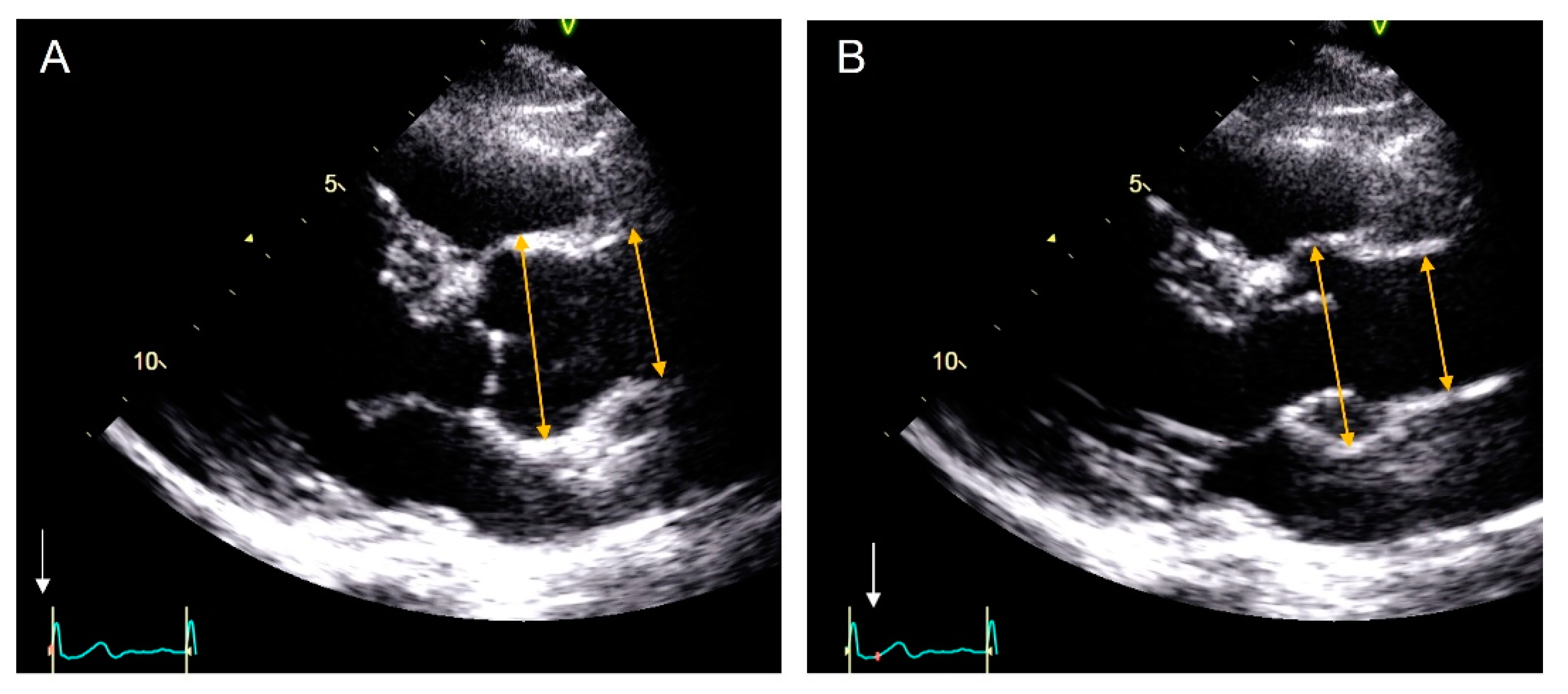
2.1. Echocardiography
2.2. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Median Aortic Diameters and Mean of the Differences Using Both Techniques
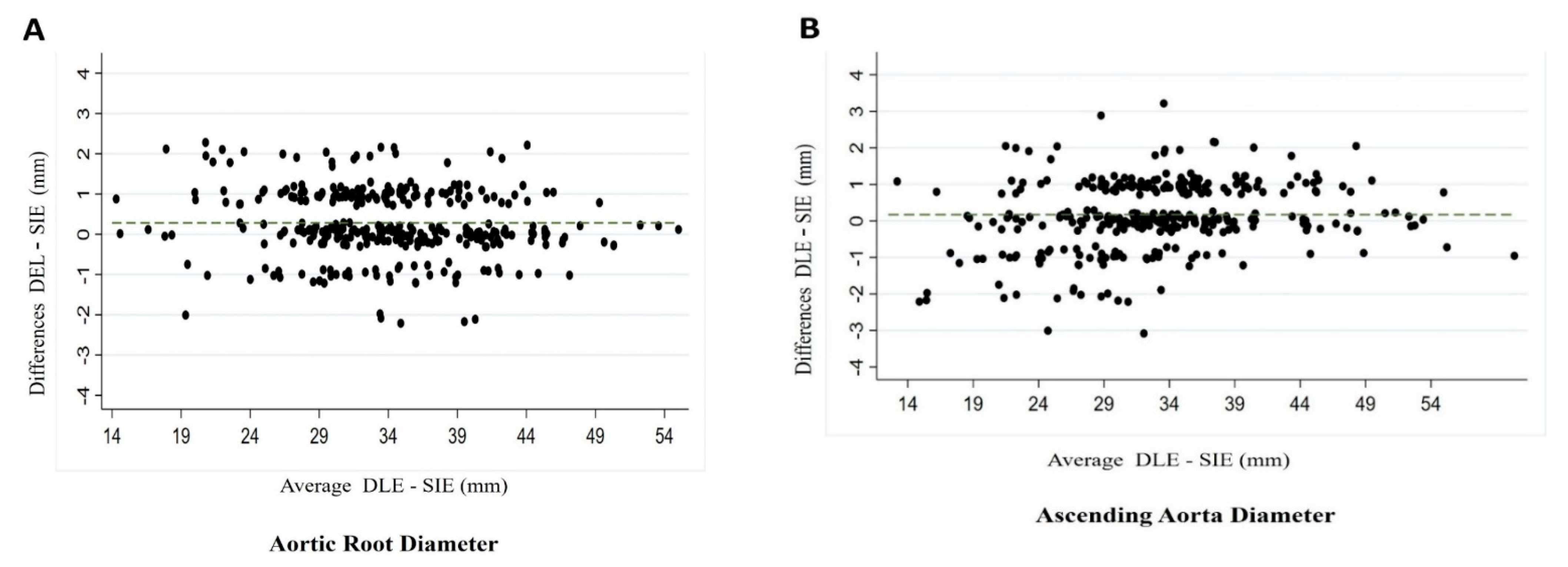
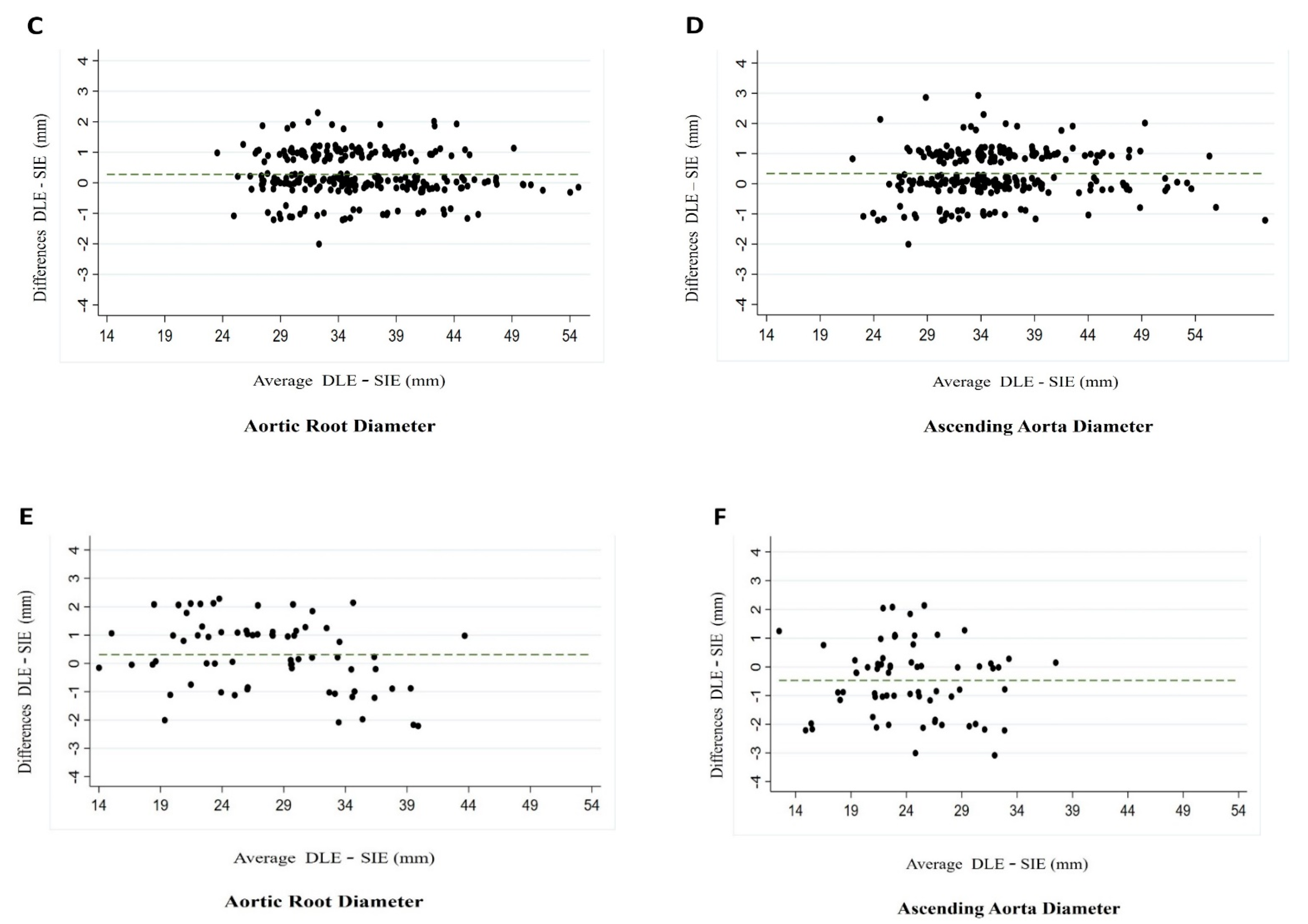
3.3. Intraobserver and Interobserver Variability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DLE | Diastolic leading edge |
| SIE | Systolic inner edge |
| TTE | Transthoracic echocardiography |
| CT | Computed tomography |
| CMR | Cardiac magnetic resonance |
References
- Goldstein, S.A.; Evangelista, A.; Abbara, S.; Arai, A.; Asch, F.M.; Badano, L.P.; Bolen, M.A.; Connolly, H.M.; Cuéllar-Calàbria, H.; Czerny, M.; et al. Multimodality imaging of diseases of the thoracic aorta in adults: From the American Society of Echocardiography and the European Association of Cardiovascular Imaging: Endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2015, 28, 119–182. [Google Scholar] [CrossRef] [PubMed]
- Campens, L.; Demulier, L.; De Groote, K.; Vandekerckhove, K.; De Wolf, D.; Roman, M.J.; Devereux, R.B.; De Paepe, A.; De Backer, J. Reference values for echocardiographic assessment of the diameter of the aortic root and ascending aorta spanning all age categories. Am. J. Cardiol. 2014, 114, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, L.; Colan, S.D.; Frommelt, P.C.; Ensing, G.J.; Kendall, K.; Younoszai, A.K.; Lai, W.W.; Geva, T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: A report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J. Am. Soc. Echocardiogr. 2010, 23, 465–495. [Google Scholar] [CrossRef] [PubMed]
- Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Bersin, R.M.; Carr, V.F.; Casey, D.E., Jr.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; Kazerooni, E.A.; et al. ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J. Am. Coll. Cardiol. 2010, 55, e27–e129, Erratum in 2013, 62, 1039–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saura, D.; Dulgheru, R.; Caballero, L.; Bernard, A.; Kou, S.; Gonjilashvili, N.; Athanassopoulos, G.D.; Barone, D.; Baroni, M.; Cardim, N.; et al. Two-dimensional transthoracic echocardiographic normal reference ranges for proximal aorta dimensions: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 167–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bossone, E.; Yuriditsky, E.; Desale, S.; Ferrara, F.; Vriz, O.; Asch, F.M. Normal Values and Differences in Ascending Aortic Diameter in a Healthy Population of Adults as Measured by the Pediatric versus Adult American Society of Echocardiography Guidelines. J. Am. Soc. Echocardiogr. 2016, 29, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Son, M.K.; Chang, S.A.; Kwak, J.H.; Lim, H.J.; Park, S.J.; Choi, J.O.; Lee, S.C.; Park, S.W.; Kim, D.K.; Oh, J.K. Comparative measurement of aortic root by transthoracic echocardiography in normal Korean population based on two different guidelines. Cardiovasc. Ultrasound 2013, 11, 28. [Google Scholar] [CrossRef] [Green Version]
- Tsang, J.F.; Lytwyn, M.; Farag, A.; Zeglinski, M.; Wallace, K.; da Silva, M.; Bohonis, S.; Walker, J.R.; Tam, J.W.; Strzelczyk, J.; et al. Multimodality imaging of aortic dimensions: Comparison of transthoracic echocardiography with multidetector row computed tomography. Echocardiography 2012, 29, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Boraita, A.; Heras, M.E.; Morales, F.; Marina-Breysse, M.; Canda, A.; Rabadan, M.; Barriopedro, M.I.; Varela, A.; de la Rosa, A.; Tuñón, J. Reference Values of Aortic Root in Male and Female White Elite Athletes According to Sport. Circ. Cardiovasc. Imaging 2016, 9, e005292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, B.T.; Kwon, A.; Scalia, G.M. The New Dimension in Aortic Measurements—Use of the Inner Edge Measurement for the Thoracic Aorta in Australian Patients. Heart Lung Circ. 2015, 24, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Kabirdas, D.; Scridon, C.; Brenes, J.C.; Hernandez, A.V.; Novaro, G.M.; Asher, C.R. Accuracy of transthoracic echocardiography for the measurement of the ascending aorta: Comparison with transesophageal echocardiography. Clin. Cardiol. 2010, 33, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Mirea, O.; Maffessanti, F.; Gripari, P.; Tamborini, G.; Muratori, M.; Fusini, L.; Claudia, C.; Fiorentini, C.; Plesea, I.E.; Pepi, M. Effects of aging and body size on proximal and ascending aorta and aortic arch: Inner edge-to-inner edge reference values in a large adult population by two-dimensional transthoracic echocardiography. J. Am. Soc. Echocardiogr. 2013, 26, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Maffessanti, F.; Kocabay, G.; Peluso, D.; Dal Bianco, L.; Piasentini, E.; Jose, S.P.; Iliceto, S.; Badano, L.P. Ascending aorta diameters measured by echocardiography using both leading edge-to-leading edge and inner edge-to-inner edge conventions in healthy volunteers. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 415–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bons, L.R.; Duijnhouwer, A.L.; Boccalini, S.; van den Hoven, A.T.; van der Vlugt, M.J.; Chelu, R.G.; McGhie, J.S.; Kardys, I.; van den Bosch, A.E.; Siebelink, H.J.; et al. Intermodality variation of aortic dimensions: How, where and when to measure the ascending aorta. Int. J. Cardiol. 2019, 276, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J.P.; Petersen, E.; Nikorowitsch, J.; Senftinger, J.; Sinning, C.; Theissen, M.; Petersen, J.; Reichenspurner, H.; Girdauskas, E. Transthoracic echocardiographic reference values of the aortic root: Results from the Hamburg City Health Study. Int. J. Cardiovasc. Imaging 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Palomares, J.F.; Teixidó-Tura, G.; Galuppo, V.; Cuéllar, H.; Laynez, A.; Gutiérrez, L.; González-Alujas, M.T.; García-Dorado, D.; Evangelista, A. Multimodality Assessment of Ascending Aortic Diameters: Comparison of Different Measurement Methods. J. Am. Soc. Echocardiogr. 2016, 29, 819–826.e4. [Google Scholar] [CrossRef] [PubMed]
- Blondheim, D.S.; Vassilenko, L.; Glick, Y.; Asif, A.; Nachtigal, A.; Meisel, S.R.; Shochat, M.; Shotan, A.; Zeina, A.R. Aortic dimensions by multi-detector computed tomography vs. echocardiography. J Cardiol. 2016, 67, 365–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, S.P.; Monaghan, M.J. Tissue harmonic imaging for standard left ventricular measurements: Fundamentally flawed? Eur. J. Echocardiogr. 2006, 7, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prihadi, E.; Delgado, V. Multimodality Imaging of the Aorta: Implications for Patient Surveillance. J. Am. Soc. Echocardiogr. 2016, 29, 838–841. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Adults (n = 260) | Pediatrics (n = 68) | Total Population (n = 328) | p | |
|---|---|---|---|---|---|
| Age (year) | 65.18 (51.78;75.26) | 10.88 (5.92;14.12) | 59.86 (27.88;72.21) | <0.001 | |
| Male | 144 (55.4%) | 45 (66.2%) | 189 (57.6%) | 0.11 | |
| Diagnosis of Inherited Diseases | Marfan Syndrome | 67 (25.76%) | 31 (45.59%) | 98 (29.88%) | |
| Loeys-Dietz/ Ehler-Danlos Syndrome | 19 (7.31%) | 4 (5.88%) | 23 (7.01%) | ||
| Familial aortopathy | 21 (8.08%) | 5 (7.35%) | 26 (7.93%) | ||
| Bicuspid aortic valve | 88 (33.85%) | 28 (41.18%) | 116 (35.36%) | ||
| Hypertrophic cardiomyopathy | 49 (18.85%) | 0 (0%) | 49 (14.94%) | ||
| Arrhythmogenic Diseases | 16 (6.15%) | 0 (0%) | 16 (4.89%) |
| DEL (mm) | SIE (mm) | p | Mean of the Differences (mm) | |
|---|---|---|---|---|
| Adult population (n = 260) | ||||
| Aortic root | 35.83 (5.84) | 35.51 (5.90) | 0.534 | 0.27 (CI −1.15; 1.70) |
| Ascending aorta | 35.62 (6.42) | 35.23 (6.36) | 0.486 | 0.33 (CI −1.20; 1.88) |
| Children population (n = 68) | ||||
| Aortic root | 27.99 (6.51) | 27.61 (6.78) | 0.739 | 0.30 (CI −1.99; 2.61) |
| Ascending aorta | 24.09 (4.95) | 24.52 (5.04) | 0.600 | 0.47 (CI −2.87; 1.93) |
| Total population (n = 328) | ||||
| Aortic root | 34.16 (6.78) | 33.88 (6.90) | 0.600 | 0.28 (CI −1.36; 1.93) |
| Ascending aorta | 33.20 (7.75) | 33.02 (7.53) | 0.763 | 0.17 (CI −1.69; 2.03) |
| Adult Guidelines (DLE) | Pediatric Guidelines (SIE) | |||||
|---|---|---|---|---|---|---|
| Aortic root | ICC | p | 95% CI | ICC | p | 95% CI |
| Interobserver variability | 0.97 | <0.001 | 0.95−0.99 | 0.93 | <0.001 | 0.88−0.98 |
| Intraobserver variability | 0.97 | <0.001 | 0.95−0.99 | 0.89 | <0.001 | 0.82−0.97 |
| Ascending aorta | ICC | p | 95% CI | ICC | p | 95% CI |
| Interobserver variability | 0.81 | <0.001 | 0.66−0.96 | 0.94 | <0.001 | 0.90−0.99 |
| Intraobserver variability | 0.94 | <0.001 | 0.89−0.99 | 0.87 | <0.001 | 0.77−0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Servato, M.L.; Teixidó-Turá, G.; Sabate-Rotes, A.; Galian-Gay, L.; Gutiérrez, L.; Valente, F.; Fernandez-Galera, R.; Casas, G.; López-Sainz, A.; González-Alujas, M.T.; et al. Are Aortic Root and Ascending Aorta Diameters Measured by the Pediatric versus the Adult American Society of Echocardiography Guidelines Interchangeable? J. Clin. Med. 2021, 10, 5290. https://doi.org/10.3390/jcm10225290
Servato ML, Teixidó-Turá G, Sabate-Rotes A, Galian-Gay L, Gutiérrez L, Valente F, Fernandez-Galera R, Casas G, López-Sainz A, González-Alujas MT, et al. Are Aortic Root and Ascending Aorta Diameters Measured by the Pediatric versus the Adult American Society of Echocardiography Guidelines Interchangeable? Journal of Clinical Medicine. 2021; 10(22):5290. https://doi.org/10.3390/jcm10225290
Chicago/Turabian StyleServato, Maria Luz, Gisela Teixidó-Turá, Anna Sabate-Rotes, Laura Galian-Gay, Laura Gutiérrez, Filipa Valente, Ruben Fernandez-Galera, Guillem Casas, Angela López-Sainz, M. Teresa González-Alujas, and et al. 2021. "Are Aortic Root and Ascending Aorta Diameters Measured by the Pediatric versus the Adult American Society of Echocardiography Guidelines Interchangeable?" Journal of Clinical Medicine 10, no. 22: 5290. https://doi.org/10.3390/jcm10225290






