Diagnostic Benefit of High b-Value Computed Diffusion-Weighted Imaging in Patients with Hepatic Metastasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Sample
2.2. Magnetic Resonance Imaging Studies
2.3. Qualitative and Quantitative Analysis Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Quantitative Analysis
3.2. Qualitative Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Approval
References
- Namasivayam, S.; Martin, D.R.; Saini, S. Imaging of liver metastases: MRI. Cancer Imaging 2007, 7, 2–9. [Google Scholar] [CrossRef]
- Hanna, R.F.; Miloushev, V.Z.; Tang, A.; Finklestone, L.A.; Brejt, S.Z.; Sandhu, R.S.; Santillan, C.S.; Wolfson, T.; Gamst, A.; Sirlin, C.B. Comparative 13-year meta-analysis of the sensitivity and positive predictive value of ultrasound, CT, and MRI for detecting hepatocellular carcinoma. Abdom. Radiol. 2016, 41, 71–90. [Google Scholar] [CrossRef]
- Karaosmanoglu, A.D.; Onur, M.R.; Ozmen, M.N.; Akata, D.; Karcaaltincaba, M. Magnetic Resonance Imaging of Liver Metastasis. Semin. Ultrasound CT MR 2016, 37, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Kaya, B.; Koc, Z. Diffusion-weighted MRI and optimal b-value for characterization of liver lesions. Acta Radiol. 2014, 55, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, V.; Das, C.J.; Sharma, R.; Gupta, A.K. Diffusion weighted imaging: Technique and applications. World J. Radiol. 2016, 8, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.B.; Zhang, L.; Huo, X.; Liu, G.; Ni, H.; Zhang, S.; Zhu, W.; Yin, J. The Diagnostic Ability of rs-DWI to Detect Subtle Acute Infarction Lesion in the Different Regions of the Brain and the Comparison between Different b-Values. BioMed Res. Int. 2018, 2018, 7069192. [Google Scholar] [CrossRef]
- Ablefoni, M.; Ullrich, S.; Surov, A.; Hoffmann, K.-T.; Meyer, H.-J. Diagnostic benefit of high b-value computed diffusion-weighted imaging in acute brainstem infarction. J. Neuroradiol. 2020. [CrossRef]
- Zhou, J.; Chen, E.; Xu, H.; Ye, Q.; Li, J.; Ye, S.; Cheng, Q.; Zhao, L.; Su, M.-Y.; Wang, M. Feasibility and Diagnostic Performance of Voxelwise Computed Diffusion-Weighted Imaging in Breast Cancer. J. Magn. Reson. Imaging 2019, 49, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Meyer, H.J.; Wienke, A. Correlation between apparent diffusion coefficient (ADC) and cellularity is different in several tumors: A meta-analysis. Oncotarget 2017, 8, 59492–59499. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Long, L.R.; Antani, S.; Thoma, G.R. Histology image analysis for carcinoma detection and grading. Comput. Methods Programs Biomed. 2012, 107, 538–556. [Google Scholar] [CrossRef]
- Shenoy-Bhangle, A.; Baliyan, V.; Kordbacheh, H.; Guimaraes, A.R.; Kambadakone, A. Diffusion weighted magnetic resonance imaging of liver: Principles, clinical applications and recent updates. World J. Hepatol. 2017, 9, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Vilgrain, V.; Esvan, M.; Ronot, M.; Caumont-Prim, A.; Aubé, C.; Chatellier, G. A meta-analysis of diffusion-weighted and gadoxetic acid-enhanced MR imaging for the detection of liver metastases. Eur. Radiol. 2016, 26, 4595–4615. [Google Scholar] [CrossRef] [PubMed]
- Colagrande, S.; Castellani, A.; Nardi, C.; Lorini, C.; Calistri, L.; Filippone, A. The role of diffusion-weighted imaging in the detection of hepatic metastases from colorectal cancer: A comparison with unenhanced and Gd-EOB-DTPA enhanced MRI. Eur. J. Radiol. 2016, 85, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Más-Estellés, F.; Mateos-Fernández, M.; Carrascosa-Bisquert, B.; Facal de Castro, F.; Puchades-Román, I.; Morera-Pérez, C. Contemporary non-echo-planar diffusion-weighted imaging of middle ear cholesteatomas. Radiographics 2012, 32, 1197–1213. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.R.; Tamada, T.; Takahashi, S.; Tanaka, U.; Sofue, K.; Kanda, T.; Nogami, M.; Ohno, Y.; Hinata, N.; Fujisawa, M.; et al. Computed Diffusion-Weighted Imaging in Prostate Cancer: Basics, Advantages, Cautions, and Future Prospects. Korean J. Radiol. 2018, 19, 832–837. [Google Scholar] [CrossRef]
- Moribata, Y.; Kido, A.; Fujimoto, K.; Himoto, Y.; Kurata, Y.; Shitano, F.; Kiguchi, K.; Konishi, I.; Togashi, K. Feasibility of Computed Diffusion Weighted Imaging and Optimization of b-value in Cervical Cancer. Magn. Reson. Med. Sci. 2017, 16, 66–72. [Google Scholar] [CrossRef]
- Higaki, T.; Nakamura, Y.; Tatsugami, F.; Kaichi, Y.; Akagi, M.; Akiyama, Y.; Baba, Y.; Iida, M.; Awai, K. Introduction to the Technical Aspects of Computed Diffusion-weighted Imaging for Radiologists. Radiographics 2018, 38, 1131–1144. [Google Scholar] [CrossRef]
- Kawahara, S.; Isoda, H.; Fujimoto, K.; Shimizu, H.; Furuta, A.; Arizono, S.; Ohno, T.; Yamashita, R.; Ono, A.; Togashi, K. Additional benefit of computed diffusion-weighted imaging for detection of hepatic metastases at 1.5T. Clin. Imaging 2016, 40, 481–485. [Google Scholar] [CrossRef]
- Shimizu, H.; Isoda, H.; Fujimoto, K.; Kawahara, S.; Furuta, A.; Shibata, T.; Togashi, K. Comparison of acquired diffusion weighted imaging and computed diffusion weighted imaging for detection of hepatic metastases. Eur. J. Radiol. 2013, 82, 453–458. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Testa, M.L.; Chojniak, R.; Sene, L.S.; Damascena, A.S.; Guimarães, M.D.; Szklaruk, J.; Marchiori, E. Is DWI/ADC a useful tool in the characterization of focal hepatic lesions suspected of malignancy? PLoS ONE 2014, 9, e101944. [Google Scholar] [CrossRef]
- Yu, J.-S.; Chung, J.-J.; Kim, J.H.; Cho, E.-S.; Kim, D.J.; Ahn, J.-H.; Kim, K.W. Detection of small intrahepatic metastases of hepatocellular carcinomas using diffusion-weighted imaging: Comparison with conventional dynamic MRI. Magn. Reson. Imaging 2011, 29, 985–992. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, B.; Wang, H.; Zhang, Y.; Yi, X.; Liao, W.; Zhao, L. Diagnostic performance of magnetic resonance imaging for colorectal liver metastasis: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 1969. [Google Scholar] [CrossRef]
- Xiong, H.; Zeng, Y.-L. Standard-b-Value Versus Low-b-Value Diffusion-Weighted Imaging in Hepatic Lesion Discrimination: A Meta-analysis. J. Comput. Assist. Tomogr. 2016, 40, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Göya, C.; Hamidi, C.; Önder, H.; Inal, A.; Tekbaş, G.; Firat, U.; Çetinçakmak, M.G. Primary and Metastatic Liver Malignancy: Utility Low and High B Value (1600–2000) in 3 Tesla MRI. Hepatogastroenterology 2015, 62, 962–965. [Google Scholar] [PubMed]
- Fukumoto, W.; Nakamura, Y.; Higaki, T.; Tatsugami, F.; Iida, M.; Awai, K. Additional Value of Diffusion-weighted MRI to Gd-EOB-DTPA-enhanced Hepatic MRI for the Detection of Liver Metastasis: The Difference Depending on the Experience of the Radiologists. Hiroshima J. Med. Sci. 2015, 64, 15–21. [Google Scholar] [PubMed]
- Arita, Y.; Yoshida, S.; Waseda, Y.; Takahara, T.; Ishii, C.; Ueda, R.; Kwee, T.C.; Miyahira, K.; Ishii, R.; Okuda, S.; et al. Diagnostic value of computed high b-value whole-body diffusion-weighted imaging for primary prostate cancer. Eur. J. Radiol. 2021, 137, 109581. [Google Scholar] [CrossRef]
- Jendoubi, S.; Wagner, M.; Montagne, S.; Ezziane, M.; Mespoulet, J.; Comperat, E.; Estellat, C.; Baptiste, A.; Renard-Penna, R. MRI for prostate cancer: Can computed high b-value DWI replace native acquisitions? Eur. Radiol. 2019, 29, 5197–5204. [Google Scholar] [CrossRef]
- DelPriore, M.R.; Biswas, D.; Hippe, D.S.; Zecevic, M.; Parsian, S.; Scheel, J.R.; Rahbar, H.; Partridge, S.C. Breast Cancer Conspicuity on Computed Versus Acquired High b-Value Diffusion-Weighted MRI. Acad. Radiol. 2021, 28, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Bickel, H.; Polanec, S.H.; Wengert, G.; Pinker, K.; Bogner, W.; Helbich, T.H.; Baltzer, P.A. Diffusion-Weighted MRI of Breast Cancer: Improved Lesion Visibility and Image Quality Using Synthetic b-Values. J. Magn. Reson. Imaging. 2019, 50, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, S.; Kromrey, M.L.; Motosugi, U.; Onishi, H. Optimal target b-value on computed diffusion-weighted magnetic resonance imaging for visualization of pancreatic ductal adenocarcinoma and focal autoimmune pancreatitis. Abdom. Radiol. 2021, 46, 636–646. [Google Scholar] [CrossRef]

| Characteristics | All Patients | |
|---|---|---|
| n | % | |
| 92 | 100 | |
| Gender | ||
| Female | 39 | 42.4 |
| Male | 53 | 57.6 |
| Age | Mean: 60.8 years Range: 29–83 years | |
| Primary tumors | ||
| Colorectal cancer | 38 | 41.3 |
| Malignant melanoma | 11 | 12 |
| Breast cancer | 7 | 7.6 |
| Neuroendocrine tumor | 7 | 7.6 |
| Pancreatic cancer | 6 | 6.4 |
| Lung cancer | 4 | 4.2 |
| Cholangiocarcinoma | 3 | 3.3 |
| Cervical cancer | 3 | 3.3 |
| Adenocarcinoma of the esophagogastric junction | ||
| Gastric cancer | 2 | 2.2 |
| Gallbladder carcinoma | 2 | 2.2 |
| Renal cell cancer | 2 | 2.2 |
| Leiomyosarcoma | 2 | 2.2 |
| Urothelial carcinoma | 2 | 2.2 |
| Testicular cancer | 1 | 1.1 |
| Gastrointestinal stromal tumors | 1 | 1.1 |
| Hepatic metastasis | 965 | 100 (100% of all patients) |
| Maximal transverse diameters <1 cm | 291 (32 patients) | 30.2 (34.8% of all patients) |
| Maximal transverse diameters ≥1 cm | 674 (60 patients) | 69.8 (65.2% of all patients) |
| Affected liver segments out of all 92 patients | ||
| Segment I | 25 | 6.9 |
| Segment II | 42 | 11.6 |
| Segment III | 41 | 11.3 |
| Segment IV | 49 | 13.5 |
| Segment V | 47 | 13.0 |
| Segment VI | 51 | 14.1 |
| Segment VII | 53 | 14.6 |
| Segment VIII | 54 | 15.0 |
| 1.5 T MRI Scanner | ||
|---|---|---|
| Parameters | DWI (n = 93) | HP (n = 93) |
| FOV (mm × mm) | 295 × 449 | 300 × 400 |
| Matrix | 134 × 88 | 320 × 180 |
| ST (mm) | 5 | 3 |
| Number of Slices | 114 | 72 |
| TR (ms) | 7900 | 3.56 |
| TE (ms) | 52 | 1.36 |
| Flip angle (°) | 90 | 10 |
| b-values (s/mm2) | (n = 40, 43.5%) 50, 400 and 800 | |
| (n = 52, 56.5%) 50, 400 and 1000 | ||
| HP | DWI | c-DWI b 1000 | c-DWI b 1500 | c-DWI b 2000 | c-DWI b 3000 | c-DWI b 4000 | c-DWI b 5000 | |
|---|---|---|---|---|---|---|---|---|
| c-DWI derived from DWI b 800 images | ||||||||
| Volume: cm3 (IQR) | 30 (6–50) | 25 (10–60) | 27.5 (10–56) | 25 (10–50) | 17.5 (5–35) | 20 (5–34) | 25 (15–25) | 20 (14–20) |
| p-value (comparison with acquired DWI b 800 images) | 0.82 | 0.4 | 0.05 | 0.001 | 0.001 | 0.5 | 0.5 | |
| c-DWI derived from DWI b-1000 images | ||||||||
| Volume: cm3 (IQR) | 12 (6–29) | 12 (5–31) | 11 (5–25) | 10 (4–24) | 9 (4–19) | 5 (2–14) | 0 | 0 |
| p-value (comparison with acquired DWI b 1000 images) | 0.76 | 0.023 | 0.001 | 0.001 | 0.001 |
| HP | DWI | c-DWI b 1000 | c-DWI b 1500 | c-DWI b 2000 | c-DWI b 3000 | c-DWI b 4000 | c-DWI b 5000 | |
|---|---|---|---|---|---|---|---|---|
| c-DWI derived from DWI b 800 images | ||||||||
| Volume: cm3 (IQR) | 39 (16–61) | 30 (93–60) | 37.5 (90–62) | 30 (91–58) | 30 (89–75) | 25 (86–185) | 0 | 0 |
| p-value (comparison with acquired DWI b 800 images) | 0.91 | 0.7 | 0.08 | 0.005 | 0.03 | |||
| c-DWI derived from DWI b 1000 images | ||||||||
| Volume: cm3 (IQR) | 94 (9–111) | 120 (10–113) | 110 (10–90) | 107 (8–85) | 119 (8–92) | 149 (3–108) | 0 | 0 |
| p-value (comparison with acquired DWI b 1000 images) | 0.1 | 0.01 | 0.003 | 0.001 | 0.005 |
| HP | DWI | c-DWI b 1000 | c-DWI b 1500 | c-DWI b 2000 | c-DWI b 3000 | c-DWI b 4000 | c-DWI b 5000 | |
|---|---|---|---|---|---|---|---|---|
| c-DWI derived from DWI b 800 images | ||||||||
| Volume: cm3 (IQR) | 4.6 (2.5–8) | 4.8 (8–10) | 4.8 (8–10) | 4.6 (6.9–10) | 3.4 (4.9–7.3) | 1.9 (2.2–2.9) | not measurable | not measurable |
| p-value (comparison with acquired DWI b 800 images) | 0.1 | 0.9 | 0.2 | 0.007 | 0 | |||
| c-DWI derived from DWI b 1000 images | ||||||||
| Volume: cm3 (IQR) | 12 (6–29) | 12 (5–31) | 11 (5–25) | 10 (4–24) | 9 (4–19) | 5 (2–14) | not measurable | not measurable |
| p-value (comparison with acquired DWI b 1000 images) | 0.1 | 1 | 0.6 | 0.06 | 0.4 |
| DWI | c-DWI b 1000 | c-DWI b 1500 | c-DWI b 2000 | c-DWI b 3000 | c-DWI b 4000 | c-DWI b 5000 | |
|---|---|---|---|---|---|---|---|
| c-DWI derived from DWI b 800 images (n = 12) | |||||||
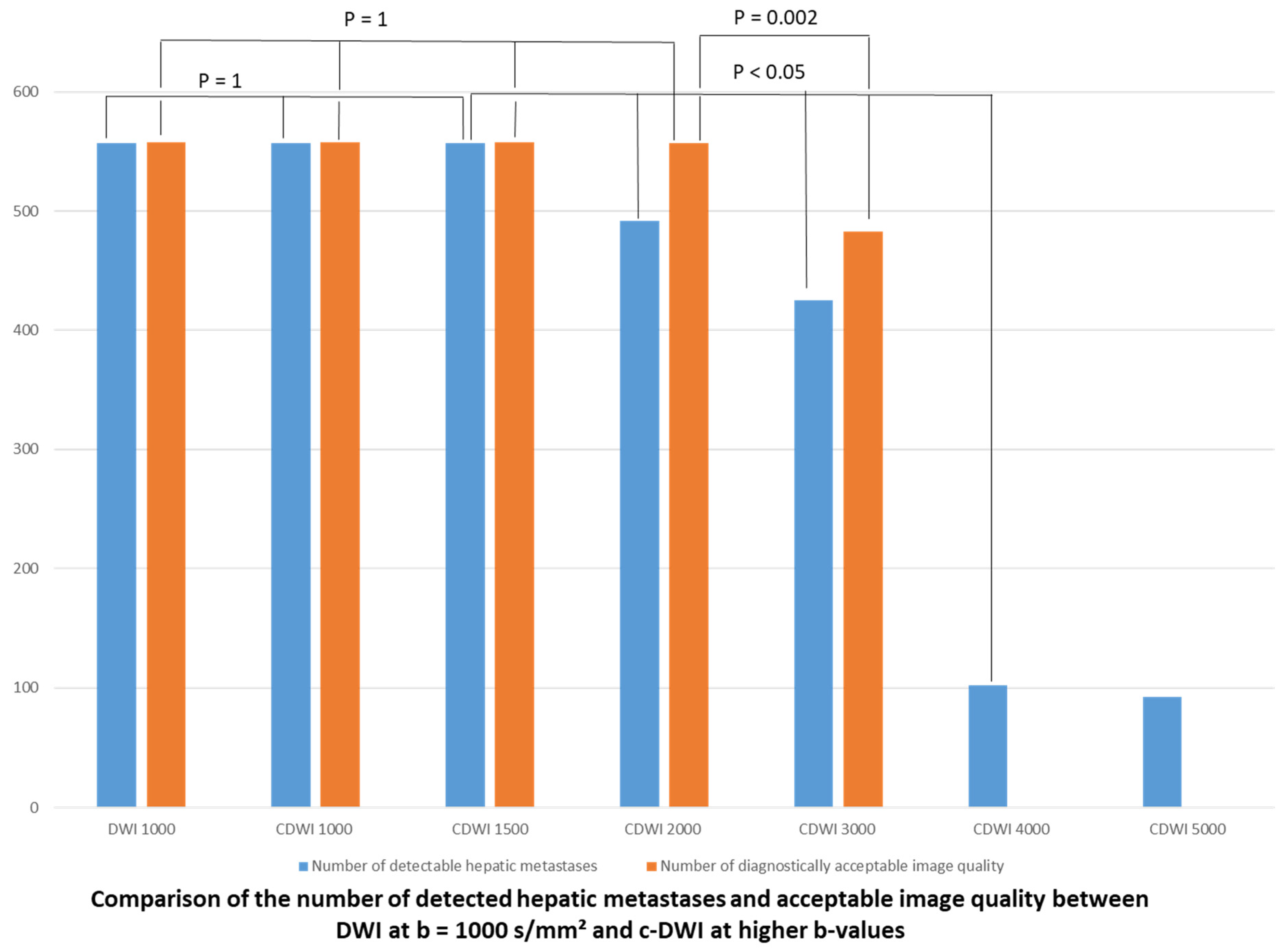
| Number of detected metastases | 106 | 106 | 106 | 106 | 78 | 30 | 30 |
| p-value (comparison with acquired DWI b 800 images) | 1 | 1 | 1 | 0.1 | 0.002 | 0.002 | |
| c-DWI derived from DWI b 1000 images | |||||||
| Number of detected metastases | 184 | 184 | 184 | 176 | 161 | 42 | 36 |
| p-value (comparison with acquired DWI b 1000 images) | 1 | 1 | 0.5 | 0.06 | 0 | 0 |
| DWI | c-DWI b 1000 | c-DWI b 1500 | c-DWI b 2000 | c-DWI b 3000 | c-DWI b 4000 | c-DWI b 5000 | |
|---|---|---|---|---|---|---|---|
| c-DWI derived from DWI b 800 images (n = 12) | |||||||
| Number of detected metastases | 147 | 147 | 147 | 129 | 105 | 0 | 0 |
| p-value (comparison with acquired DWI b 800 images) | 1 | 1 | 0.5 | 0.008 | |||
| c-DWI derived from DWI b 1000 images | |||||||
| Number of detected metastases | 100 | 100 | 100 | 80 | 66 | 0 | 0 |
| p-value (comparison with acquired DWI b 1000 images) | 1 | 1 | 0.3 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ablefoni, M.; Surup, H.; Ehrengut, C.; Schindler, A.; Seehofer, D.; Denecke, T.; Meyer, H.-J. Diagnostic Benefit of High b-Value Computed Diffusion-Weighted Imaging in Patients with Hepatic Metastasis. J. Clin. Med. 2021, 10, 5289. https://doi.org/10.3390/jcm10225289
Ablefoni M, Surup H, Ehrengut C, Schindler A, Seehofer D, Denecke T, Meyer H-J. Diagnostic Benefit of High b-Value Computed Diffusion-Weighted Imaging in Patients with Hepatic Metastasis. Journal of Clinical Medicine. 2021; 10(22):5289. https://doi.org/10.3390/jcm10225289
Chicago/Turabian StyleAblefoni, Maxime, Hans Surup, Constantin Ehrengut, Aaron Schindler, Daniel Seehofer, Timm Denecke, and Hans-Jonas Meyer. 2021. "Diagnostic Benefit of High b-Value Computed Diffusion-Weighted Imaging in Patients with Hepatic Metastasis" Journal of Clinical Medicine 10, no. 22: 5289. https://doi.org/10.3390/jcm10225289
APA StyleAblefoni, M., Surup, H., Ehrengut, C., Schindler, A., Seehofer, D., Denecke, T., & Meyer, H.-J. (2021). Diagnostic Benefit of High b-Value Computed Diffusion-Weighted Imaging in Patients with Hepatic Metastasis. Journal of Clinical Medicine, 10(22), 5289. https://doi.org/10.3390/jcm10225289








