Correlation between Polysomnographic Parameters and Tridimensional Changes in the Upper Airway of Obstructive Sleep Apnea Patients Treated with Mandibular Advancement Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Sample Identification and Selection
2.3. Treatment Procedures
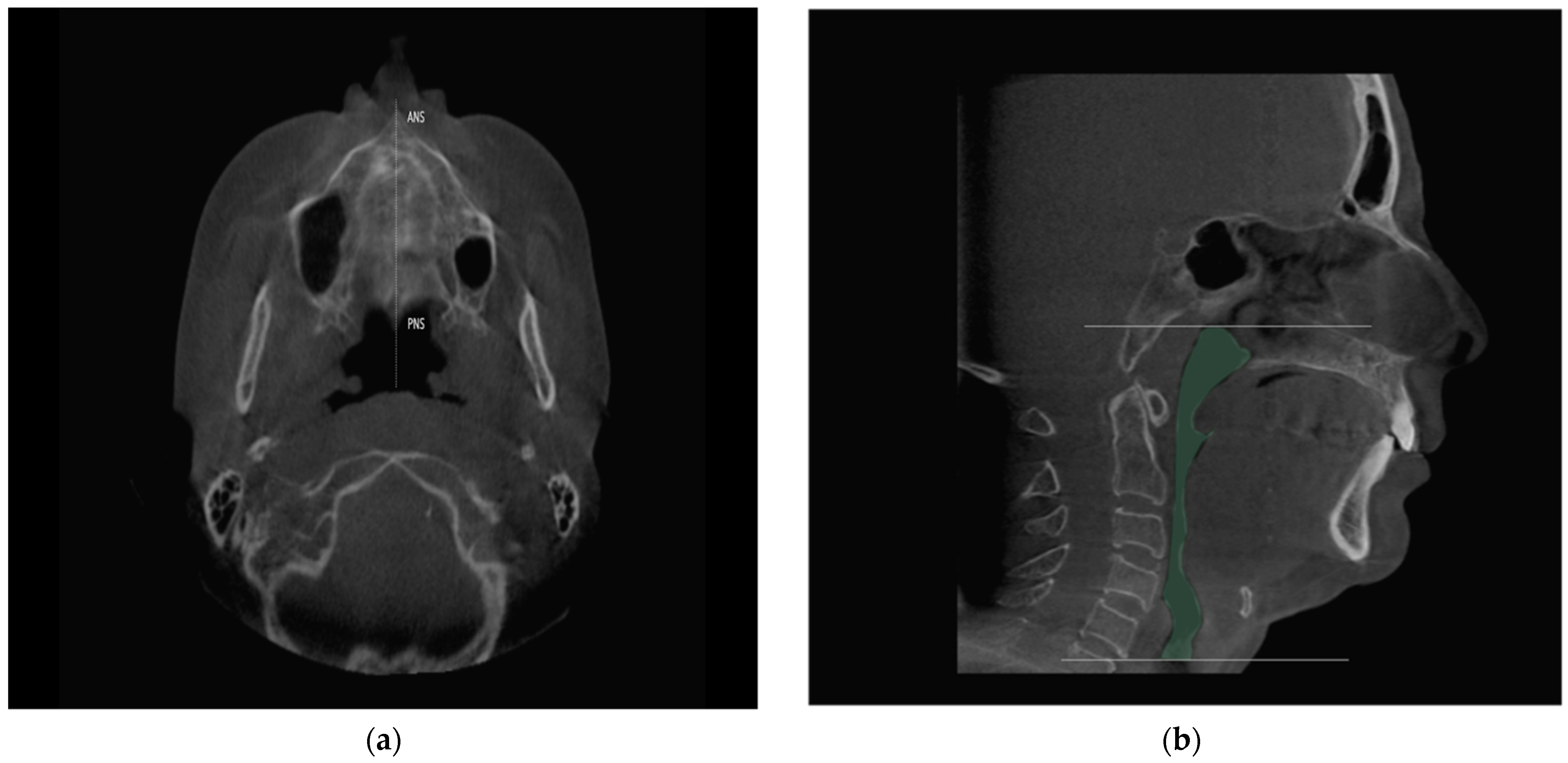
2.4. Study Variables and Data Collection
2.5. Statistical Analysis
3. Results
3.1. Descriptive Analysis of the Sample
3.2. Impact of Mandibular Advancement upon the Respiratory and Neurophysiological Parameters
3.3. Impact of Mandibular Advancement upon the Structure of the Airway
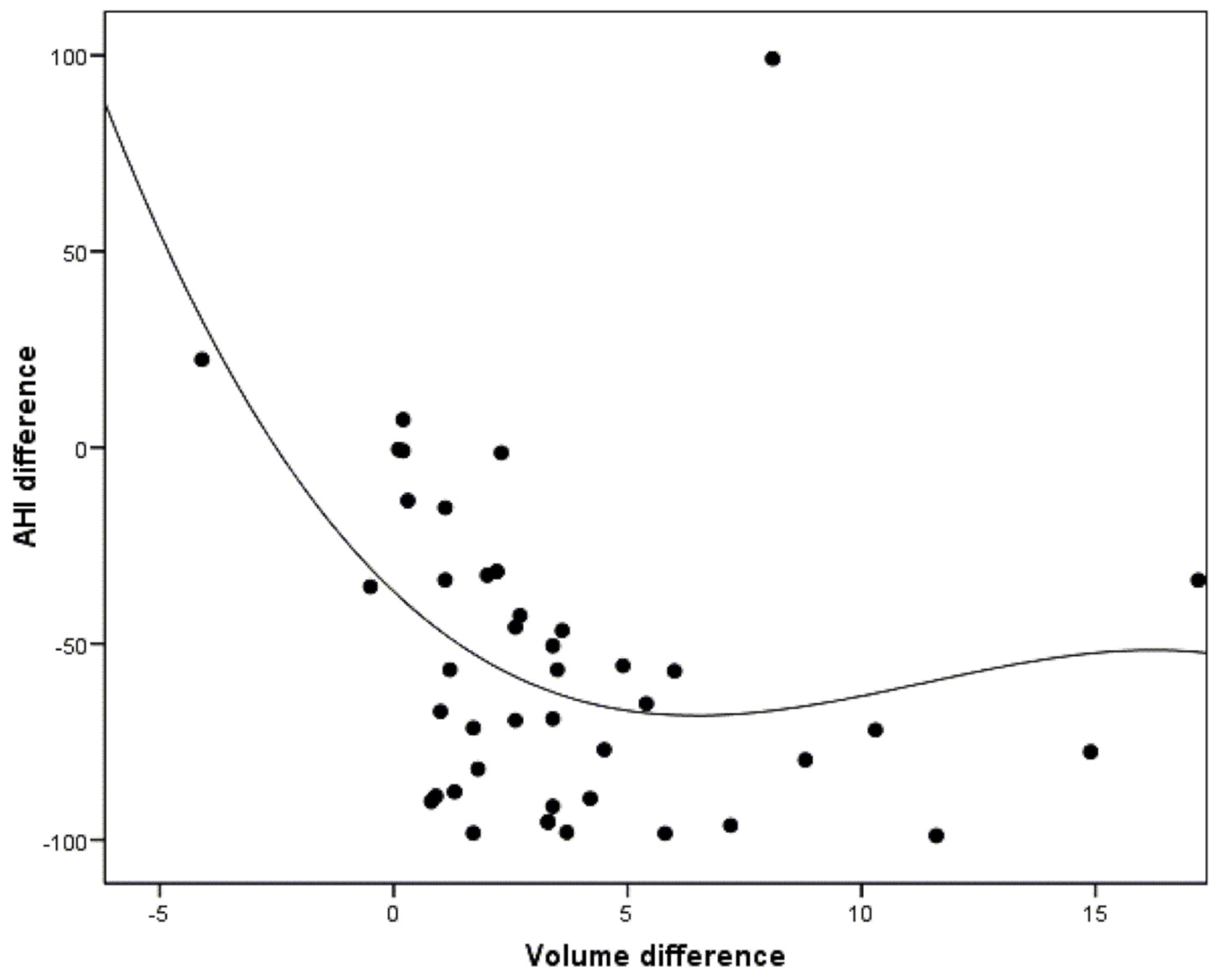
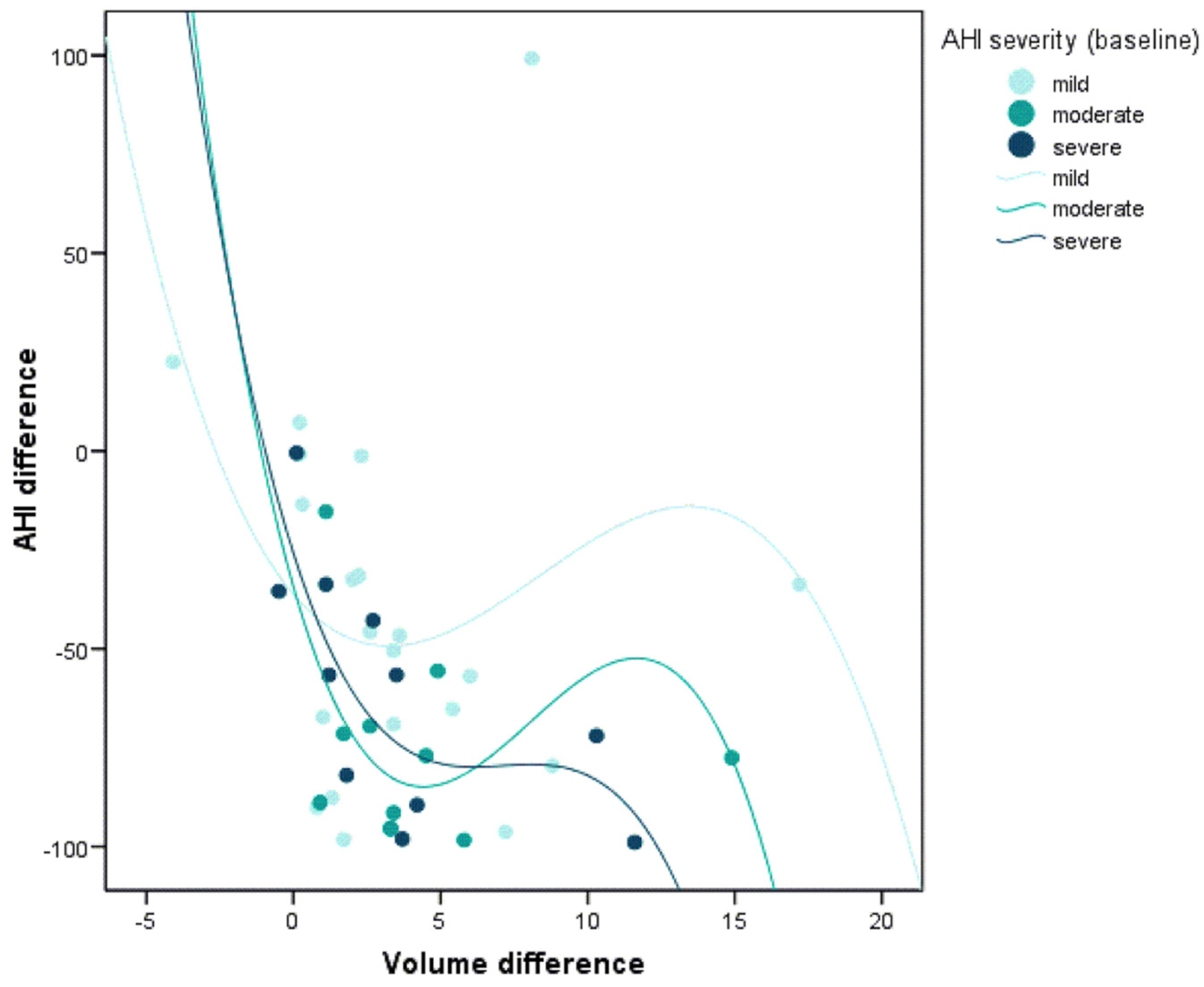
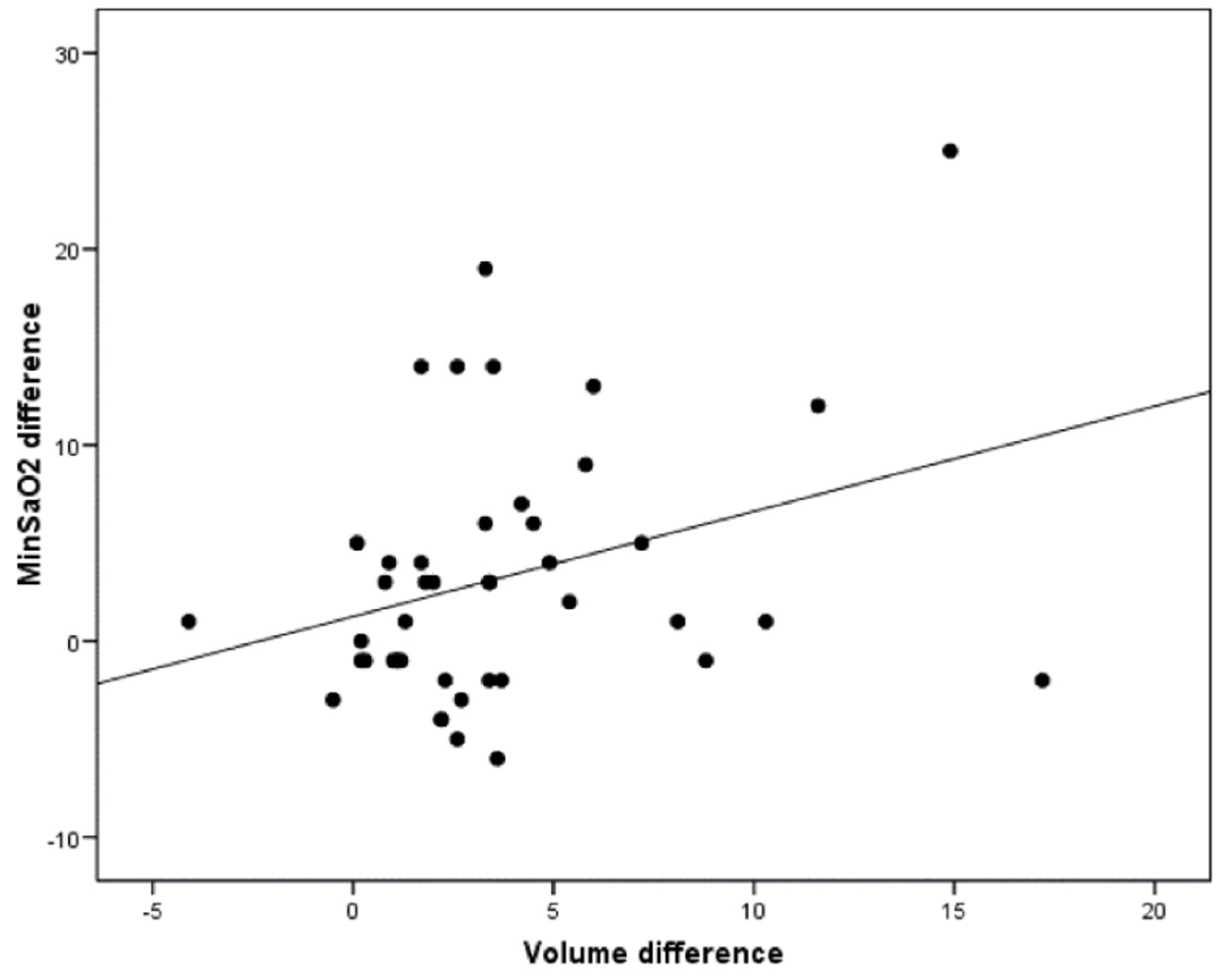
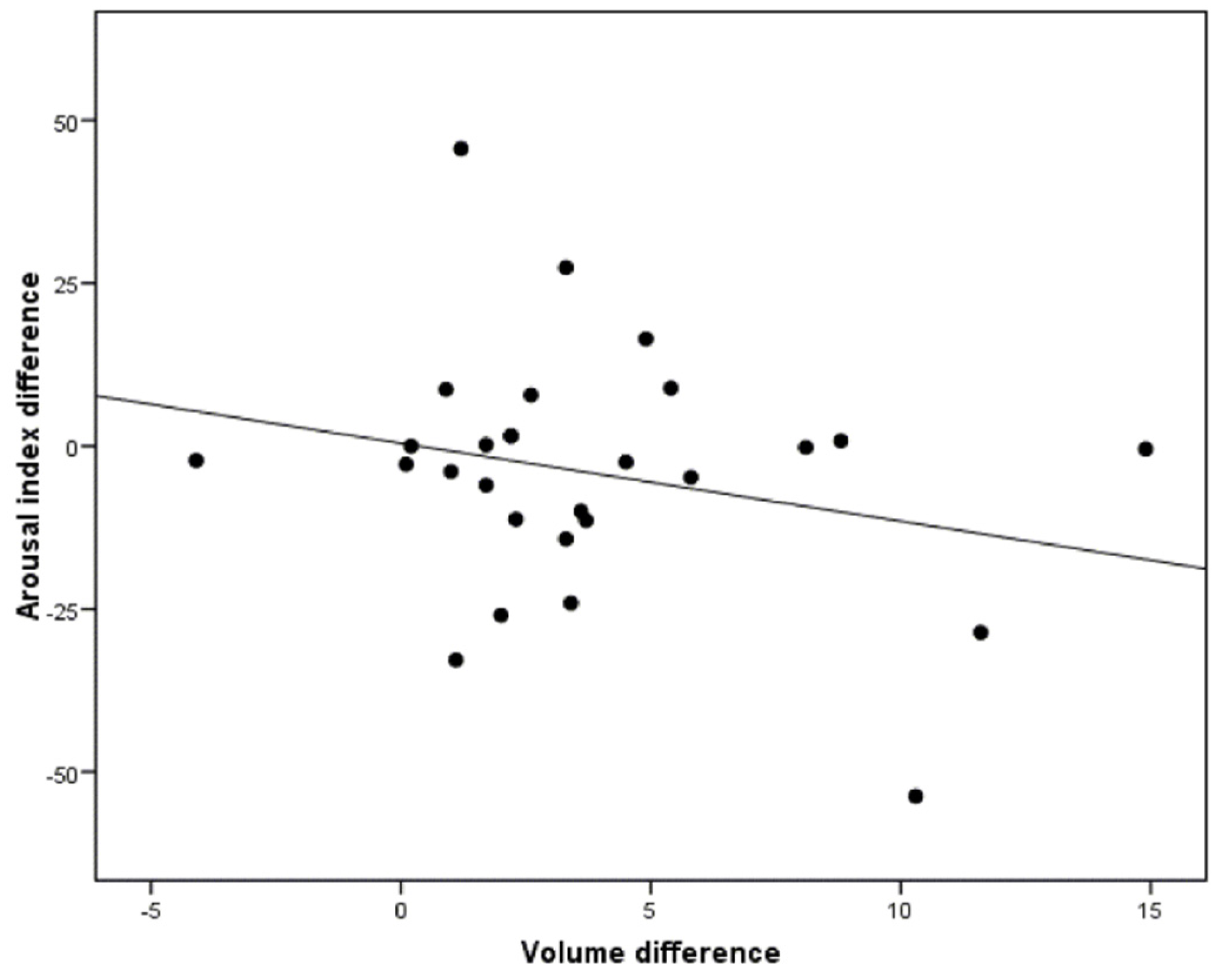
3.4. Correlation between the Morphological Changes in the Airway and the Polysomnographic Parameters
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berry, R.B.; Brooks, R.; Gamaldo, C.; Harding, S.M.; Lloyd, R.M.; Quan, S.F.; Troester, M.T.; Vaughn, B.V. AASM Scoring Manual Updates for 2017 (Version 2.4). J. Clin. Sleep Med. 2017, 15, 665–666. [Google Scholar] [CrossRef]
- Michael, J.; Sateia, M.D. International Classification of Sleep Disorders—Third Edition. Highlights and Modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef]
- Dalewski, B.; Kamińska, A.; Syrico, A.; Kałdunska, A.; Pałka, Ł.; Sobolewska, E. The Usefulness of Modified Mallampati Score and CT Upper Airway Volume Measurements in Diagnosing OSA among Patients with Breathing-Related Sleep Disorders. Appl. Sci. 2021, 11, 3764. [Google Scholar] [CrossRef]
- Ramar, K.; Dort, L.C.; Katz, S.G.; Lettieri, C.J.; Harrod, C.G.; Thomas, S.M.; Chervin, R.D. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: An update for 2015. J. Clin. Sleep Med. 2015, 11, 773–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mediano, O.; González-Mangado, N.; Montserrat, J.M.; Alonso-Álvarez, M.L.; Almendros, I.; Alonso-Fernández, A.; Barbé, F.; Borsini, E.; Caballero-Eraso, C.; Cano-Pumarega, I.; et al. International consensus document on obstructive sleep apnea. Arch. Bronconeumol. 2021, 21, 115. [Google Scholar] [CrossRef]
- Lugaresi, E.; Cirignotta, F.; Coccagna, G.; Baruzzi, A. Snoring and the obstructive apnea syndrome. Electroencephalogr. Clin. Neurophysiol. 1982, 35, 421–430. [Google Scholar]
- Iannella, G.; Maniaci, A.; Magliulo, G.; Cocuzza, S.; La Mantia, I.; Cammaroto, G.; Greco, A.; Vicini, C. Current challenges in the diagnosis and treatment of obstructive sleep apnea syndrome in the elderly. Pol. Arch. Intern. Med. 2020, 130, 649–654. [Google Scholar] [CrossRef] [Green Version]
- Shucard, D.W.; Juryeda, S. Obstructive sleep apnea—An overview of the disorder and consequences. Semin. Orthod. 2004, 10, 63–72. [Google Scholar] [CrossRef]
- Ahrens, A.; McGrath, C.; Hägg, U. Subjective efficacy of oral appliance design features in the management of obstructive sleep apnea: A systematic review. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 559–576. [Google Scholar] [CrossRef]
- Barnes, M.; McEvoy, R.D.; Banks, S.; Tarquinio, N.; Murray, C.G.; Vowles, N.; Pierce, R.J. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am. J Respir. Crit. Care Med. 2004, 170, 656–664. [Google Scholar] [CrossRef]
- Lam, B.; Sam, K.; Mok, W.Y.; Cheung, M.T.; Fong, D.Y.; Lam, J.C.; Lam, D.C.; Yam, L.Y.; Lp, M.S. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax 2007, 62, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Kohler, M.; Smith, D.; Tippett, V.; Stradling, J.R. Predictors of long-term compliance with continuous positive airway pressure. Thorax 2010, 65, 829–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannella, G.; Magliulo, G.; Di Luca, M.; De Vito, A.; Meccariello, G.; Cammaroto, G.; Pelucchi, S.; Bonsembiante, A.; Maniaci, A.; Vicini, C. Lateral pharyngoplasty techniques for obstructive sleep apnea syndrome: A comparative experimental stress test of two different techniques. Eur. Arch. Otorhinolaryngol. 2020, 277, 1793–1800. [Google Scholar] [CrossRef]
- Rashwan, M.S.; Montevecchi, F.; Cammaroto, G.; Badr El Deen, M.; Iskander, N.; El Hennawi, D.; El Tabbakh, M.; Meccariello, G.; Gobbi, R.; Stomeo, F.; et al. Evolution of soft palate surgery techniques for obstructive sleep apnea patients: A comparative study for single-level palatal surgeries. Clin. Otolaryngol. 2018, 43, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Ramon, M.A.; Sampol, G.; Anitua, E.; Cobo, J.; De-Carlos, F.; González, M.; Macias, E.; Monasterio, C.; Nogués, L.; Teran, J.; et al. Guía Española de Práctica Clínica Sobre la Utilización de Dispositivos de Avance Mandibular en el Tratamiento de Pacientes Adultos Diagnosticados de Síndrome de Apneas-Hipopneas del Sueño. Guía de Práctica Clínica en el Sistema Nacional de Salud. 2017. Available online: https://ses.org.es (accessed on 1 September 2021).
- Mehta, A.; Petocz, P.; Darendellier, M.A.; Cistulli, P.A. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2001, 163, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Gotsopoulos, H.; Kelly, J.J.; Cistulli, P.A. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: A randomized, controlled trial. Sleep 2004, 27, 934–941. [Google Scholar] [CrossRef] [Green Version]
- Pitsis, A.J.; Darendeliler, M.A.; Gotsopoulos, H.; Petocz, P.; Cistulli, P.A. Effect of vertical dimension on efficacy of oral appliance therapy in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2002, 166, 860–864. [Google Scholar] [CrossRef]
- Ghazal, A.; Sorichter, S.; Jonas, I.; Rose, E.C. A randomized prospective long-term study of two oral appliances for sleep apnoea treatment. J. Sleep Res. 2009, 18, 321–328. [Google Scholar] [CrossRef]
- Cakirer, B.; Hans, M.; Graham, G.; Aylor, J.; Tishler, V.; Redline, S. The relationship between craniofacial morphology and obstructive sleep apena in Whites and in African-Americans. Am. J. Respir. Crit. Care Med. 2001, 163, 947–950. [Google Scholar] [CrossRef]
- Srinivas, M.S.; Zachary, A.; Thomas, B.D.; Leonard, B.K. Cephalometric measurement of upper airway length correlates with the presence and severity of obstructive sleep apnea. J. Oral Maxillofac. Surg. 2010, 68, 2846–2855. [Google Scholar] [CrossRef]
- Hsu, W.E.; Wu, T.Y. Comparison of upper airway measurement by lateral cephalogram in upright position and CBCT in supine position. J. Dent. Sci. 2019, 14, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Obelenis-Ryan, D.P.; Bianchi, J.; Ignácio, J.; Wolford, L.M.; Gonçalves, J.R. Cone-beam computed tomography airway measurements: Can we trust them? Am. J. Orthod. Dentofac. Orthop. 2019, 156, 53–60. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Marcus, C.L.; Vaughn, B. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, 2nd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2015; pp. 154–196. [Google Scholar] [CrossRef] [Green Version]
- Esteller, E.; Carrasco, M.; Díaz-Herrera, M.Á.; Vila, J.; Sampol, G.; Juvanteny, J.; Sieira, R.; Farré, A.; Vilaseca, I. Clinical Practice Guideline recommendations on examination of the upper airway for adults with suspected obstructive sleep apnoea-hypopnoea syndrome. Acta Otorrinolaringol. Esp. 2019, 70, 364–372. [Google Scholar] [CrossRef]
- Aarab, G.; Lobbezoo, F.; Hamburger, H.L.; Naeije, M. Effects of an oral appliance with different mandibular protrusion positions at a constant vertical dimension on obstructive sleep apnea. Clin. Oral Investig. 2010, 14, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Wee, J.H.; Lim, J.H.; Gelera, J.E. Comparison of success criteria based on long-term symptoms and new-onset hypertension in mandibular advancement device treatment for obstructive sleep apnoea: Observational cohort study. BMJ Open 2018, 8, 216–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leite, F.G.; Rodrigues, R.C.; Ribeiro, F.R.; Eckeli, A.L.; Regalo, S.C.; Sousa, L.G.; Fernandes, R.M.F.; Valera, F.C.P. The use of a mandibular repositioning device for obstructive sleep apnea. Eur. Arch. Otorhinolaryngol. 2014, 271, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Giannasi, L.C.; Magini, M.; de-Oliveira, C.S.; de-Oliveira, L.V. Treatment of obstructive sleep apnea using an adjustable mandibular repositioning appliance fitted to a total prosthesis in a maxillary edentulous patient. Sleep Breath 2008, 12, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.R.; Parker, J.A.; Hodges, J.S.; Lowe, A.A.; Fegurson, K.A. Effect of a titration polysomnogram on treatment success with a mandibular repositioning appliance. J. Clin. Sleep Med. 2009, 5, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Dieltjens, M.; Vanderveken, O.M.; Hamans, E. Treatment of obstructive sleep apnea using a custom-made titratable duobloc oral appliance: A prospective clinical study. Sleep Breath 2013, 17, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Pahkala, R.; Seppä, J.; Myllykangas, R.; Tervaniemi, J.; Vartiainen, V.M.; Suominen, A.L.; Muraja-Murro, A. The impact of oral appliance therapy with moderate mandibular advancement on obstructive sleep apnea and upper airway volume. Sleep Breath 2020, 24, 865–873. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Eckert, D.J.; Van-der-Stelt, P.F.; Guo, J.; Ge, S.; Emami, E.; Almeida, F.R.; Huynh, N.T. Phenotypes of responders to mandibular advancement device therapy in obstructive sleep apnea patients: A systematic review and meta-analysis. Sleep Med. Rev. 2020, 49, 101229. [Google Scholar] [CrossRef]
- Lan, W.C.; Chang, W.D.; Tsai, M.H.; Tsou, Y.A. Trans-oral robotic surgery versus coblation tongue base reduction for obstructive sleep apnea syndrome. PeerJ 2019, 7, 7812. [Google Scholar] [CrossRef] [PubMed]
- Di Luca, M.; Iannella, G.; Montevecchi, F.; Magliulo, G.; De Vito, A.; Cocuzza, S.; Maniaci, A.; Meccariello, G.; Cammaroto, G.; Sgarzani, R.; et al. Use of the transoral robotic surgery to treat patients with recurrent lingual tonsillitis. Int. J. Med. Robot. 2020, 16, 2106. [Google Scholar] [CrossRef]
- Fleury, B.; Rakotonanahary, D.; Pêtelle, B. Mandibular advancement titration for obstructive sleep apnea: Optimization of procedure by combining clinical and oximetric parameters. Chest 2004, 125, 1761–1767. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.D.; Keropian, B.; Pillar, G. Effects of the full breath solution appliance for the tratment of osbstuctive sleep apnea: A preliminary study. Cranio 2009, 27, 109–117. [Google Scholar] [CrossRef]
- Aarab, G.; Lobbezoo, F.; Wicks, D.J.; Hamburger, H.L.; Naeije, M. Short-term effects of a mandibular advancement device on obstructive sleep apnoea: An open-label pilot trial. J. Oral Rehabil. 2005, 32, 564–570. [Google Scholar] [CrossRef]
- Dal-Fabbro, C.; Garbuio, S.; D’Almeida, V.; Cintra, F.D.; Tufik, S.; Bittencourt, L. Mandibular advancement device and CPAP upon cardiovascular parameters in OSA. Sleep Breath 2014, 18, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Durán-Cantolla, J.; Crovetto-Martínez, R.; Alkhraisat, M.H.; Crovetto, M.; Municio, A.; Kutz, R.; Aizpuru, F.; Miranda, E.; Anitua, E. Efficacy of mandibular advancement device in the treatment of obstructive sleep apnea syndrome: A randomized controlled crossover clinical trial. Med. Oral Patol. Oral Cir. Bucal 2015, 20, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.A.; Ono, T.; Lowe, A.A.; Keenan, S.P.; Fleetham, J.A. A randomized crossover study of an oral appliance vs nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apnea. Chest 1996, 109, 1269–1275. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Liu, Y.; Gao, Y. Three-dimensional upper-airway changes associated with various amounts of mandibular advancement in awake apnea patients. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 661–668. [Google Scholar] [CrossRef]
- Cossellu, G.; Biagi, R.; Sarcina, M.; Montellaro, C.; Farronato, G. Three dimensional evaluation of upper airway in patients with obstructive sleep apnea syndrome during oral appliance therapy. J. Craniofac. Surg. 2015, 26, 745–748. [Google Scholar] [CrossRef]
- Shete, C.S.; Wasundhara, A.B. Three-dimensional upper airway changes with mandibular advancement device in patients with obstructive sleep apnea. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 941–948. [Google Scholar] [CrossRef]
- Chan, A.S.; Sutherland, K.; Schwab, R.J.; Zeng, B.; Petocz, P.; Lee, R.W.; Darendeliler, M.A.; Cistulli, P.A. The effect of mandibular advancement on upper airway structure in obstructive sleep apnoea. Thorax 2010, 65, 726–732. [Google Scholar] [CrossRef] [Green Version]
- Haskell, J.A.; McCrillis, J.; Haskell, B.S.; Scheetz, J.P. Effects of Mandibular advancement device (MAD) on airway dimensions assessed with cone-beam computed tomography. Semin. Orthod. 2009, 15, 132–158. [Google Scholar] [CrossRef]
- Tsuiki, S.; Hiyama, S.; Ono, T.; Imamura, N.; Ishiwata, Y.; Kuroda, T.; Lowe, A. Effects of a titratable oral appliance on supine airway size in awake non-apneic individuals. Sleep 2001, 24, 554–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.K.; Goldman, M.; Koyal, S.; Clark, G. Effect of jaw and head position on airway resistance in obstructive sleep apnea. Sleep Breath 2000, 4, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Otsuka, R.; Ono, T.; Honda, E.; Sasaki, T.; Kuroda, T. Effect of titrated mandibular advancement and jaw opening on the upper airway in nonapneic men: A magnetic resonance imaging and cephalometric study. Am. J. Orthod. Dentofac. Orthop. 2004, 125, 191–199. [Google Scholar] [CrossRef]
- L’Estrange, P.R.; Battagel, J.M.; Harkness, B.; Spratley, M.H.; Nolan, P.J.; Jorgensen, G.I. A method of studying adaptive changes of the oropharynx to variation in mandibular position in patients with obstructive sleep apnoea. J. Oral Rehabil. 1996, 23, 699–711. [Google Scholar] [CrossRef]
- Tsuiki, S.; Isono, S.; Ishikawa, T.; Yamashiro, Y.; Tatsumi, K.; Nishino, T. Anatomical balance of the upper airway and obstructive sleep apnea. Anesthesiology 2008, 108, 1009–1015. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, K.; Chan, A.S.; Cistulli, P.A. Three-dimensional assessment of anatomical balance and oral appliance treatment outcome in obstructive sleep apnoea. Sleep Breath 2016, 20, 903–910. [Google Scholar] [CrossRef]
- Liang, X.N.; Lu, Z.Q.; Wu, P.A.; Guan, Y.F.; Zhou, P.; Zeng, J.X. Relationship between upper airway volume and polysomnography parameters of patients with obstructive sleep apnea hypopnea syndrome. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2018, 32, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, K.; Takaya, H.; Qian, J.; Petocz, P.; Ng, A.T.; Cistulli, P.A. Oral appliance treatment response and polysomnographic phenotypes of obstructive sleep apnea. J. Clin. Sleep Med. 2015, 11, 861–868. [Google Scholar] [CrossRef]
- Marco-Pitarch, R.; García-Selva, M.; Plaza-Espín, A.; Puertas-Cuesta, J.; Agustín-Panadero, R.; Fernández-Julián, E.; Marco-Algarra, J.; Fons-Font, A. Dimensional analysis of the upper airway in obstructive sleep apnoea syndrome patients treated with mandibular advancement device: A bi- and three-dimensional evaluation. J. Oral Rehabil. 2021, 48, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Vos, W.; De-Backer, J.; Devolder, A.; Vanderveken, O.; Verhulst, S.; Salgado, R.; Germonpre, P.; Partoens, B.; Wuyts, F.; Parizel, P.; et al. Correlation between severity of sleep apnea and upper airway morphology based on advanced anatomical and functional imaging. J. Biomech. 2007, 40, 2207–2213. [Google Scholar] [CrossRef]
- Tsuiki, S.; Ono, T.; Kuroda, T. Mandibular advancement modulates respiratory-related genioglossus electromyographic activity. Sleep Breath 2000, 4, 53–57. [Google Scholar] [CrossRef] [PubMed]
- White, D.P. Pathogenesis of obstructive and central sleep apnea. Am. J. Respir. Crit. Care Med. 2005, 172, 1363–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, B.A.; White, D.P. Control of the pharyngeal musculature during wakefulness and sleep: Implications in normal controls and sleep apnea. Head Neck 2011, 33, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezzanotte, W.S.; Tangel, D.G.; White, D.P. Waking genioglossal electromyogram in sleep apnea patients versus normal controls: A neuromuscular compensatory mechanism. J. Clin. Investig. 1992, 89, 1571–1579. [Google Scholar] [CrossRef]
- Fogel, R.B.; Trinder, J.; White, D.P.; Malhotra, A.; Raneri, J.; Schory, K.; Kleverlaan, D.; Pierce, R.J. The effect of sleep onset on upper airway muscle activity in patients with sleep apnoea versus controls. J. Physiol. 2005, 564, 549–562. [Google Scholar] [CrossRef]



| Demographic Characteristics | Baseline Measure |
|---|---|
| Gender, n (%) | |
| Men | 24 (53.3) |
| Women | 21 (46.7) |
| Age, years, mean (SD) 1 | 54.1 (10.2) |
| BMI, kg/m2, mean (SD) 2 | 25.6 (2.7) |
| AHI, events/r, mean (SD) 3 | 21.9 (16.6) |
| MinSaO2, mean (SD) 4 | 84.6 (6.9) |
| Arousal index | 18.1 (14.7) |
| OSA severity category, n (%) 5 | |
| Mild (5 ≤ AHI <15) | 23 (51.1) |
| Moderate (15 ≤ AHI <30) | 11 (24.4) |
| Severe (30 ≤ AHI) | 11 (24.4) |
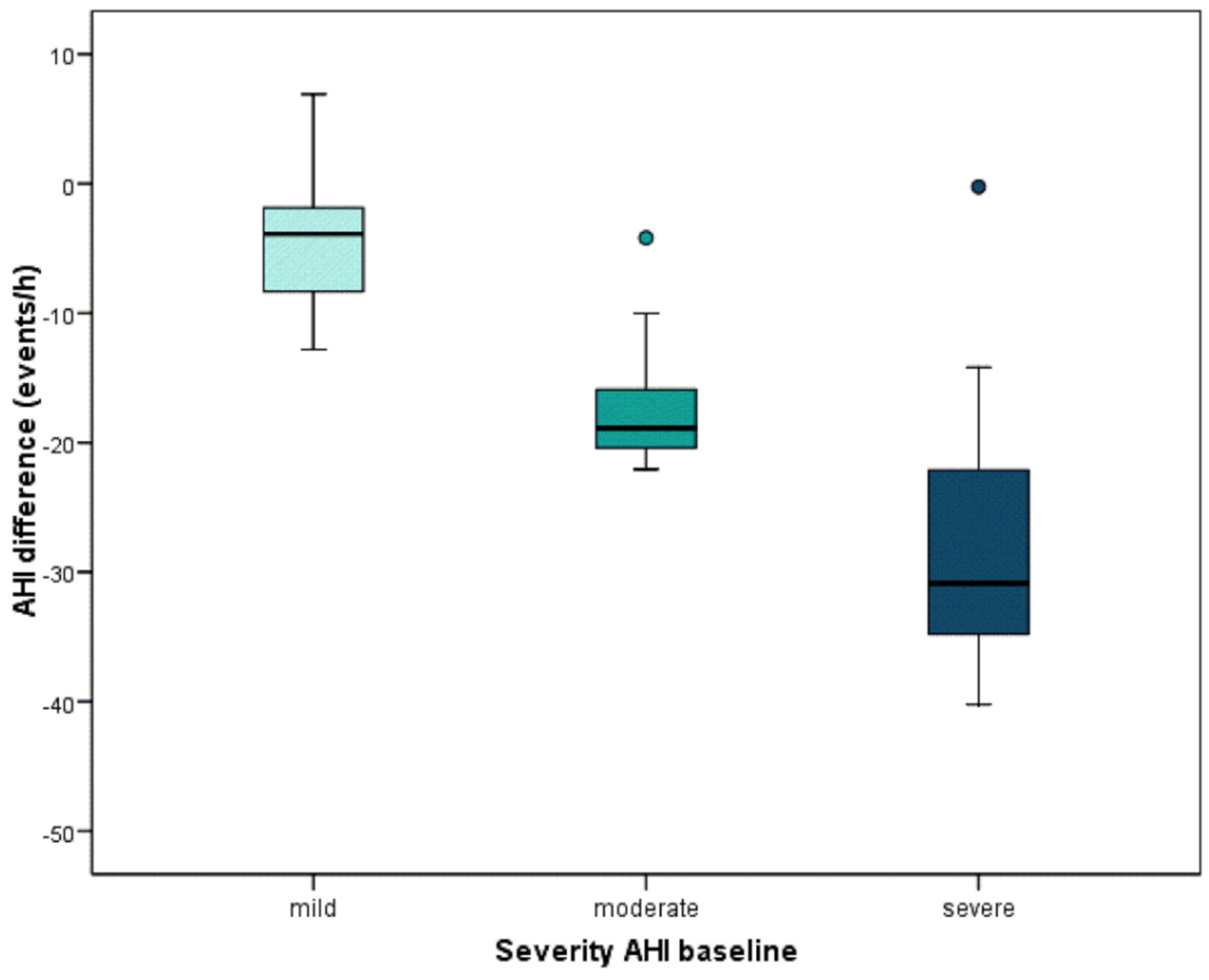
| AHI Severity | ||||
|---|---|---|---|---|
| Total | Mild | Moderate | Severe | |
| N | 45 | 23 | 11 | 11 |
| mean (SD) | −12.8 (11.8) | −4.2 (4.5) | −17.0 (5.5) | −26.6 (11.9) |
| minimum | −40.2 | −12.8 | −22.1 | −40.2 |
| maximum | 6.9 | 6.9 | −4.2 | −0.3 |
| percentile 25 | −20.9 | −8.5 | −21.0 | −36.8 |
| median | −9.7 | −3.9 | −18.9 | −30.9 |
| percentile 75 | −3.9 | −1.8 | −14.0 | −20.9 |
| Baseline | After MAD Tritation | p-Value 1 | |
|---|---|---|---|
| Total airway volume (cm3), mean ± SD | 20.6 ± 6.8 | 24.9 ± 8.8 | p < 0.001 |
| Researcher 1 1st Measurement | Researcher 1 2nd Measurement | Bland and Altman | ICC | ||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean difference | SD | 95% confidence interval | |
| 15.5 ± 5.9 | 15.2 ± 5.7 | 10.3 | 360.02 | −695.32; 715.96 | 0.99 |
| Researcher 2 1st measurement | Researcher 2 2nd measurement | Bland and Altman | ICC | ||
| Mean ± SD | Mean ± SD | Mean difference | SD | 95% confidence interval | |
| 15.8 ± 6.8 | 15.5 ± 5.1 | 74.3 | 637.57 | −1175.36; 1323.94 | 0.99 |
| Researcher 1 1st measurement | Researcher 2 1st measurement | Bland and Altman | ICC | ||
| Mean ± SD | Mean ± SD | Mean difference | SD | 95% confidence interval | |
| 15.5 ± 5.9 | 15.8 ± 6.8 | −306.28 | 1573.3 | −3390.36; 2777.53 | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camañes-Gonzalvo, S.; Marco-Pitarch, R.; Plaza-Espín, A.; Puertas-Cuesta, J.; Agustín-Panadero, R.; Fons-Font, A.; Fons-Badal, C.; García-Selva, M. Correlation between Polysomnographic Parameters and Tridimensional Changes in the Upper Airway of Obstructive Sleep Apnea Patients Treated with Mandibular Advancement Devices. J. Clin. Med. 2021, 10, 5255. https://doi.org/10.3390/jcm10225255
Camañes-Gonzalvo S, Marco-Pitarch R, Plaza-Espín A, Puertas-Cuesta J, Agustín-Panadero R, Fons-Font A, Fons-Badal C, García-Selva M. Correlation between Polysomnographic Parameters and Tridimensional Changes in the Upper Airway of Obstructive Sleep Apnea Patients Treated with Mandibular Advancement Devices. Journal of Clinical Medicine. 2021; 10(22):5255. https://doi.org/10.3390/jcm10225255
Chicago/Turabian StyleCamañes-Gonzalvo, Sara, Rocío Marco-Pitarch, Andrés Plaza-Espín, Javier Puertas-Cuesta, Rubén Agustín-Panadero, Antonio Fons-Font, Carla Fons-Badal, and Marina García-Selva. 2021. "Correlation between Polysomnographic Parameters and Tridimensional Changes in the Upper Airway of Obstructive Sleep Apnea Patients Treated with Mandibular Advancement Devices" Journal of Clinical Medicine 10, no. 22: 5255. https://doi.org/10.3390/jcm10225255
APA StyleCamañes-Gonzalvo, S., Marco-Pitarch, R., Plaza-Espín, A., Puertas-Cuesta, J., Agustín-Panadero, R., Fons-Font, A., Fons-Badal, C., & García-Selva, M. (2021). Correlation between Polysomnographic Parameters and Tridimensional Changes in the Upper Airway of Obstructive Sleep Apnea Patients Treated with Mandibular Advancement Devices. Journal of Clinical Medicine, 10(22), 5255. https://doi.org/10.3390/jcm10225255








