The Role of CAR-T Cells in Peritoneal Carcinomatosis from Gastric Cancer: Rationale, Experimental Work, and Clinical Applications
Abstract
:1. Introduction
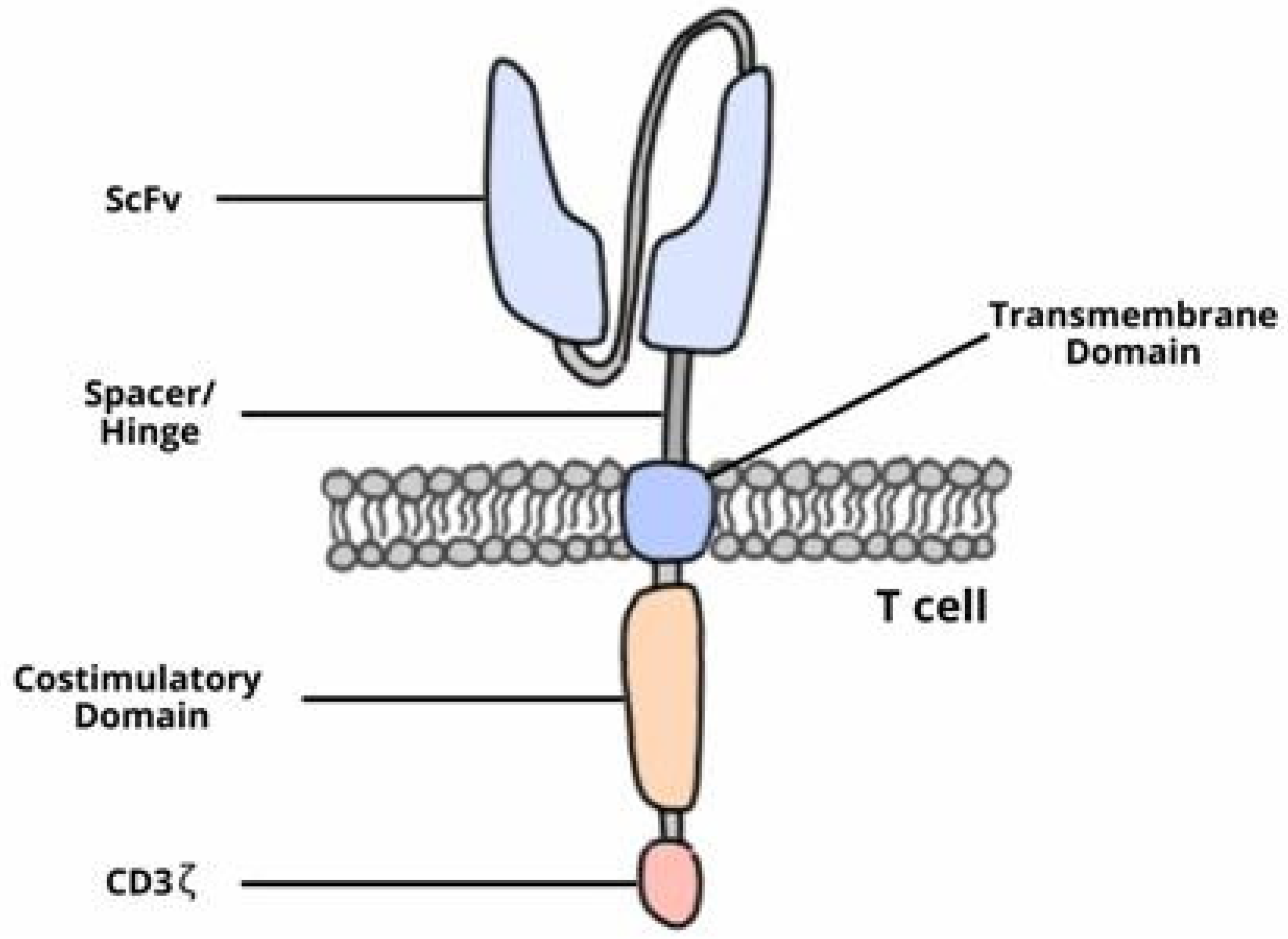
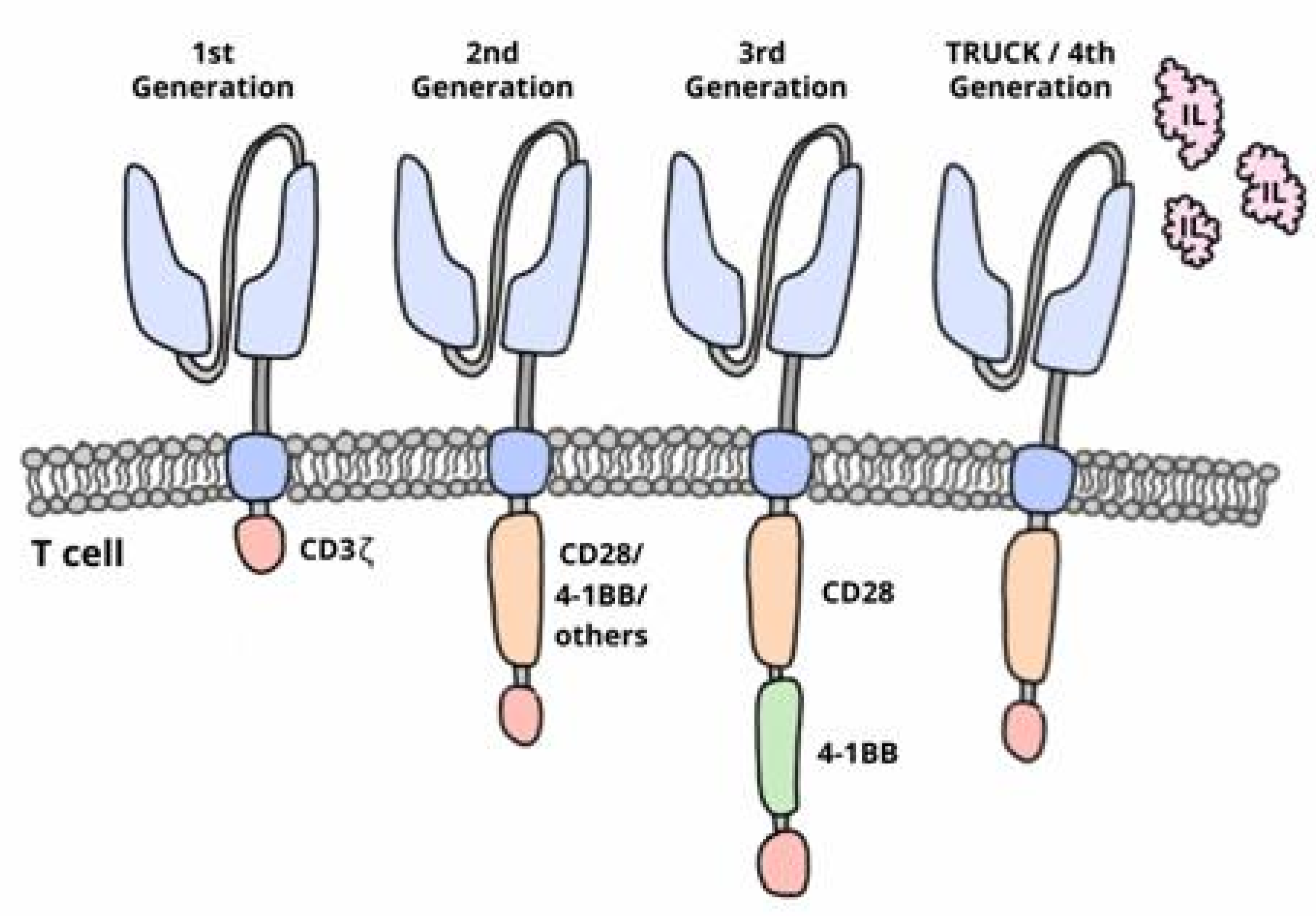
1.1. Chimeric Antigen Receptor-T (CAR-T) Cell Therapy
1.2. Adverse Effects Associated with CAR-T Cell Therapy
2. Rationale
2.1. Safety and Efficacy of CAR-T Therapy for Advanced Gastric Cancer: Ongoing Clinical Trials
2.2. Carcinoembryonic Antigen (CEA)
2.3. HER2 (Human Epidermal Growth Factor Receptor 2)
2.4. ICAM (Intercellular Adhesion Molecule 1)
2.5. Claudin 18.2 (CLDN18.2)
2.6. EpCAM
2.7. CAR-T in Peritoneal Carcinomatosis of Gastric Origin
3. Experimental Works and Clinical Applications
3.1. Route of CAR-T Administration Cells in PC
3.2. CAR-T in Gastric PC
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [Green Version]
- Durães, C.; Almeida, G.; Seruca, R.; Oliveira, C.; Carneiro, F. Biomarkers for gastric cancer: Prognostic, predictive or targets of therapy? Virchows Arch. 2014, 464, 367–378. [Google Scholar] [CrossRef]
- Orditura, M.; Galizia, G.; Sforza, V.; Gambardella, V.; Fabozzi, A.; Laterza, M.M.; Andreozzi, F.; Ventriglia, J.; Savastano, B.; Mabilia, A.; et al. Treatment of gastric cancer. World J. Gastroenterol. 2014, 20, 1635–1649. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.S.; Seshadri, R.A.; Kepenekian, V.; Vaudoyer, D.; Passot, G.; Glehen, O. Survival outcomes after Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from gastric cancer: A systematic review. Pleura Peritoneum 2016, 1, 67–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coccolini, F.; Gheza, F.; Lotti, M.; Virzì, S.; Iusco, D.; Ghermandi, C.; Melotti, R.; Baiocchi, G.; Giulini, S.M.; Ansaloni, L.; et al. Peritoneal carcinomatosis. World J. Gastroenterol. 2013, 19, 6979–6994. [Google Scholar] [CrossRef] [PubMed]
- Ponterio, E.; De Maria, R.; Haas, T.L. Identification of Targets to Redirect CAR T Cells in Glioblastoma and Colorectal Cancer: An Arduous Venture. Front. Immunol. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Lordick, F.; Allum, W.; Carneiro, F.; Mitry, E.; Tabernero, J.; Tan, P.; Van Cutsem, E.; van de Velde, C.; Cervantes, A. Unmet needs and challenges in gastric cancer: The way forward. Cancer Treat. Rev. 2014, 40, 692–700. [Google Scholar] [CrossRef] [Green Version]
- Long, B.; Qin, L.; Zhang, B.; Li, Q.; Wang, L.; Jiang, X.; Ye, H.; Zhang, G.; Yu, Z.; Jiao, Z. CAR T-cell therapy for gastric cancer: Potential and perspective (Review). Int. J. Oncol. 2020, 56, 889–899. [Google Scholar] [CrossRef]
- Yang, X.-J.; Huang, C.-Q.; Suo, T.; Mei, L.-J.; Yang, G.-L.; Cheng, F.-L.; Zhou, Y.-F.; Xiong, B.; Yonemura, Y.; Li, Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: Final results of a phase III randomized clinical trial. Ann. Surg. Oncol. 2011, 18, 1575–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, R.S.; Al-Adra, D.P.; Nagendran, J.; Campbell, S.; Shi, X.; Haase, E.; Schiller, D. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: A systematic review of survival, mortality, and morbidity. J. Surg. Oncol. 2011, 104, 692–698. [Google Scholar] [CrossRef]
- Ji, Z.H.; Zhang, Y.; Li, Y. Intra-operative hyperthermic intraperitoneal chemotherapy for prevention and treatment of peritoneal metastases from gastric cancer: A narrative review. J. Gastrointest. Oncol. 2021, 12, S70–S78. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Lucas, F.A.M.; Cristovam, S.N. HER2 testing in gastric cancer: An update. World J. Gastroenterol. 2016, 22, 4619–4625. [Google Scholar]
- Gross, G.; Waks, T.; Eshhar, Z. Expression of immunoglobulin-T cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA 1989, 86, 10024–10028. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.C.; Abramson, J.S. Patient selection for chimeric antigen receptor (CAR) T-cell therapy for aggressive B-cell non-Hodgkin lymphomas. Leuk. Lymphoma 2020, 61, 2561–2567. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.S. Anti-CD19 CAR T-Cell Therapy for B-Cell Non-Hodgkin Lymphoma. Transfus. Med. Rev. 2020, 34, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Nastoupil, L.J.; Jain, M.; Feng, L.; Spiegel, J.Y.; Ghobadi, A.; Lin, Y.; Dahiya, S.; Lunning, M.; Lekakis, L.; Reagan, P.; et al. Standard-of-Care AxicabtageneCiloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results From the US Lymphoma CAR T Consortium. J. Clin. Oncol. 2020, 38, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.; Sharif, S.; Simmonds, M.; Claxton, L.; Hodgson, R. Tisagenlecleucel for the Treatment of Relapsed or Refractory B-cell Acute Lymphoblastic Leukaemia in People Aged up to 25 Years: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. Pharmacoeconomics 2019, 37, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Thistlethwaite, F.C.; Gilham, D.E.; Guest, R.D.; Rothwell, D.G.; Pillai, M.; Burt, D.J.; Byatte, A.J.; Kirillova, N.; Valle, J.W.; Sharma, S.K.; et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol. Immunother. 2017, 66, 1425–1436. [Google Scholar] [CrossRef]
- Brocker, T. Chimeric Fv-zeta or Fv-epsilon receptors are not sufficient to induce activation or cytokine production in peripheral T cells. Blood 2000, 96, 1999–2001. [Google Scholar] [CrossRef] [PubMed]
- Eshhar, Z.; Waks, T.; Gross, G.; Schindler, D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA 1993, 90, 720–724. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Zhao, L.; Zhang, Y.; Qin, Y.; Guan, Y.; Zhang, T.; Liu, C.; Zhou, J. Understanding the Mechanisms of Resistance to CAR T-Cell Therapy in Malignancies. Front. Oncol. 2019, 9, 1237. [Google Scholar] [CrossRef] [Green Version]
- Knochelmann, H.M.; Smith, A.S.; Dwyer, C.J.; Wyatt, M.M.; Mehrotra, S.; Paulos, C.M. CAR T Cells in Solid Tumors: Blueprints for Building Effective Therapies. Front. Immunol. 2018, 9, 1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sur, D.; Havasi, A.D.; Cainap, C.; Samasca, G.; Burz, C.; Balacescu, O.; Lupan, I.; Deleanu, D.; Irimie, A. Chimeric Antigen Receptor T-Cell Therapy for Colorectal Cancer. J. Clin. Med. 2020, 9, 182. [Google Scholar] [CrossRef] [Green Version]
- Finney, H.M.; Akbar, A.N.; Lawson, A.D.G. Activation of resting human primary T cells with chimeric receptors: Costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J. Immunol. 2004, 172, 104–113. [Google Scholar] [CrossRef]
- Finney, H.M.; Lawson, A.D.; Bebbington, C.R.; Weir, A.N. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J. Immunol. 1998, 161, 2791–2797. [Google Scholar]
- Pulè, M.A.; Straathof, K.C.; Dotti, G.; Heslop, H.E.; Rooney, C.M.; Brenner, M.K. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol. Ther. 2005, 12, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Milone, M.C.; Fish, J.D.; Carpenito, C.; Carroll, R.G.; Binder, G.K.; Teachey, D.; Samanta, M.; Lakhal, M.; Gloss, B.; Danet-Desnoyers, G.; et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 2009, 17, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Newick, K.; O’Brien, S.; Moon, E.; Albelda, S.M. CAR T Cell Therapy for Solid Tumors. Annu. Rev. Med. 2017, 68, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 2015, 385, 517–528. [Google Scholar] [CrossRef]
- Gardner, R.A.; Finney, O.; Annesley, C.; Brakke, H.; Summers, C.; Leger, K.; Bleakley, M.; Brown, C.; Mgebroff, S.; Kelly-Spratt, K.S.; et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 2017, 129, 3322–3331. [Google Scholar] [CrossRef]
- Tasian, S.K.; Gardner, R.A. CD19-redirected chimeric antigen receptor-modified T cells: A promising immunotherapy for children and adults with B-cell acute lymphoblastic leukemia (ALL). Ther. Adv. Hematol. 2015, 6, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Marks, I.; Srinivasarao, M.; Kanduluru, A.K.; Mahalingam, S.M.; Liu, X.; Chu, H.; Low, P.S. Use of a single CAR T cell and several bispecific adapters facilitates eradication of multiple antigenically different solid tumors. Cancer Res. 2019, 79, 387–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, C.S.M.; Dardalhon, V.; Devaud, C.; Taylor, N.; Darcy, P.K.; Kershaw, M.H. CAR T-cell therapy of solid tumors. Immunol. Cell Biol. 2017, 95, 356–363. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef] [Green Version]
- Grotz, T.E.; Fournier, K.F.; Mansfield, P.F. Patient Selection for Cytoreductive Surgery. Surg. Oncol. Clin. N. Am. 2018, 27, 443–462. [Google Scholar] [CrossRef]
- Holzinger, A.; Abken, H. CAR T cells targeting solid tumors: Carcinoembryonic antigen (CEA) proves to be a safe target. Cancer Immunol. Immunother. 2017, 66, 1505–1507. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.C.; Burga, R.A.; Mccormack, E.; Wang, L.J.; Mooring, W.; Point, G.R.; Khare, P.D.; Thorn, M.; Ma, Q.; Stainken, B.F.; et al. Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor—Modified T-cell Therapy for CEA þ Liver Metastases. Clin. Cancer Res. 2015, 21, 3149–3160. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wang, Z.; Yang, Z.; Wang, M.; Li, S.; Li, Y.; Zhang, R.; Xiong, Z.; Wei, Z.; Shen, J.; et al. Phase I Escalating-Dose Trial of CAR-T Therapy Targeting CEA+ Metastatic Colorectal Cancers. Mol. Ther. 2017, 25, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, Z.; Peng, M.; Fu, S.; Xue, Z.; Zhang, R. CAR-T cell therapy in gastrointestinal tumors and hepatic carcinoma: From bench to bedside. Oncoimmunology 2016, 5, e1251539. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Iqbal, N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol. Biol. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Han, Y.; Liu, C.; Li, G.; Li, J.; Lv, X.; Shi, H.; Liu, J.; Liu, S.; Yan, P.; Wang, S.; et al. Antitumor effects and persistence of a novel HER2 CAR T cells directed to gastric cancer in preclinical models. Am. J. Cancer Res. 2018, 8, 106–119. [Google Scholar] [PubMed]
- Maruo, Y.; Gochi, A.; Kaihara, A.; Shimamura, H.; Yamada, T.; Tanaka, N.; Orita, K. ICAM-1 expression and the soluble ICAM-1 level for evaluating the metastatic potential of gastric cancer. Int. J. Cancer 2002, 100, 486–490. [Google Scholar] [CrossRef] [Green Version]
- Jung, M.; Yang, Y.; McCloskey, J.E.; Zaman, M.; Vedvyas, Y.; Zhang, X.; Stefanova, D.; Gray, K.D.; Min, I.M.; Zarnegar, R.; et al. Chimeric Antigen Receptor T Cell Therapy Targeting ICAM-1 in Gastric Cancer. Mol. Ther.-Oncolytics 2020, 18, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Osanai, M.; Takasawa, A.; Murata, M.; Sawada, N. Claudins in cancer: Bench to bedside. Pflug. Arch. 2017, 469, 55–67. [Google Scholar] [CrossRef]
- Jiang, H.; Shi, Z.; Wang, P.; Wang, C.; Yang, L.; Du, G.; Zhang, H.; Shi, B.; Jia, J.; Li, Q.; et al. Claudin18.2-specific chimeric antigen receptor engineered T cells for the treatment of gastric cancer. J. Natl. Cancer Inst. 2019, 111, 409–418. [Google Scholar] [CrossRef]
- Zhan, X.; Wang, B.; Li, Z.; Li, J.; Wang, H.; Chen, L.; Jiang, H.; Wu, M.; Xiao, J.; Peng, X.; et al. Phase I trial of Claudin 18.2-specific chimeric antigen receptor T cells for advanced gastric and pancreatic adenocarcinoma. J. Clin. Oncol. 2019, 37 (Suppl. 15), 2509. [Google Scholar] [CrossRef]
- Bębnowska, D.; Grywalska, E.; Niedźwiedzka-Rystwej, P.; Sosnowska-Pasiarska, B.; Smok-Kalwat, J.; Pasiarski, M.; Góźdź, S.; Roliński, J.; Polkowski, W. CAR-T Cell Therapy—An Overview of Targets in Gastric Cancer. J. Clin. Med. 2020, 9, 1894. [Google Scholar] [CrossRef]
- Manzanedo, I.; Pereira, F.; Rihuete Caro, C.; Pérez-Viejo, E.; Serrano, Á.; Gutiérrez Calvo, A.; Regueira, F.M.; Casado-Adam, Á.; Cascales-Campos, P.A.; Arteaga, X.; et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for gastric cancer with peritoneal carcinomatosis: Multicenter study of Spanish group of peritoneal oncologic surgery (GECOP). Ann. Surg. Oncol. 2019, 26, 2615–2621. [Google Scholar] [CrossRef] [PubMed]
- Rau, B.; Brandl, A.; Piso, P.; Pelz, J.; Busch, P.; Demtröder, C.; Schüle, S.; Schlitt, H.; Roitman, M.; Tepel, J.; et al. Peritoneal metastasis in gastric cancer: Results from the German database. Gastric Cancer 2020, 23, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhao, H.; Natalia, A.; Lim, C.Z.J.; Ho, N.R.Y.; Ong, C.J.; Teo, M.C.C.; So, J.B.Y.; Shao, H. Exosome-templated nanoplasmonics for multiparametric molecular profiling. Sci. Adv. 2020, 6, eaba2556. [Google Scholar] [CrossRef]
- Ohzawa, H.; Kimura, Y.; Saito, A.; Yamaguchi, H.; Miyato, H.; Sakuma, Y.; Horie, H.; Hosoya, Y.; Lefor, A.K.; Sata, N.; et al. Ratios of miRNAs in Peritoneal Exosomes are Useful Biomarkers to Predict Tumor Response to Intraperitoneal Chemotherapy in Patients with Peritoneal Metastases from Gastric Cancer. Ann. Surg. Oncol. 2020, 27, 5057–5064. [Google Scholar] [CrossRef]
- Katz, S.; Point, G.R.; Cunetta, M.; Thorn, M.; Guha, P.; Espat, N.J.; Boutros, C.; Hanna, N.; Junghans, R.P. Regional CAR-T cell infusions for peritoneal carcinomatosis are superior to systemic delivery. Cancer Gene Ther. 2016, 23, 142–148. [Google Scholar] [CrossRef]
- Murad, J.P.; Kozlowska, A.K.; Lee, H.J.; Ramamurthy, M.; Chang, W.-C.; Yazaki, P.; Colcher, D.; Shively, J.; Cristea, M.; Forman, S.J.; et al. Effective targeting of TAG72+peritoneal ovarian tumors via regional delivery of CAR-engineered T cells. Front. Immunol. 2018, 9, 2268. [Google Scholar] [CrossRef] [PubMed]
- Ang, W.X.; Li, Z.; Chi, Z.; Du, S.H.; Chen, C.; Tay, J.C.K.; Toh, H.C.; Connolly, J.E.; Xu, X.H.; Wang, S. Intraperitoneal immunotherapy with T cells stably and transiently expressing anti-EpCAM CAR in xenograft models of peritoneal carcinomatosis. Oncotarget 2017, 8, 13545–13559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Olmo, D.; Campos, P.V.; Barambio, J.; Gomez-Heras, S.G.; Vega-Clemente, L.; Olmedillas-Lopez, S.; Guadalajara, H.; Garcia-Arranz, M. Intraperitoneal collagenase as a novel therapeutic approach in an experimental model of colorectal peritoneal carcinomatosis. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Benmebarek, M.R.; Karches, C.H.; Cadilha, B.L.; Lesch, S.; Endres, S.; Kobold, S. Killing mechanisms of chimeric antigen receptor (CAR) T cells. Int. J. Mol. Sci. 2019, 20, 1283. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Zhao, R.; Wu, D.; Zheng, D.; Wu, Z.; Shi, J.; Wei, X.; Wu, Q.; Long, Y.; Lin, S.; et al. Mesothelin is a target of chimeric antigen receptor T cells for treating gastric cancer. J. Hematol. Oncol. 2019, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]

| Target Antigen | Identifier | Gastric Cancer Condition | Trial Phase | CAR-T Infusion Route | Start-Completion Date | Study Status | Country |
|---|---|---|---|---|---|---|---|
| EpCAM | NCT02725125 | EpCAM-positive relapsed or refractory advanced gastric adenocarcinoma | I/II | Intravenous | November 2015–November 2019 | Unknown | China |
| NCT03013712 | EpCAM-positive relapsed or refractory advanced gastric adenocarcinoma | I/II | Intravenous | January 2017–December 2020 | Unknown | China | |
| Claudin18.2 | NCT04467853 | Claudin18.2-positive advanced gastric adenocarcinoma | I | Intravenous | September 2020–November 2024 | Recruiting | China |
| NCT03890198 | Claudin18.2-positive advanced gastric adenocarcinoma | I | Intravenous | April 2019–March 2020 | Early discontinuation | China | |
| NCT04404595 | Claudin18.2-positive advanced gastric adenocarcinoma | I | Intravenous | October 2020–September 2035 | Recruiting | United States | |
| NCT04581473 | Claudin18.2-positive advanced gastric adenocarcinoma | I/II | Intravenous | October 2020–December 2022 | Recruiting | China | |
| HER2 | NCT04650451 | HER2-positive advanced or metastatic gastric adenocarcinoma | I/II | Intravenous | December 2020–January 2025 | Recruiting | United States |
| NCT04511871 | HER2-positive relapsed or refractory advanced gastric adenocarcinoma | I | Intravenous | July 2020–January 2023 | Recruiting | China | |
| NCT02713984 | HER2-positive relapsed or refractory advanced gastric adenocarcinoma | I/II | Intravenous | March 2019–July 2019 | Withdrawn | China | |
| NCT03740256 | HER2-positive advanced or metastatic gastric adenocarcinoma | I | Intravenousin combination with intratumorCAdVEC (oncolytic adenovirus) | June 2021–December 2038 | Recruiting | United States | |
| Mesothelin | NCT03941626 | Mesothelin-positive advanced gastric adenocarcinoma, unresectable or refractory to chemoradiotherapy | I/II | intravenous | September 2019–December 2021 | Recruiting | China |
| NCT03638206 | Mesothelin-positive relapsed or refractory advanced gastric adenocarcinoma | I/II | intravenous | Marah 2018–March 2023 | Recruiting | China | |
| NCT04348643 | CEA-positive relapsed or refractory advanced gastric adenocarcinoma | I/II | intravenous | February 2020–April 2023 | Recruiting | China | |
| CEA | NCT02349724 | CEA-positive relapsed or refractory advanced gastric adenocarcinoma | I | intravenous | December 2014–December 2019 | Unknown | China |
| MUC1 | NCT02617134 | MUC1-positive relapsed or refractory advanced gastric adenocarcinoma | I/II | intravenous | November 2015–November 2018 | Unknown | China |
| CD276 | NCT04864821 | CD276-positive advanced gastric adenocarcinoma | I | Intravenous and intratumor | May 2021–May 2023 | Recruitment not begun | China |
| ROR2 | NCT03960060 | ROR2-positive relapsed or refractory advanced gastric adenocarcinoma | I | intravenous | May 2019–June 2023 | Recruitment closed | China |
| CD44v6 | NCT04427449 | CD44v6-positive advanced gastric adenocarcinoma | I/II | Intravenous | June 2020–December 2023 | Recruiting | China |
| Identifier | Trial Phase | Target Antigen | Gastric Cancer Condition | CAR-T Infusion Route | Start-Completion Date |
Study Status | Country |
|---|---|---|---|---|---|---|---|
| NCT03563326 | I | EpCAM | Peritoneal metastasis | Intraperitoneal | Aug 2018-Dec 2022 | Recruiting | China |
| NCT03682744 | I | CEA | Peritoneal metastases or malignant ascites | Intraperitoneal | Sep 2018-Mar 2021 | Active, recruitment closed | United States |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, S.; Villarejo-Campos, P.; García-Olmo, D. The Role of CAR-T Cells in Peritoneal Carcinomatosis from Gastric Cancer: Rationale, Experimental Work, and Clinical Applications. J. Clin. Med. 2021, 10, 5050. https://doi.org/10.3390/jcm10215050
Qian S, Villarejo-Campos P, García-Olmo D. The Role of CAR-T Cells in Peritoneal Carcinomatosis from Gastric Cancer: Rationale, Experimental Work, and Clinical Applications. Journal of Clinical Medicine. 2021; 10(21):5050. https://doi.org/10.3390/jcm10215050
Chicago/Turabian StyleQian, Siyuan, Pedro Villarejo-Campos, and Damián García-Olmo. 2021. "The Role of CAR-T Cells in Peritoneal Carcinomatosis from Gastric Cancer: Rationale, Experimental Work, and Clinical Applications" Journal of Clinical Medicine 10, no. 21: 5050. https://doi.org/10.3390/jcm10215050
APA StyleQian, S., Villarejo-Campos, P., & García-Olmo, D. (2021). The Role of CAR-T Cells in Peritoneal Carcinomatosis from Gastric Cancer: Rationale, Experimental Work, and Clinical Applications. Journal of Clinical Medicine, 10(21), 5050. https://doi.org/10.3390/jcm10215050







