The Main Role of Diaphragm Muscle as a Mechanism of Hypopressive Abdominal Gymnastics to Improve Non-Specific Chronic Low Back Pain: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Sample Size Calculation
2.3. Participants
2.4. Procedures
Hypopressive Abdominal Gymnastics (HAG)
2.5. Outcome Measures
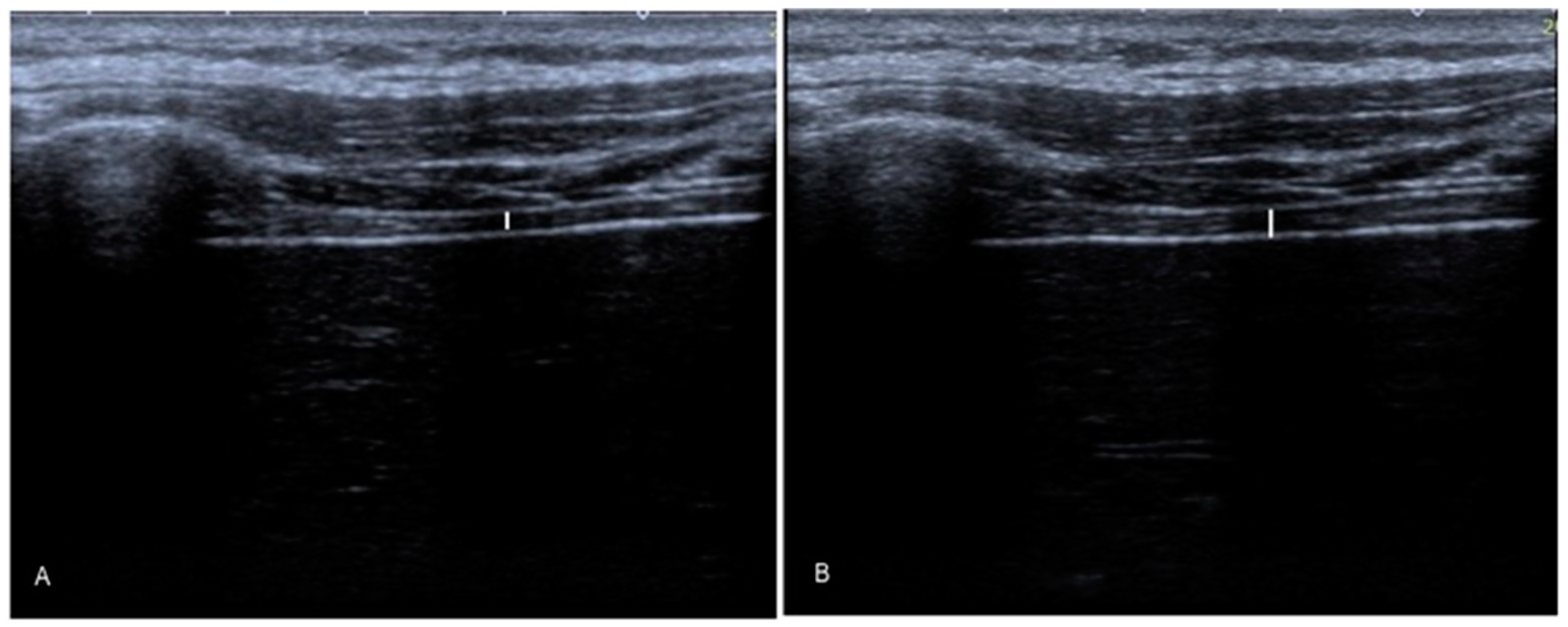
2.5.1. Diaphragm Thickness
2.5.2. Inspiratory Muscle Strength
2.5.3. Pain Intensity
2.5.4. Pain Threshold
2.5.5. Questionnaires
- −
- Physical Activity Questionnaire (IPAQ). This tool is formed of 4 questions about specific types of physical activities, for example, walking as well as vigorous and moderate activities, the frequency as well as the duration of each specific activity type and the time spent seated per day in each week. Data calculated using the IPAQ were converted into MET-min/week (named metabolic equivalents) by calculating the minutes per week for each category of the activities within their specific metabolic equivalents (walking corresponded to 3.3 METs; moderate physical activity corresponded to 4 METs; vigorous physical activity corresponded to 8 METs). Physical activity levels for each individual were ranked following IPAQ’s recommendations, which describe the physical activity categories as follows: Category I (considered as low physical activity) that corresponds to individuals who do not fulfil the criteria of the other 2 categories, considered as inactive; Category II (considered as moderate physical activity) that corresponds to individuals who meet 1 of the following criteria: 3 and/or more days of vigorous physical activity for at least 20 min per day, or 5 and/or more days of any combination of walking and vigorous or moderate physical activity, which reaches a total physical activity of at least 600 MET-min/week; Category II (considered as high physical activity) that corresponds to subjects who met 1 of the following criteria: vigorous activity of at least 5 days, reaching a total physical activity of 1500 MET-min/week, or 7 and/or more days of any combination of walking and moderate and/or vigorous activity that reaches a total physical activity of at least 3000 MET-min/week.
- −
- Roland–Morris Disability Questionnaire (RMDQ). This reliable and validated questionnaire presents 24 items which measure limitations under daily life activities secondary to LBP. The RMDQ Spanish version presents good comprehensibility, reliability and internal consistency and is considered as a useful and adequate instrument to determine disability originated by LBP [41].
- −
- Central Sensitization Inventory (CSI). This questionnaire allows the identification of symptoms related to central sensitization. This tool consists of two parts. The first part of this questionnaire analyzes 25 symptoms scored from 4 (always) to 0 (never), the total score being from 0 to 100 points. A score greater than 40 may be considered as the cut-off for detecting a central sensitization syndrome. The second part consists of questions about possible conditions that patients have been previously diagnosed with related to central sensitization [20].
- −
- Tampa Scale of Kinesiophobia-11 Items (TSK-11). The Spanish adaptation of the TSK-11 scale was used. The literature has demonstrated the validity and reliability of this test in assessing the level of kinesiophobia, especially for patients with chronic non-specific LBP. Unlike the original TSK, the TSK-11 is an abbreviated version with only eleven items, with four possible responses: strongly agree, agree, disagree and strongly disagree. The scoring range varies from 11 to 44 points, with some articles considering a significant difference as a difference of 4 points after treatment [20].
2.6. Statistical Analysis
3. Results
3.1. Baseline Data
3.2. Between-Group Comparisons for Outcome Measurement Differences
3.3. Multivariate Linear Regression Models
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Safiri, S.; Kolahi, A.A.; Cross, M.; Carson-Chahhoud, K.; Almasi-Hashiani, A.; Kaufman, J.; Mansournia, M.A.; Sepidarkish, M.; Ashrafi-Asgarabad, A.; Hoy, D.; et al. Global, regional, and national burden of other musculoskeletal disorders 1990–2017: Results from the Global Burden of Disease Study 2017. Rheumatology 2020, 72, 1916–1927. [Google Scholar] [CrossRef]
- Friedman, B.W.; Chilstrom, M.; Bijur, P.E.; Gallagher, E.J. Diagnostic testing and treatment of low back pain in United States emergency departments: A national perspective. Spine 2010, 35, E1406-11. [Google Scholar] [CrossRef] [Green Version]
- Koes, B.W.; van Tulder, M.; Lin, C.-W.C.; Macedo, L.G.; McAuley, J.; Maher, C. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur. Spine J. 2010, 19, 2075–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellido-Fernández, L.; Jiménez-Rejano, J.J.; Chillón-Martínez, R.; Gómez-Benítez, M.A.; De-La-Casa-Almeida, M.; Rebollo-Salas, M. Effectiveness of Massage Therapy and Abdominal Hypopressive Gymnastics in Nonspecific Chronic Low Back Pain: A Randomized Controlled Pilot Study. Evid. Based. Complement. Alternat. Med. 2018, 2018, 3684194. [Google Scholar] [CrossRef]
- Bliven, K.C.; Anderson, B.E. Core Stability Training for Injury Prevention. Sport. Health A Multidiscip. Approach 2013, 5, 514–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolar, P.; Sulc, J.; Kyncl, M.; Sanda, J.; Neuwirth, J.; Bokarius, A.V.; Kriz, J.; Kobesova, A. Stabilizing function of the diaphragm: Dynamic MRI and synchronized spirometric assessment. J. Appl. Physiol. 2010, 109, 1064–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocjan, J.; Gzik-Zroska, B.; Nowakowska, K.; Burkacki, M.; Suchoń, S.; Michnik, R.; Czyżewski, D.; Adamek, M. Impact of diaphragm function parameters on balance maintenance. PLoS ONE 2018, 13, e0208697. [Google Scholar] [CrossRef] [Green Version]
- Faulkner, J.A. Power output of the human diaphragm. Am. Rev. Respir. Dis. 1986, 134, 1081–1083. [Google Scholar]
- Miyamoto, K.; Shimizu, K.; Masuda, K. Fast MRI used to evaluate the effect of abdominal belts during contraction of trunk muscles. Spine 2002, 27, 1749–1755. [Google Scholar] [CrossRef]
- Kolar, P.; Neuwirth, J.; Sanda, J.; Suchanek, V.; Svata, Z.; Volejnik, J.; Pivec, M. Analysis of diaphragm movement during tidal breathing and during its activation while breath holding using MRI synchronized with spirometry. Physiol. Res. 2009, 58, 383–392. [Google Scholar] [CrossRef]
- Hodges, P.W.; Butler, J.E.; McKenzie, D.K.; Gandevia, S.C. Contraction of the human diaphragm during rapid postural adjustments. J. Physiol. 1997, 505, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Kolar, P.; Sulc, J.; Kyncl, M.; Sanda, J.; Cakrt, O.; Andel, R.; Kumagai, K.; Kobesova, A. Postural function of the diaphragm in persons with and without chronic low back pain. J. Orthop. Sports Phys. Ther. 2012, 42, 352–362. [Google Scholar] [CrossRef] [Green Version]
- Added, M.A.N.; Costa, L.O.P.; Fukuda, T.Y.; de Freitas, D.G.; Salomão, E.C.; Monteiro, R.L.; da Costa, L.C.M. Efficacy of adding the Kinesio Taping method to guideline-endorsed conventional physiotherapy in patients with chronic nonspecific low back pain: A randomised controlled trial. BMC Musculoskelet. Disord. 2013, 14, 301. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Zhao, J.; Zhao, L.; Liu, Z.; Zhang, J.; Sun, D.; Song, L.; Xia, Y. Pelvic floor muscle exercise for chronic low back pain. J. Int. Med. Res. 2013, 41, 146–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliday, M.H.; Pappas, E.; Hancock, M.J.; Clare, H.A.; Pinto, R.Z.; Robertson, G.; Ferreira, P.H. A Randomized Controlled Trial Comparing the McKenzie Method to Motor Control Exercises in People With Chronic Low Back Pain and a Directional Preference. J. Orthop. Sports Phys. Ther. 2016, 46, 514–522. [Google Scholar] [CrossRef] [Green Version]
- Finta, R.; Nagy, E.; Bender, T. The effect of diaphragm training on lumbar stabilizer muscles: A new concept for improving segmental stability in the case of low back pain. J. Pain Res. 2018, 11, 3031–3045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ithamar, L.; de Moura Filho, A.G.; Benedetti Rodrigues, M.A.; Duque Cortez, K.C.; Machado, V.G.; de Paiva Lima, C.R.O.; Moretti, E.; Lemos, A. Abdominal and pelvic floor electromyographic analysis during abdominal hypopressive gymnastics. J. Bodyw. Mov. Ther. 2018, 22, 159–165. [Google Scholar] [CrossRef]
- Navarro Brazález, B.; Sánchez Sánchez, B.; Prieto Gómez, V.; De La Villa Polo, P.; McLean, L.; Torres Lacomba, M. Pelvic floor and abdominal muscle responses during hypopressive exercises in women with pelvic floor dysfunction. Neurourol. Urodyn. 2020, 39, 793–803. [Google Scholar] [CrossRef]
- Resende, A.P.M.; Bernardes, B.T.; Stüpp, L.; Oliveira, E.; Castro, R.A.; Girão, M.J.; Sartori, M.G. Pelvic floor muscle training is better than hypopressive exercises in pelvic organ prolapse treatment: An assessor-blinded randomized controlled trial. Neurourol. Urodyn. 2019, 38, 876–877. [Google Scholar] [CrossRef]
- Navarro-Brazález, B.; Prieto-Gómez, V.; Prieto-Merino, D.; Sánchez-Sánchez, B.; McLean, L.; Torres-Lacomba, M. Effectiveness of Hypopressive Exercises in Women with Pelvic Floor Dysfunction: A Randomised Controlled Trial. J. Clin. Med. 2020, 9, 1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jose-Vaz, L.A.; Andrade, C.L.; Cardoso, L.C.; Bernardes, B.T.; Pereira-Baldon, V.S.; Resende, A.P.M. Can abdominal hypropressive technique improve stress urinary incontinence? an assessor-blinded randomized controlled trial. Neurourol. Urodyn. 2020, 39, 2314–2321. [Google Scholar] [CrossRef]
- Soriano, L.; González-Millán, C.; Álvarez Sáez, M.M.; Curbelo, R.; Carmona, L. Effect of an abdominal hypopressive technique programme on pelvic floor muscle tone and urinary incontinence in women: A randomised crossover trial. Physiotherapy 2020, 108, 37–44. [Google Scholar] [CrossRef]
- Boussuges, A.; Gole, Y.; Blanc, P. Diaphragmatic motion studied by m-mode ultrasonography: Methods, reproducibility, and normal values. Chest 2009, 135, 391–400. [Google Scholar] [CrossRef]
- Harper, C.J.; Shahgholi, L.; Cieslak, K.; Hellyer, N.J.; Strommen, J.A.; Boon, A.J. Variability in diaphragm motion during normal breathing, assessed with B-mode ultrasound. J. Orthop. Sports Phys. Ther. 2013, 43, 927–931. [Google Scholar] [CrossRef] [Green Version]
- Testa, A.; Soldati, G.; Giannuzzi, R.; Berardi, S.; Portale, G.; Gentiloni Silveri, N. Ultrasound M-mode assessment of diaphragmatic kinetics by anterior transverse scanning in healthy subjects. Ultrasound Med. Biol. 2011, 37, 44–52. [Google Scholar] [CrossRef]
- Goligher, E.C.; Laghi, F.; Detsky, M.E.; Farias, P.; Murray, A.; Brace, D.; Brochard, L.J.; Sebastien-Bolz, S.; Rubenfeld, G.D.; Kavanagh, B.P.; et al. Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: Feasibility, reproducibility and validity. Intensive Care Med. 2015, 41, 642–649. [Google Scholar] [CrossRef]
- Vostatek, P.; Novák, D.; Rychnovský, T.; Rychnovská, Š. Diaphragm Postural Function Analysis Using Magnetic Resonance Imaging. PLoS ONE 2013, 8, e56724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo-Lobo, C.; Almazán-Polo, J.; Becerro-de-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Palomo-López, P.; Rodríguez-Sanz, D.; López-López, D. Ultrasonography comparison of diaphragm thickness and excursion between athletes with and without lumbopelvic pain. Phys. Ther. Sport 2019, 37, 128–137. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. Trials 2010, 11, 32. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Janssens, L.; Brumagne, S.; McConnell, A.K.; Hermans, G.; Troosters, T.; Gayan-Ramirez, G. Greater diaphragm fatigability in individuals with recurrent low back pain. Respir. Physiol. Neurobiol. 2013, 188, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Whittaker, J.L.; Warner, M.B.; Stokes, M. Comparison of the Sonographic Features of the Abdominal Wall Muscles and Connective Tissues in Individuals with and without Lumbopelvic Pain. J. Orthop. Sport. Phys. Ther. 2013, 43, 11–19. [Google Scholar] [CrossRef]
- Caufried, M. (Ed.) Gymnastique Adominale Hypopressive; Hôpital du Valais: Sion, Switzerland, 1997. [Google Scholar]
- Rebullido, T.R.; Pinsach, P. Hypopressive Techniques, 1st ed.; Cardeñoso: Vigo, Spain, 2015. [Google Scholar]
- Graham, B.L.; Steenbruggen, I.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; Miller, M.R.; et al. Standardization of spirometry 2019 update an official American Thoracic Society and European Respiratory Society technical statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; Macintyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Pinto, L.M.; Morogan, A.; Bourbeau, J. The COPD assessment test: A systematic review. Eur. Respir. J. 2014, 44, 873–884. [Google Scholar] [CrossRef] [Green Version]
- Dülger, E.; Bilgin, S.; Bulut, E.; İnal İnce, D.; Köse, N.; Türkmen, C.; Çetin, H.; Karakaya, J. The effect of stabilization exercises on diaphragm muscle thickness and movement in women with low back pain. J. Back Musculoskelet. Rehabil. 2018, 31, 323–329. [Google Scholar] [CrossRef]
- Keating, L.; Lubke, C.; Powell, V.; Young, T.; Souvlis, T.; Jull, G. Mid-thoracic tenderness: A comparison of pressure pain threshold between spinal regions, in asymptomatic subjects. Man. Ther. 2001, 6, 34–39. [Google Scholar] [CrossRef]
- Waller, R.; Straker, L.; O’Sullivan, P.; Sterling, M.; Smith, A. Reliability of pressure pain threshold testing in healthy pain free young adults. Scand. J. Pain 2015, 9, 38–41. [Google Scholar] [CrossRef]
- Kovacs, F.M.; Llobera, J.; Gil Del Real, M.T.; Abraira, V.; Gestoso, M.; Fernández, C.; Primaria Group, K.-A. Validation of the spanish version of the Roland-Morris questionnaire. Spine 2002, 27, 538–542. [Google Scholar] [CrossRef] [Green Version]
- Kelley, K.; Preacher, K.J. On Effect Size. Psychol. Methods 2012, 17, 137–152. [Google Scholar] [CrossRef]
- Schepens, T.; Dianti, J. Diaphragm protection: What should we target? Curr. Opin. Crit. Care 2020, 26, 35–40. [Google Scholar] [CrossRef]
- Gea, J.; Zhu, E.; Gáldiz, J.B.; Comtois, N.; Salazkin, I.; Fiz, J.A.; Grassino, A. Functional consequences of eccentric contractions of the diaphragm. Arch. Bronconeumol. 2009, 45, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Bellido-Fernández, L.; Jiménez-Rejano, J.-J.; Chillón-Martínez, R.; Lorenzo-Muñoz, A.; Pinero-Pinto, E.; Rebollo-Salas, M. Clinical relevance of massage therapy and abdominal hypopressive gymnastics on chronic nonspecific low back pain: A randomized controlled trial. Disabil. Rehabil. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barnett, W.H.; Abdala, A.P.; Paton, J.F.R.; Rybak, I.A.; Zoccal, D.B.; Molkov, Y.I. Chemoreception and neuroplasticity in respiratory circuits. Exp. Neurol. 2017, 287, 153–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo-Lobo, C.; Diez-Vega, I.; Martínez-Pascual, B.; Fernández-Martínez, S.; de la Cueva-Reguera, M.; Garrosa-Martín, G.; Rodríguez-Sanz, D. Tensiomyography, sonoelastography, and mechanosensitivity differences between active, latent, and control low back myofascial trigger points: A cross-sectional study. Medicine 2017, 96, e6287. [Google Scholar] [CrossRef]
- Tkachuk, G.A.; Harris, C.A. Psychometric properties of the tampa scale for kinesiophobia-11 (TSK-11). J. Pain 2012, 13, 970–977. [Google Scholar] [CrossRef]
- Abodonya, A.M.; Abdelbasset, W.K.; Awad, E.A.; Elalfy, I.E.; Salem, H.A.; Elsayed, S.H. Inspiratory muscle training for recovered COVID-19 patients after weaning from mechanical ventilation: A pilot control clinical study. Medicine 2021, 100, e25339. [Google Scholar] [CrossRef] [PubMed]
- Krenn, C.; Horvath, K.; Jeitler, K.; Zipp, C.; Siebenhofer-Kroitzsch, A.; Semlitsch, T. Management of non-specific low back pain in primary care—A systematic overview of recommendations from international evidence-based guidelines. Prim. Health Care Res. Dev. 2020, 21, e64. [Google Scholar] [CrossRef]

| Baseline Data | Intervention (n = 20) Mean ± SD (95% CI) | Control (n = 20) Mean ± SD (95% CI) |
|---|---|---|
| Age (years) | 23.25 ± 4.52 (21.13–25.36) | 23.90 ± 7.36 (20.45–27.34) |
| Weight (kg) | 66.02 ± 11.11 (60.82–71.22) | 66.40 ± 11.63 (60.95–71.84) |
| Height (cm) | 168.25 ± 8.44 (164.29–172.20) | 167.85 ± 7.25 (164.45–171.24) |
| Left diaphragm thickness at Tins (cm) | 1.35 ± 0.41 (1.15–1.55) | 1.37 ± 0.43 (1.16–1.58) |
| Left diaphragm thickness at Texp (cm) | 1.05 ± 0.37 (0.87–1.22) | 1.12 ± 0.37 (0.94–1.29) |
| Left diaphragm thickness at Tins-exp (cm) | 0.31 ± 0.15 (0.24–0.38) | 0.25 ± 0.13 (0.19–0.31) |
| Right diaphragm thickness at Tins (cm) | 1.35 ± 0.36 (1.17–1.52) | 1.58 ± 0.60 (0.90–3.10) |
| Right diaphragm thickness at Texp (cm) | 1.07 ± 0.29 (0.93–1.20) | 1.21 ± 0.43 (1.00–1.41) |
| Right diaphragm thickness at Tins-exp (cm) | 0.27 ± 0.22 (0.17–0.38) | 0.36 ± 0.31 (0.22–0.51) |
| PImax (%) | 98.75 ± 25.79 (86.67–110.82) | 96.70 ± 16.99 (88.74–104.65) |
| PPT (kg/cm2) | 4.44 ± 1.50 (3.73–5.14) | 5.49 ± 2.17 (4.47–6.51) |
| NRS (score) | 6.10 ± 1.55 (5.37–6.82) | 5.70 ± 2.17 (4.68–6.71) |
| CSI (score) | 27.35 ± 11.74 (21.85–32.84) | 24.75 ± 11.17 (19.51–29.98) |
| TSK-11 (scores) | 21.85 ± 5.33 (19.35–24.34) | 21.40 ± 5.15 (18.98–23.81) |
| RMQ (scores) | 3.30 ± 2.40 (2.17–4.42) | 2.90 ± 1.68 (2.11–3.68) |
| IPAQ (METs/min/week) | 2547.63 ± 2279.95 (1480.67–3614.78) | 3196.02 ± 2713.71 (1925.96–4466.08) |
| Outcome Differences after Treatment | Intervention (n = 20) Mean ± SD (95% CI) | Control (n = 20) Mean ± SD (95% CI) | Mean Difference (95% CI) | Statistics | p-Value | Effect Size (Cohen d) |
|---|---|---|---|---|---|---|
| Left diaphragm thickness at Tins (cm) | 0.24 ± 0.21 (0.14–0.34) | 0.04 ± 0.13 (−0.02–0.10) | 0.20 (0.08–0.32) | U = 94.500 | 0.004 † | d = 1.14 |
| Left diaphragm thickness at Texp (cm) | 0.04 ± 0.26 (−0.07–0.16) | 0.05 ± 0.14 (−0.01–0.12) | −0.01 (−0.14–0.12) | U = 200.500 | 1.000 † | d = 0.04 |
| Left diaphragm thickness at Tins-exp (cm) | 0.20 ± 0.21 (0.09–0.30) | −0.01 ± 0.07 (−0.04–0.02) | 0.21 (0.10–0.32) | U = 62.000 | <0.001 † | d = 1.34 |
| Right diaphragm thickness at Tins (cm) | 0.33 ± 0.32 (0.17–0.48) | −0.08 ± 0.26 (−0.20–0.04) | 0.41 (0.21–0.60) | t = 4.364 | <0.001 * | d = 1.40 |
| Right diaphragm thickness at Texp (cm) | 0.08 ± 0.34 (−0.08–0.24) | 0.00 ± 0.20 (−0.09–0.09) | 0.08 (−0.10 –0.26) | t = 0.890 | 0.379 * | d = 0.28 |
| Right diaphragm thickness at Tins-exp (cm) | 0.26 ± 0.20 (0.16–0.36) | −0.07 ± 0.22 (−0.18–0.03) | 0.34 (0.20–0.48) | t = 4.929 | <0.001 * | d = 1.56 |
| PImax (%) | 20.45 ± 11.83 (14.91–25.98) | −2.80 ± 8.00 (−6.54–0.94) | 23.25 (16.75–29.74) | t = 7.278 | <0.001 * | d = 2.30 |
| PPT (kPa) | 0.69 ± 1.48 (0.00–1.39) | 0.42 ± 1.21 (−0.14–0.99) | 0.27 (−0.59 –1.13) | t = 0.631 | 0.631 * | d = 0.19 |
| NRS (score) | −2.20 ± 1.73 (−3.01–−1.38) | 0.05 ± 2.11 (−0.93–1.03) | −2.25 (−3.48–−1.01) | t = −3.679 | 0.001 * | d = 1.16 |
| CSI (score) | −5.80 ± 8.69 (−9.86–−1.73) | 0.15 ± 5.82 (−2.57–2.87) | −5.95 (−10.68–1.21) | t = −2.543 | 0.015 * | d = 0.80 |
| TSK-11 (scores) | −1.45 ± 4.17 (−3.40–0.50) | 0.55 ± 4.44 (−1.52–2.62) | −2.00 (−4.75–0.75) | t = −1.467 | 0.150 * | d = 0.46 |
| RMQ (scores) | −1.30 ± 1.94 (−2.21–−0.38) | 0.10 ± 1.97 (−0.82–1.02) | −1.40 (−2.65–−0.14) | U = 276.000 | 0.040 † | d = 0.71 |
| IPAQ (METs/min/week) | −29.63 ± 1321.60 (−648.16–588.90) | −356.42 ± 1834.94 (−1215.20–502.35) | 326.79 (−696.84–1350.43) | t = 0.646 | 0.522 * | d = 0.20 |
| Outcome Measurement Differences | Model (β) | R2 Change | Model R2 |
|---|---|---|---|
| Left diaphragm thickness at Tins (cm) | 0.450 −0.205 * Group | 0.250 ‡ | 0.250 |
| Left diaphragm thickness at Tins-exp (cm) | 0.414 −0.214 * Group | 0.312 ‡ | 0.312 |
| Right diaphragm thickness at Tins (cm) | 0.740 −0.410 * Group | 0.334 ‡ | 0.334 |
| Right diaphragm thickness at Tins-exp (cm) | 0.217 −0.341 * Group | 0.390 ‡ | 0.390 |
| PImax (%) | 43.700 −23.250 * Group | 0.582 ‡ | 0.582 |
| NRS (score) | −4.450 +2.250 * Group | 0.263 ‡ | 0.263 |
| CSI (score) | −13.340 +2.117 * PPT at baseline | 0.266 ‡ | 0.266 |
| RMQ (score) | −2.700 +1.400 * Group | 0.118 † | 0.118 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente-Campos, D.; Sanchez-Jorge, S.; Terrón-Manrique, P.; Guisard, M.; Collin, M.; Castaño, B.; Rodríguez-Sanz, D.; Becerro-de-Bengoa-Vallejo, R.; Chicharro, J.L.; Calvo-Lobo, C. The Main Role of Diaphragm Muscle as a Mechanism of Hypopressive Abdominal Gymnastics to Improve Non-Specific Chronic Low Back Pain: A Randomized Controlled Trial. J. Clin. Med. 2021, 10, 4983. https://doi.org/10.3390/jcm10214983
Vicente-Campos D, Sanchez-Jorge S, Terrón-Manrique P, Guisard M, Collin M, Castaño B, Rodríguez-Sanz D, Becerro-de-Bengoa-Vallejo R, Chicharro JL, Calvo-Lobo C. The Main Role of Diaphragm Muscle as a Mechanism of Hypopressive Abdominal Gymnastics to Improve Non-Specific Chronic Low Back Pain: A Randomized Controlled Trial. Journal of Clinical Medicine. 2021; 10(21):4983. https://doi.org/10.3390/jcm10214983
Chicago/Turabian StyleVicente-Campos, Davinia, Sandra Sanchez-Jorge, Pablo Terrón-Manrique, Marion Guisard, Marion Collin, Borja Castaño, David Rodríguez-Sanz, Ricardo Becerro-de-Bengoa-Vallejo, José López Chicharro, and César Calvo-Lobo. 2021. "The Main Role of Diaphragm Muscle as a Mechanism of Hypopressive Abdominal Gymnastics to Improve Non-Specific Chronic Low Back Pain: A Randomized Controlled Trial" Journal of Clinical Medicine 10, no. 21: 4983. https://doi.org/10.3390/jcm10214983
APA StyleVicente-Campos, D., Sanchez-Jorge, S., Terrón-Manrique, P., Guisard, M., Collin, M., Castaño, B., Rodríguez-Sanz, D., Becerro-de-Bengoa-Vallejo, R., Chicharro, J. L., & Calvo-Lobo, C. (2021). The Main Role of Diaphragm Muscle as a Mechanism of Hypopressive Abdominal Gymnastics to Improve Non-Specific Chronic Low Back Pain: A Randomized Controlled Trial. Journal of Clinical Medicine, 10(21), 4983. https://doi.org/10.3390/jcm10214983







