The Role of Rehabilitative Ultrasound Imaging Technique in the Lumbopelvic Region as a Diagnosis and Treatment Tool in Physiotherapy: Systematic Review, Meta-Analysis and Meta-Regression
Abstract
1. Introduction
1.1. Rationale
1.2. Objectives
2. Materials and Methods
2.1. Eligibility Criteria and Information Sources
2.2. Search Strategy
2.3. Selection Process and Data Collection
2.4. Data Items
2.5. Study Risk of Bias Assessment
2.6. Synthesis Methods
3. Results
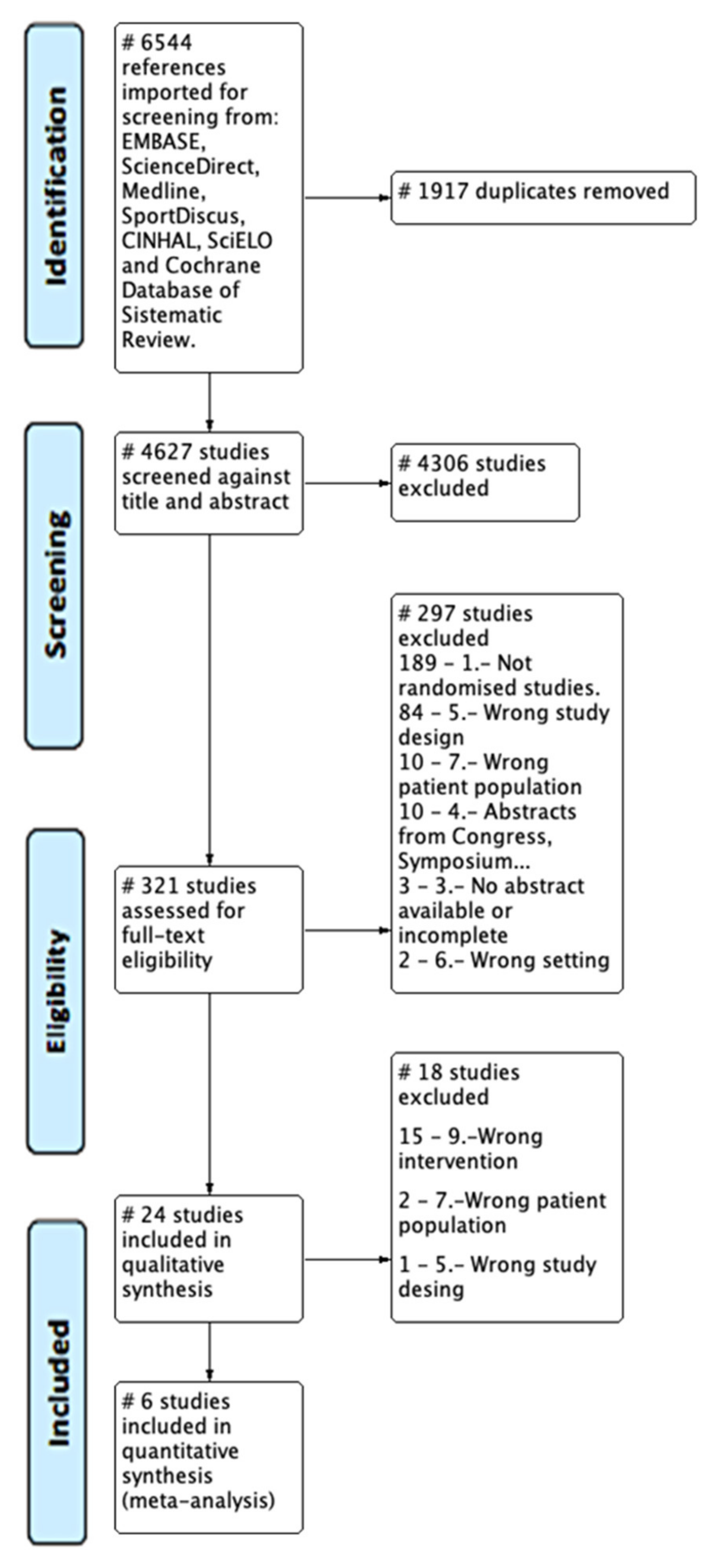
3.1. Study Selection (Flow of Studies)
3.2. Study Characteristics and Risk of Bias
3.2.1. Lumbar
3.2.2. Abdominal
3.2.3. Pelvic Floor
3.3. Results of Syntheses
4. Discussion
Assessment of Techniques. Biofeedback
5. Conclusions
Future Lines of Research
6. Other Information
Registration and Protocol
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teyhen, D. Rehabilitative Ultrasound Imaging Symposium San Antonio, TX, 8–10 May 2006. J. Orthop. Sport Phys. Ther. 2006, 36, A1–A3. [Google Scholar] [CrossRef]
- ISEAPT. International Society for Electrophysical Agents in Physical Therapy (ISEAPT) World Confederation for Physical Therapy. 2011. Available online: https://www.wcpt.org/iseapt (accessed on 7 December 2018).
- Fernández-Carnero, S.; Calvo-Lobo, C.; Garrido-Marin, A.; Arias-Buría, J. 2nd Rehabilitative Ultrasound Imaging Symposium in Physiotherapy—Madrid, Spain, 3–5 June 2016. Br. J. Sports Med. 2018, 52 (Suppl. 2), A1–A6. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Ellis, R.; Hodges, P.W.; OSullivan, C.; Hides, J.; Fernandez-Carnero, S.; Arias-Buria, J.L.; Teyhen, D.S.; Stokes, M.J. Imaging with ultrasound in physical therapy: What is the PT’s scope of practice? A competency-based educational model and training recommendations. Br. J. Sports Med. 2019, 53, 100193. [Google Scholar] [CrossRef]
- Young, A.; Stokes, M.; Crowe, M. Size and strength of the quadriceps muscles of old and young women. Eur. J. Clin. Investig. 1984, 14, 282–287. [Google Scholar] [CrossRef]
- Young, A.; Stokes, M.; Crowe, M. The size and strength of the quadriceps muscles of old and young men. Clin. Physiol. 1985, 5, 145–154. [Google Scholar] [CrossRef]
- Young, A.; Stokes, M.; Round, J.M.; Edwards, R.H. The effect of high-resistance training on the strength and cross-sectional area of the human quadriceps. Eur. J. Clin. Investig. 1983, 13, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Hides, J.A.; Richardson, C.A.; Jull, G.A. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine 1996, 21, 2763–2769. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.L.; Warner, M.B.; Stokes, M.J. Induced transducer orientation during ultrasound imaging: Effects on abdominal muscle thickness and bladder position. Ultrasound Med. Biol. 2009, 35, 1803–1811. [Google Scholar] [CrossRef]
- Woolf, A.D. Bone and Joint Decade report: Moving together beyond the decade. Preface. Best Pract. Res. Clin. Rheumatol. 2012, 26, 167–168. [Google Scholar] [CrossRef] [PubMed]
- Patrick, N.; Emanski, E.; Knaub, M.A. Acute and Chronic Low Back Pain. Med. Clin. N. Am. 2014, 98, 777–789. [Google Scholar] [CrossRef]
- Airaksinen, O.; Brox, J.I.; Cedraschi, C.; Hildebrandt, J.; Klaber-Moffett, J.; Kovacs, F.; Mannion, A.F.; Reis, S.; Staal, J.B.; Ursin, H.; et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur. Spine J. 2006, 15 (Suppl. S2), S192–S300. [Google Scholar] [CrossRef]
- Mohseni-Bandpei, M.A.; Fakhri, M.; Ahmad-Shirvani, M.; Bagheri-Nessami, M.; Khalilian, A.R.; Shayesteh-Azar, M.; Mohseni-Bandpei, H. Low back pain in 1,100 Iranian pregnant women: Prevalence and risk factors. Spine J. 2009, 9, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.F.; Muller, R.; Grant, W.D. Low Back Pain in Australian Adults: The Economic Burden. Asia Pac. J. Public Health 2003, 15, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Maniadakis, N.; Gray, A. The economic burden of back pain in the UK. Pain 2000, 84, 95–103. [Google Scholar] [CrossRef]
- Nakashima, H.; Yukawa, Y.; Suda, K.; Yamagata, M.; Ueta, T.; Kato, F. Abnormal findings on magnetic resonance images of the cervical spines in 1211 asymptomatic subjects. Spine 2015, 40, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Herzog, R.; Elgort, D.R.; Flanders, A.E.; Moley, P.J. Variability in diagnostic error rates of 10 MRI centers performing lumbar spine MRI examinations on the same patient within a 3-week period. Spine J. 2017, 17, 554–561. [Google Scholar] [CrossRef]
- Waris, E.; Eskelin, M.; Hermunen, H.; Kiviluoto, O.; Paajanen, H. Disc degeneration in low back pain: A 17-year follow-up study using magnetic resonance imaging. Spine 2007, 32, 681–684. [Google Scholar] [CrossRef]
- Liu, I.S.; Chai, H.M.; Yang, J.L.; Wang, S.F. Inter-session reliability of the measurement of the deep and superficial layer of lumbar multifidus in young asymptomatic people and patients with low back pain using ultrasonography. Man. Ther. 2013, 18, 481–486. [Google Scholar] [CrossRef]
- Hides, J.A.; Miokovic, T.; Belavy, D.L.; Stanton, W.R.; Richardson, C.A. Ultrasound imaging assessment of abdominal muscle function during drawing-in of the abdominal wall: An intrarater reliability study. J. Orthop. Sports Phys. Ther. 2007, 37, 480–486. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Chai, H.-M.; Yang, J.-L.; Lin, Y.-J.; Wang, S.-F. Reliability and Validity of Transversus Abdominis Measurement at the Posterior Muscle-Fascia Junction with Ultrasonography in Asymptomatic Participants. J. Manip. Physiol. Ther. 2015, 38, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.M.; Behbahani, R.B.; Lorestani, L.; Azari, A. Original article: Assessment of pelvic floor muscle function in women with and without low back pain using transabdominal ultrasound. Man. Ther. 2010, 15, 235–239. [Google Scholar] [CrossRef] [PubMed]
- de Abreu Etienne, M.; de Oliveira, A.L.; da Silva Carramão, S.; Macea, J.R.; Aoki, T.; Auge, A.P.F. Pubococcygeal activity on perineal ultrasound in incontinent women. Int. Urogynecol. J. 2011, 22, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Hebert, J.J.; Koppenhaver, S.L.; Parent, E.C.; Fritz, J.M. A systematic review of the reliability of rehabilitative ultrasound imaging for the quantitative assessment of the abdominal and lumbar trunk muscles. Spine 2009, 34, E848–E856. [Google Scholar] [CrossRef]
- Koppenhaver, S.L.; Hebert, J.J.; Parent, E.C.; Fritz, J.M. Rehabilitative ultrasound imaging is a valid measure of trunk muscle size and activation during most isometric sub-maximal contractions: A systematic review. Aust. J. Physiother. 2009, 55, 153–169. [Google Scholar] [CrossRef]
- Schwartz, R.G.; Rohan, J.; Hayden, F. Diagnostic paraspinal musculoskeletal ultrasonography. J. Back Musculoskelet. Rehabil. 1999, 12, 25. [Google Scholar] [CrossRef]
- Cheng, C.; MacIntyre, N.J. Real-Time Ultrasound Imaging in Physiotherapy Evaluation and Treatment of Transversus Abdominus and Multifidus Muscles in Individuals with Low-Back Pain. Crit. Rev. Phys. Rehabil. Med. 2010, 22, 279–300. [Google Scholar] [CrossRef]
- Tubaro, A.; Koelbl, H.; Laterza, R.; Khullar, V.; de Nunzio, C. Ultrasound imaging of the pelvic floor: Where are we going? Neurourol. Urodyn. 2011, 30, 729–734. [Google Scholar] [CrossRef]
- Chipchase, L.; Thoirs, K.; Jedrzejczak, A. The effectiveness of real time ultrasound as a biofeedback tool for muscle retraining. Phys. Ther. Rev. 2009, 14, 124–131. [Google Scholar] [CrossRef]
- Carnero, S.F.; Buria, J.A.; Zaldivar, J.C.; Quiñones, A.L.; Calvo-Lobo, C.; Saborido, C.M. Rehabilitative Ultrasound Imaging Evaluation in Physiotherapy: Piloting a Systematic Review. Appl. Sci. 2019, 9, 181. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011. Available online: https://handbook-5-1.cochrane.org/ (accessed on 2 January 2020).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Veritas Health Innovation Melbourne Australia. Covidence Systematic Review Software. 2020. Available online: https://www.covidence.org/ (accessed on 19 August 2020).
- The Cochrane Collaboration. Review Manager Web (RevMan Web). The Cochrane Collaboration. 2020. Available online: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman (accessed on 30 November 2021).
- Stata Corporation. College Station TX: StataCorp LLC. Stata Statistical Software: Release 16, 2019. 2019. Available online: https://www.stata.com/ (accessed on 30 November 2021).
- Hebert, J.J.; Fritz, J.M.; Thackeray, A.; Koppenhaver, S.L.; Teyhen, D. Early multimodal rehabilitation following lumbar disc surgery: A randomised clinical trial comparing the effects of two exercise programmes on clinical outcome and lumbar multifidus muscle function. Br. J. Sports Med. 2015, 49, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Van, K.; Hides, J.A.; Richardson, C.A. The use of real-time ultrasound imaging for biofeedback of lumbar multifidus muscle contraction in healthy subjects. J. Orthop. Sport Phys. Ther. 2006, 36, 920–925. [Google Scholar] [CrossRef]
- Ferreira, P.H.; Ferreira, M.L.; Maher, C.G.; Refshauge, K.; Herbert, R.D.; Hodges, P.W. Changes in recruitment of transversus abdominis correlate with disability in people with chronic low back pain. Br. J. Sport Med. 2010, 44, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, R.J.; Grindstaff, T.L.; Croy, T.; Ingersoll, C.D.; Saliba, S.A. The Effect of Traditional Bridging or Suspension-Exercise Bridging on Lateral Abdominal Thickness in Individuals with Low Back Pain. J. Sport Rehabil. 2012, 21, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Teyhen, D.; Miltenberger, C.; Deiters, H.; Del Toro, Y.; Pulliam, J.; Childs, J.D.; Boyles, R.E.; Flynn, T.W. The use of ultrasound imaging of the abdominal drawing-in maneuver in subjects with low back pain. J. Orthop. Sport Phys. Ther. 2005, 35, 346–355. [Google Scholar] [CrossRef]
- Bruno Teixeira, B.; Ana Paula Magalhães, R.; Liliana, S.; Emerson, O.; Rodrigo Aquino, C.; Zsuzsanna Ilona Katalin, J.d.B.; Batista Castello Girãoet, M.J.; Gracio Ferreira Sartori, M. Efficacy of pelvic floor muscle training and hypopressive exercises for treating pelvic organ prolapse in women: Randomized controlled trial/Eficácia do treinamento da musculatura do assoalho pélvico e de exercícios hipopressivos para o tratamento do pr. Sao Paulo Med. J. 2012, 130, 5–9. [Google Scholar] [CrossRef]
- Halliday, M.H.; Pappas, E.; Hancock, M.J.; Clare, H.A.; Pinto, R.Z.; Robertson, G.; Ferreira, P.H. A Randomized Controlled Trial Comparing the McKenzie Method to Motor Control Exercises in People With Chronic Low Back Pain and a Directional Preference. J. Orthop. Sports Phys. Ther. 2016, 46, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, N.; Mohseni Bandpei, M.A.; Mosallanezhad, Z.; Rahgozar, M.; Jaberzadeh, S. The Effect of 2 Different Exercise Programs on Pain Intensity and Muscle Dimensions in Patients With Chronic Low Back Pain: A Randomized Controlled Trial. J. Manip. Physiol. Ther. 2017, 41, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, M.; Sarrafzadeh, J.; Jamshidi, A.; Zarabi, V.; Pourahmadi, M.R. The effect of core stability and general exercise on abdominal muscle thickness in non-specific chronic low back pain using ultrasound imaging. Physiother. Theory Pract. 2016, 32, 277–283. [Google Scholar] [CrossRef]
- Hides, J.A.; Richardson, C.A.; Jull, G.A. Magnetic resonance imaging and ultrasonography of the lumbar multifidus muscle: Comparison of two different modalities. Spine 1995, 20, 54–58. [Google Scholar] [CrossRef]
- Wachi, M.; Suga, T.; Higuchi, T.; Misaki, J.; Tsuchikane, R.; Tanaka, D.; Miyake, Y.; Isaka, T. Applicability of ultrasonography for evaluating trunk muscle size: A pilot study. J. Phys. Ther. Sci. 2017, 29, 245–249. [Google Scholar] [CrossRef]
- Stokes, M.; Rankin, G.; Newham, D.J. Original article: Ultrasound imaging of lumbar multifidus muscle: Normal reference ranges for measurements and practical guidance on the technique. Man. Ther. 2005, 10, 116–126. [Google Scholar] [CrossRef]
- Hides, J.; Stokes, M.; Saide, M.; Jull, G.; Cooper, D. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine 1994, 19, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Koppenhaver, S.L.; Parent, E.C.; Teyhen, D.S.; Hebert, J.J.; Fritz, J.M. The effect of averaging multiple trials on measurement error during ultrasound imaging of transversus abdominis and lumbar multifidus muscles in individuals with low back pain. J. Orthop. Sports Phys. Ther. 2009, 39, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Wallwork, T.L.; Hides, J.A.; Stanton, W.R. Intrarater and interrater reliability of assessment of lumbar multifidus muscle thickness using rehabilitative ultrasound imaging. J. Orthop. Sport Phys. Ther. 2007, 37, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, O.; Djordjevic, A.; Konstantinovic, L. Interrater and intrarater reliability of transverse abdominal and lumbar multifidus muscle thickness in subjects with and without low back pain. J. Orthop. Sports Phys. Ther. 2014, 44, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Teyhen, D.S.; Childs, J.D.; Stokes, M.J.; Wright, A.C.; Dugan, J.L.; George, S.Z. Abdominal and lumbar multifidus muscle size and symmetry at rest and during contracted States. Normative reference ranges. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2012, 31, 1099–1110. [Google Scholar]
- Koppenhaver, S.L.; Hebert, J.J.; Fritz, J.M.; Parent, E.C.; Teyhen, D.S.; Magel, J.S. Reliability of rehabilitative ultrasound imaging of the transversus abdominis and lumbar multifidus muscles. Arch. Phys. Med. Rehabil. 2009, 90, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Abiko, T.; Takei, H.; Shimamura, R.; Abiko, Y.; Yamamoto, J.; Sakasai, T.; Soma, M.; Ogawa, D.; Yamaguchi, T.; Hata, M. Reliability of Rehabilitative Ultrasound Imaging of the Lumbar Multifidus. Rigakuryoho Kagaku 2011, 26, 693–697. [Google Scholar] [CrossRef]
- Macdonald, D.A.; Dawson, A.P.; Hodges, P.W. Behavior of the Lumbar Multifidus during Lower Extremity Movements in People With Recurrent Low Back Pain During Symptom Remission. J. Orthop. Sport Phys. Ther. 2011, 41, 155. [Google Scholar] [CrossRef]
- Coldron, Y.; Stokes, M.; Cook, K. Lumbar multifidus muscle size does not differ whether ultrasound imaging is performed in prone or side lying. Man. Ther. 2003, 8, 161–165. [Google Scholar] [CrossRef]
- Reeve, A.; Dilley, A. Effects of posture on the thickness of transversus abdominis in pain-free subjects. Man. Ther. 2009, 14, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Choi, J.D.; Kim, S.Y.; Oh, D.W.; Kim, J.K.; Park, J.W. Comparison between muscle activation measured by electromyography and muscle thickness measured using ultrasonography for effective muscle assessment. J. Electromyogr. Kinesiol. 2014, 24, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Kiesel, K.B.; Uhl, T.L.; Underwood, F.B.; Rodd, D.W.; Nitz, A.J. Original article: Measurement of lumbar multifidus muscle contraction with rehabilitative ultrasound imaging. Man. Ther. 2007, 12, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, N.; O’Sullivan, C.; Kelly, G. Multifidus muscle size and percentage thickness changes among patients with unilateral chronic low back pain (CLBP) and healthy controls in prone and standing. Man. Ther. 2014, 19, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Qiuchen, H.; Yuying, Z.; Desheng, L.I.; Degang, Y.; Ming, H.U.O.; Hitoshi, M. The Evaluation of Chronic Low Back Pain by Determining the Ratio of the Lumbar Multifidus Muscle Cross-sectional Areas of the Unaffected and Affected Sides. J. Phys. Ther. Sci. 2014, 26, 1613–1614. [Google Scholar]
- Hides, J.A.; Oostenbroek, T.; Franettovich Smith, M.M.; Mendis, M.D. The effect of low back pain on trunk muscle size/function and hip strength in elite football (soccer) players. J. Sports Sci. 2016, 34, 2303–2311. [Google Scholar] [CrossRef]
- Wallwork, T.L.; Stanton, W.R.; Freke, M.; Hides, J.A. Original Article: The effect of chronic low back pain on size and contraction of the lumbar multifidus muscle. Man. Ther. 2009, 14, 496–500. [Google Scholar] [CrossRef]
- Kiesel, K.B.; Uhl, T.; Underwood, F.B.; Nitz, A.J. Original Article: Rehabilitative ultrasound measurement of select trunk muscle activation during induced pain. Man. Ther. 2008, 13, 132–138. [Google Scholar] [CrossRef]
- Warner, M.B.; Cotton, A.M.; Stokes, M.J. Comparison of curvilinear and linear ultrasound imaging probes for measuring cross-sectional area and linear dimensions. J. Med. Eng. Technol. 2008, 32, 498–504. [Google Scholar] [CrossRef]
- Worsley, P.R.; Smith, N.; Warner, M.B.; Stokes, M. Ultrasound transducer shape has no effect on measurements of lumbar multifidus muscle size. Man. Ther. 2012, 17, 187–191. [Google Scholar] [CrossRef]
- Rho, M.; Spitznagle, T.; Van Dillen, L.; Maheswari, V.; Oza, S.; Prather, H. Gender Differences on Ultrasound Imaging of Lateral Abdominal Muscle Thickness in Asymptomatic Adults: A Pilot Study. PM R 2013, 5, 374–380. [Google Scholar] [CrossRef]
- Tahan, N.; Khademi-Kalantari, K.; Mohseni-Bandpei, M.A.; Mikaili, S.; Baghban, A.A.; Jaberzadeh, S. Measurement of superficial and deep abdominal muscle thickness: An ultrasonography study. J. Physiol. Anthropol. 2016, 35, 17. [Google Scholar] [CrossRef]
- Rankin, G.; Stokes, M.; Newham, D.J. Abdominal muscle size and symmetry in normal subjects. Muscle Nerve 2006, 34, 320–326. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Warner, M.B.; Stokes, M. Comparison of the sonographic features of the abdominal wall muscles and connective tissues in individuals with and without lumbopelvic pain. J. Orthop. Sport Phys. Ther. 2013, 43, 11–19. [Google Scholar] [CrossRef]
- Coldron, Y.; Stokes, M.J.; Newham, D.J.; Cook, K. Postpartum characteristics of rectus abdominis on ultrasound imaging. Man. Ther. 2008, 13, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, C.M.; Macauley, J.A. Concurrent Validity of Calipers and Ultrasound Imaging to Measure Interrecti Distance. J. Orthop. Sport Phys. Ther. 2013, 43, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Mota, P.; Pascoal, A.G.; Sancho, F.; Carita, A.I.; Bø, K. Technical and measurement report: Reliability of the inter-rectus distance measured by palpation. Comparison of palpation and ultrasound measurements. Man. Ther. 2013, 18, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Liaw, L.-J.; Hsu, M.-J.; Liao, C.-F.; Liu, M.-F.; Hsu, A.-T. The relationships between inter-recti distance measured by ultrasound imaging and abdominal muscle function in postpartum women: A 6-month follow-up study. J. Orthop. Sports Phys. Ther. 2011, 41, 435–443. [Google Scholar] [CrossRef]
- Teyhen, D.S.; Williamson, J.N.; Carlson, N.H.; Suttles, S.T.; O’Laughlin, S.J.; Whittaker, J.L.; Goffar, S.L.; Childs, J.D. Original article: Ultrasound Characteristics of the Deep Abdominal Muscles During the Active Straight Leg Raise Test. Arch. Phys. Med. Rehabil. 2009, 90, 761–767. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.L.; Watson, T. Reproducibility of ultrasound measurement of transversus abdominis during loaded, functional tasks in asymptomatic young adults. PM R J. Inj. Funct. Rehabil. 2012, 4, 402–412. [Google Scholar] [CrossRef]
- Larivière, C.; Gagnon, D.; De Oliveira Junior, E.; Henry, S.M.; Dumas, J.-P. Reliability of ultrasound measures of the abdominal muscles: Effect of task and transducer position. PM R 2011, 97, eS383–eS384. [Google Scholar] [CrossRef]
- Kim, K.H.; Cho, S.-H.; Goo, B.-O.; Baek, I.-H. Differences in Transversus Abdominis Muscle Function between Chronic Low Back Pain Patients and Healthy Subjects at Maximum Expiration: Measurement with Real-time Ultrasonography. J. Phys. Ther. Sci. 2013, 25, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, F.; Arab, A.M.; Salavati, M.; Jaberzadeh, S.; Hajihasani, A. Ultrasound Measurement of Abdominal Muscle Thickness With and Without Transducer Fixation During Standing Postural Tasks in Participants With and Without Chronic Low Back Pain: Intrasession and Intersession Reliability. PM R 2016, 8, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; Pengel, L.H.M.; Herbert, R.D.; Gandevia, S.C. Measurement of muscle contraction with ultrasound imaging. Muscle Nerve 2003, 27, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.H.; Ferreira, M.L.; Nascimento, D.P.; Pinto, R.Z.; Franco, M.R.; Hodges, P.W. Discriminative and reliability analyses of ultrasound measurement of abdominal muscles recruitment. Man. Ther. 2011, 16, 463–469. [Google Scholar] [CrossRef]
- McMeeken, J.M.; Beith, I.D.; Newham, D.J.; Milligan, P.; Critchley, D.J. The relationship between EMG and change in thickness of transversus abdominis. Clin. Biomech. 2004, 19, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-H.; Park, D.-J. Reliability of ultrasound in combination with surface electromyogram for evaluating the activity of abdominal muscles in individuals with and without low back pain. J. Exerc. Rehabil. 2014, 10, 230–235. [Google Scholar] [CrossRef]
- Vasseljen, O.; Fladmark, A.M.; Westad, C.; Torp, H.G. Onset in abdominal muscles recorded simultaneously by ultrasound imaging and intramuscular electromyography. J. Electromyogr. Kinesiol. 2009, 19, e23–e31. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.L.; Mclean, L.; Hodder, J.; Warner, M.B.; Stokes, M.J. Association Between Changes in Electromyographic Signal Amplitude and Abdominal Muscle Thickness in Individuals With and Without Lumbopelvic Pain. J. Orthop. Sport Phys. Ther. 2013, 43, 466–477. [Google Scholar] [CrossRef]
- Arab, A.M.; Rasouli, O.; Amiri, M.; Tahan, N. Reliability of ultrasound measurement of automatic activity of the abdominal muscle in participants with and without chronic low back pain. Chiropr. Man. Ther. 2013, 5, 104–113. [Google Scholar] [CrossRef]
- Hides, J.A.; Belavy, D.L.; Cassar, L.; Williams, M.; Wilson, S.J.; Richardson, C.A. Altered response of the anterolateral abdominal muscles to simulated weight-bearing in subjects with low back pain. Eur. Spine J. 2009, 18, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Hides, J.A.; Wong, I.; Wilson, S.J.; Belavy, D.L.; Richardson, C.A. Assessment of abdominal muscle function during a simulated unilateral weight-bearing task using ultrasound imaging. J. Orthop. Sport Phys. Ther. 2007, 37, 467–471. [Google Scholar] [CrossRef]
- Kruger, J.; Dietz, P.; Botelho, C.; Dumoulin, C. Can we “feel” with our fingers as well as we “see” with ultrasound? Neurourol. Urodyn. 2010, 29, 1176–1177. [Google Scholar] [CrossRef]
- Koelbl, H.; Bernaschek, G. A new method for sonographic urethrocystography and simultaneous pressure-flow measurements. Obstet. Gynecol. 1989, 74, 417–422. [Google Scholar] [PubMed]
- Abraham-Justice, K.E.; Houghton, M.; Hiller, J.; Kang, S.; Hopson, S.; Meck, B. The Development and Reliability of an Objective Tool for Assessment of Pelvic Floor Muscle Function Using Diagnostic Ultrasound Imaging. J. Womens Health Phys. Ther. 2011, 35, 20–21. [Google Scholar]
- Tosun, O.C.; Turan, V.; Malkoc, M.; Ergenoglu, A.M.; Yeniel, A.O.; Itil, I.M.; Mat, E.; Solmaz, U.; Ekin, A.; Gezer, C.; et al. Comparison of digital palpation against longitudinal and tranverse abdominal sonography practices in the assessment of pelvic floor muscle strength. Fiz. Rehabil. 2010, 21, 165. [Google Scholar]
- Thompson, J.A.; O’Sullivan, P.B.; Briffa, K.; Neumann, P.; Court, S. Assessment of pelvic floor movement using transabdominal and transperineal ultrasound. Int. Urogynecol. J. Pelvic. Floor Dysfunct. 2005, 16, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Sherburn, M.; Murphy, C.A.; Carroll, S.; Allen, T.J.; Galea, M.P. Investigation of transabdominal real-time ultrasound to visualise the muscles of the pelvic floor. Aust. J. Physiother. 2005, 51, 167–170. [Google Scholar] [CrossRef]
- Stafford, R.E.; Coughlin, G.; Lutton, N.J.; Hodges, P.W. Validity of Estimation of Pelvic Floor Muscle Activity from Transperineal Ultrasound Imaging in Men. PLoS ONE 2015, 10, e0144342. [Google Scholar] [CrossRef] [PubMed]
- Roll, S.C.; Kutch, J.J. Transperineal Sonography Evaluation of Muscles and Vascularity in the Male Pelvic Floor. J. Diagn. Med. Sonogr. 2013, 29, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Dresler, M.M.; Kociszewski, J.; Wlaźlak, E.; Pędraszewski, P.; Trzeciak, A.; Surkont, G. Repeatability and reproducibility of measurements of the suburethral tape location obtained in pelvic floor ultrasound performed with a transvaginal probe. J. Ultrason. 2017, 17, 101–105. [Google Scholar] [CrossRef]
- Tunn, R.; Petri, E. Introital and transvaginal ultrasound as the main tool in the assessment of urogenital and pelvic floor dysfunction: An imaging panel and practical approach. Ultrasound Obstet. Gynecol. 2003, 22, 205–213. [Google Scholar] [CrossRef]
- Frawley, H.C.; Galea, M.P.; Phillips, B.A.; Sherburn, M.; Bø, K. Effect of test position on pelvic floor muscle assessment. Int. Urogynecol. J. Pelvic. Floor Dysfunct. 2006, 17, 365–371. [Google Scholar] [CrossRef]
- Kelly, M.; Tan, B.-K.; Thompson, J.; Carroll, S.; Follington, M.; Arndt, A.; Seet, M. Healthy adults can more easily elevate the pelvic floor in standing than in crook-lying: An experimental study. Aust. J. Physiother. 2007, 53, 187–191. [Google Scholar] [CrossRef]
- Thompson, J.A.; O’Sullivan, P.B.; Briffa, N.K.; Neumann, P. Comparison of transperineal and transabdominal ultrasound in the assessment of voluntary pelvic floor muscle contractions and functional manoeuvres in continent and incontinent women. Int. Urogynecol. J. Pelvic. Floor Dysfunct. 2007, 18, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.S.; Hirakawa, H.S.; Oliveira, A.B.; Driusso, P. Relationship among vaginal palpation, vaginal squeeze pressure, electromyographic and ultrasonographic variables of female pelvic floor muscles. Braz. J. Phys. Ther. 2014, 18, 428–434. [Google Scholar] [CrossRef]
- Stachowicz, N.; Stachowicz, S.; Smoleń, A.; Morawska, D.; Kotarski, J. Sonographic evaluation of the levator ani muscle in women with stress urinary incontinence. Ginekol. Pol. 2012, 83, 669–673. [Google Scholar] [PubMed]
- Thibault-Gagnon, S.; McLean, L.; Pukall, C.; Goldfinger, C.; Chamberlain, S. Pelvic floor muscle morphology and function in women with and without provoked vestibulodynia evaluated using 3D/4D transperineal ultrasound imaging. Physiotherapy 2011, 97, eS1228. [Google Scholar]
- Panayi, D.C.; Khullar, V.; Fernando, R.; Tekkis, P. Transvaginal ultrasound measurement of bladder wall thickness: A more reliable approach than transperineal and transabdominal approaches. BJU Int. 2010, 106, 1519–1522. [Google Scholar] [CrossRef]
- Akselim, B.; Doğanay, M.; Özcan, N.; Akselim, S.; Cavkaytar, S. Correlation of bladder wall thickness and treatment success in types of urinary incontinence. Int. Urogynecol. J. 2017, 28, 417–422. [Google Scholar] [CrossRef]
- Üçer, O.; Gümüş, B.; Albaz, A.C.; Pekindil, G. Assessment of bladder wall thickness in women with overactive bladder. Turk. Urol. Derg. 2016, 42, 97–100. [Google Scholar] [CrossRef]
- Jedrzejczak, A.; Chipchase, L. The availability and usage frequency of real time ultrasound by physiotherapists in South Australia: An observational study. Physiother. Res. Int. 2008, 13, 231–240. [Google Scholar] [CrossRef]
- McKiernan, S.; Chiarelli, P.; Warren-Forward, H. A survey of diagnostic ultrasound within the physiotherapy profession for the design of future training tools. Radiography 2011, 17, 121–125. [Google Scholar] [CrossRef]
- Potter, C.L.; Cairns, M.C.; Stokes, M. Use of ultrasound imaging by physiotherapists: A pilot study to survey use, skills and training. Man. Ther. 2012, 17, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Shelly, E.; Krum, L. Methods used by physical therapists to learn pelvic floor muscle examination. Neurourol. Urodyn. 2009, 28, 821–822. [Google Scholar] [CrossRef]
- Ellis, R.; De Jong, R.; Bassett, S.; Helsby, J.; Stokes, M.; Cairns, M. Exploring the clinical use of ultrasound imaging: A survey of physiotherapists in New Zealand. Musculoskelet. Sci. Pract. 2018, 34, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Arias-Buría, J.L.; Fernández-Carnero, S.; Calvo-Lobo, C.; Albalate-Barbero, F.R.; Cobaleda-Peragón, J.; Tapia-Amores, D.; Orenga-Nebot, V.; Martínez, J. Current status ultrasound use among spanish physiotherapists: On-line survey. In Abstracts; BMJ Publishing Group Ltd and British Association of Sport and Exercise Medicine: London, UK, 2018; p. 24. [Google Scholar] [CrossRef]
- Ellis, R.; Helsby, J.; Naus, J.; Bassett, S.; Fernández-de-Las-Peñas, C.; Carnero, S.F.; Hides, J.; O’Sullivan, C.; Teyhen, D.; Stokesi, M.; et al. Exploring the use of ultrasound imaging by physiotherapists: An international survey. Musculoskelet. Sci. Pract. 2020, 49, 102213. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Chai, H.-M.; Shau, Y.-W.; Wang, C.-L.; Wang, S.-F. Original article: Increased sliding of transverse abdominis during contraction after myofascial release in patients with chronic low back pain. Man. Ther. 2016, 23, 69–75. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, P.; Twomey, L.; Allison, G.; Phyty, G.D. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine 1997, 22, 2959–2967. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.; Hodges, P.W.; Hides, J. Manipulation Association of Chartered Physiotherapists. In Therapeutic Exercise for Lumbopelvic Stabilization: A Motor Control Approach for the Treatment and Prevention of Low Back Pain; Churchill Livingstone: London, UK, 2004; 271p. [Google Scholar]
- Critchley, D.J.; Pierson, Z.; Battersby, G. Original article: Effect of pilates mat exercises and conventional exercise programmes on transversus abdominis and obliquus internus abdominis activity: Pilot randomised trial. Man. Ther. 2011, 16, 183–189. [Google Scholar] [CrossRef]
- Kaping, K.; Ang, B.O.; Rasmussen-Barr, E. The abdominal drawing-in manoeuvre for detecting activity in the deep abdominal muscles: Is this clinical tool reliable and valid? BMJ Open 2015, 5, e00871. [Google Scholar] [CrossRef]
- Pascoal, A.G.; Dionisio, S.; Cordeiro, F.; Mota, P. Inter-rectus distance in postpartum women can be reduced by isometric contraction of the abdominal muscles: A preliminary case-control study. Physiotherapy 2014, 100, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Momose, K. The relationship between transversus abdominis thickness and abdominal pressure during successful and unsuccessful abdominal draw-in maneuvers. Physiotherapy 2015, 101, eS1454–eS1455. [Google Scholar] [CrossRef][Green Version]
- Baeßler, K.; Junginger, B. Traditional Gymnastic Exercises for the Pelvic Floor Often Lead to Bladder Neck Descent—A Study Using Perineal Ultrasound. Geburtshilfe Frauenheilkd. 2017, 77, 765–770. [Google Scholar] [CrossRef]
- Navarro Brazález, B.; Torres Lacomba, M.; Arranz Martín, B.; Sánchez Méndez, O. ORIGINAL: Respuesta muscular durante un ejercicio hipopresivo tras tratamiento de fisioterapia pelviperineal: Valoración con ecografía transabdominal. Fisioterapia 2017, 39, 187–194. [Google Scholar] [CrossRef]
- Bernardes, B.T.; Resende, A.P.M.; Stüpp, L.; Oliveira, E.; Castro, R.A.; di Bella, Z.I.K.J.; Girão, M.J.B.C.; Sartori, M.G.F. Efficacy of pelvic floor muscle training and hypopressive exercises for treating pelvic organ prolapse in women: Randomized controlled trial. Sao Paulo Med. J. 2012, 130, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Finnoff, J.T.; Hall, M.M.; Adams, E.; Berkoff, D.; Concoff, A.L.; Dexter, W.; Smith, J. American Medical Society for Sports Medicine (AMSSM) position statement: Interventional musculoskeletal ultrasound in sports medicine. PM R 2015, 7, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Finnoff, J.T.; Nutz, D.J.; Henning, P.T.; Hollman, J.H.; Smith, J. Original research: Accuracy of Ultrasound-Guided versus Unguided Pes Anserinus Bursa Injections. PM R 2010, 2, 732–739. [Google Scholar] [CrossRef]
- Testa, A.; Soldati, G.; Giannuzzi, R.; Berardi, S.; Portale, G.; Gentiloni Silveri, N. Ultrasound M-Mode Assessment of Diaphragmatic Kinetics by Anterior Transverse Scanning in Healthy Subjects. Ultrasound Med. Biol. 2011, 37, 44–52. [Google Scholar] [CrossRef]
- Gerscovich, E.O.; Cronan, M.; McGahan, J.P.; Jain, K.; Jones, C.D.; McDonald, C. Ultrasonographic evaluation of diaphragmatic motion. J. Ultrasound Med. 2001, 20, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.J.; Shahgholi, L.; Cieslak, K.; Hellyer, N.J.; Strommen, J.A.; Boon, A.J. Variability in Diaphragm Motion During Normal Breathing, Assessed With B-Mode Ultrasound. J. Orthop. Sport Phys. Ther. 2013, 43, 927–931. [Google Scholar] [CrossRef] [PubMed]
| Region | Study | Design | Descriptive Statistics | Intervention | Control | Measured Outcomes |
|---|---|---|---|---|---|---|
| Abdominal | Teyhen et al. (2005) | RCT | n= 30 Age (y) 30.8 (±10.1) 31.2 (±7.5) Height (cm) 170.7 (±9.5) 169.5 (±7.3) Body mass (kg) 77.9 (±14.1) 77.3 (±8.2) | Biofeedback Trainning (BT) | Traditional Trainnig (TT) | Main outcome: Abdominal muscles thickness |
| Chon etal. (2009) | RCT | Experimental (n = 20) Control (n = 20) Age (years) 24 (±1.6) 24 (±1.9) Height (cm) 168 (±8.9) 169 (±7.9) Weight (kg) 61 (±12.0) 59 (±9.1) | ADIM (Abdominal Draw in Maneouvre) + ankle dorsiflexion | ADIM | Main outcome measures: Ultrasonography muscle thickness and electromyography activity of abdominal muscles | |
| Costa et al. (2009) | RCT | n=35 (22 female) Age (years) 53.3 (11.27) Weight (kg) 69.3 (11.49) Height (m) 1.6 (0.08) | Motor Control Exercise (MCE) | Placebo | To test the automatic recruitment of the abdominal wall muscles by real-time ultrasound imaging | |
| Bajaj et al. (2010) | Observational | RUSI group (n=11) PBU group(n=11) Age (yrs) MEAN + SD 30.90 + 8.96 32.54 + 6.57 Height (cms) MEAN + SD 163.27 + 9.59 161.30 + 10.41.Weight (kgs) MEAN + SD 59.63 +8.64 58.68 + 9.79 BMI(kg/m2) MEAN + SD 22.5 + 1.4 22.4 + 1.58 | RUSI + ADIM | PBU (Pressure Biofeedback Unit) + ADIM | The variables available for analysis were number of days and number of trials for both RUSI and PBU groups | |
| Vasseljem et al. (2010) | RCT | UGE (n=36) SE (n=36) GE (N=37) Age 40.9 (11.5) 43.4 (10.2) 36 (10.3) BMI 24.9 (3.1) 24.9 (3.1) 24.3 (2.8) | The ultrasound guided exercise (UGE) | Sling Exercise (SE) | 1. Muscle Thickness External Oblique, Internal Oblique, Transversus Abdominis (EO, IO, TrA) 2. Pain Numeric Rating Scale (NRS) | |
| Guthrie et al. (2012) | RCT | n= 51 men (18) Age (y) 23.1 ± 6.0, Height (cm) 173.6 ± 10.5, Mass (kg) 74.7 ± 14.5, BMI (kg/m2) 24.6 ± 2.8 | Traditional bridge (TB) | Suspension-exercise bridge (SE) | Main outcome: Abdominal muscles thickness by US | |
| Ferreira et al. (2014) | RCT | MCE (n = 11) GE (n = 10) SMT (n = 13) Age, years (SD) 47.5 (±17.3) 54.9 (±11.3) 45.4 (±17.7) Weight, kg (SD) 78.7 (±13.0) 70.1 (±12.0) 72.6 (±10.2) Height, cm (SD) 171.0 (±10.8) 160.7 (±6.6) 165.0 (±8.5) Female, n (%) 6 (55) 7 (70) 10 (77) | Motor Control Exercise (MCE) | General Exercise (GE) | TrA was measured using a US. Global impression of recovery. Disability was measured using the Roland Morris disability questionnaire. Pain intensity on a numerical rating scale. Function was measured with a modified patient specific functional scale | |
| Tajiri et al. (2014) | RCT | Exercise group (n= 9) 52.1 ± 9.5 Height 156.1 ± 6.2 Weight 51.9 ± 5.3 Control group (n= 6) 52.0 ± 7.6 Height 161.0 ± 7.4 Weight 55.7 ± 13.9 | TA (Transversus Addominis) + PFM (Pelvic Floor Muscle) co-contration exercise | Control Group (CG) | Authors evaluated the thickness of the TA using ultrasound | |
| Gisela Rochade et al. (2015) | RCT | Age 31 (5) 30 (8) Weight (kg) 65.2 (9.8) 68.9 (11.7) Height (m) 1.67 (0.07) 1.67 (0.11) BMI (m2/kg) 23.2 (2.0) 24.5 (2.8) | Pilates | Strength | The aim of this study was to compare the effects of Pilates mat exercises and a conventional strength training programme on the activity of TrA and OI. They used ultrasound measures of muscle thickness as a proxy of muscle activity | |
| Gong et al. (2016) | RCT | Training group (n = 15) 27.35 ± 6.16 164.47 ± 8.32 57.70 ± 8.06 Control group (n = 15) 27.88 ± 6.99 165.00 ± 8.22 59.05 ± 9.96 | Running in place | ADIM | Ultrasonography was used to examine the abdominal muscle thicknesses before and after running in place. | |
| Halliday et al. (2016) | RCT | Age (years) Mckenzie: 48.8 (±12.1) MCE: 48.3 (±14.2) Sex (males); n (%) McKenzie: Males 7 (20.0%) MCE: Males 7 (20.0%) | Mckenzie (MKZ) | Motor Control Exercise (MCE) | 1. Muscle Thickness (EO, IO, TrA) 2. Patient Specific Functional Scale 3. Pain (VAS) | |
| Hoppes et al. (2016) | RCT | n= 34 16 Male, 18 Female Age CG 27 ± 5 MCE 29 ± 5 Weight CG 70.53 ± 15.42 MCE 70.86 ± 10.83 Height CG 1.73 ± 0.11 MCE 1.73 ± 0.12 BMI CG 23.27 ± 2.88 MCE 23.66 ± 2.59 | Motor Control Exercise (MCE) | Control Group (CG) | The measures during the pre- and post-intervention assessments included ultrasound imaging of abdominal muscle thickness | |
| Shamsi et al. (2016) | Q-RCT | Core stability exercise group General exercise group n= 22 n= 21 Male: 7 Male: 6 Female: 15 Female: 15 Age (year) 39.2 ±11.7 Height (cm) 166.4 ±9.1 Weight (kg) 70.1 ±15.1 | Motor Control Exercise (MCE) | General Exercise | Using ultrasound imaging, four transabdominal muscle thicknesswere measured before and after the intervention. Disability and pain were measured as secondary outcomes | |
| Nabavi et al. (2017) | RCT | Stabilization Group Routine Group Mean Standard Age (y) 40.75 ±8.23 34.05 ±10.75 Height (m) 1.68 ±0.08 1.65 ±0.08 Weight (kg) 70.15 ±14.53 72.05 ±10.77 Body mass index (kg/m2) 24.86 ±4.39 26.39 ±3.21 | MCE (Motor Control Exercise)+electrotherapy (N=20) | General Exercise + Electrotherapy | Pain intensity, using a visual analog scale, and muscle dimensions of both right and left transverse abdominis and lumbar multifidus muscles, using rehabilitative ultrasonography | |
| Worth et al. (2007) | RCT | Male 6 (60.0%) 4 (44.4%) Female 4 (40.0%) 5 (55.6%) Age (years) 37.0 ±11.5 33.1 ±13.5 Height (m) 1.74±0.14 1.73±0.12 Weight (kg) 79.0 ± 9.08 73,2 ±14.89 | AHE (Abdominal Hollowing Exercise) | AHE + RTUS (Real Time Ultraound) | NPI= Numeric Pain index TCi = Typicai Clinicai Instruction Group TCi + US = Typical Clinical Instruction augmented with Real Time Ultrasound Group | |
| Lumbar | Hides et al. (1996) | RCT | Age 30.9 and 30.65 Height 173.3 cm and 170.1 cm Weight 73.53 Kg and 71.05 Kg. | Medical Treatment + Specific localized exercise therapy (T+SET) | Medical Treatment (MT) | Pain, McGill Pain Questionnarie (MPQ), VAS and daily pain diaries. The Roland Morris Disabiliy Index. Range of motion, and size of the multifidus cross-sectional area (CSA) |
| Van et al. (2005) | RCT | Group 1 (knowledge of results [KR] alone) contained 10 females and 3 males (mean ± SD, 19.1 ± 2.1 years) and group 2 (KR plus visual feedback) contained 9 females and 3 males (mean ± SD, 19.9 ± 2.2 years). | Clinical instructions for multifidus muscle contraction + Provision of visual biofeedback using real-time ultrasound imaging | Clinical instructions for multifidus muscle contraction | Multifidus muscle thickness | |
| Akbari et al. (2008) | RCT | MCE (n = 25) GE(n = 24). Age 39.6 ± 3.5b 40 ± 3.6. Height (cm) 171.2 ± 2.7 172.08 ± 2.2 0.2 Weight (kg) 73.7 ± 3.1 74.6 ± 2.4 0.26 BMI (kg/m2) 25.2 ± 1.7 25.21 ± 1.02 | Motor Control Exercise (MCE) | General Exercise (GE) | 1. Muscle Thickness Transversus Abdominis and Lumbar Multifidus (TA and LM) 2. Activity limitation (AL) was assessed using Back Performance Scale (BPS). 3. Pain measurement Visual Analog Scale (VAS) | |
| Hebert et al. (2015) | RCT | MT (n=20) MT+SET (n=21) Age 31 ± 7.9 - 30.9 ± 6.5 Height 173.3 - 170.1 Weight 73.53 - 71.05 | Specific Trunk Exercises (MCE) | General Trunk Exercise (GE) | 1. Pain: McGill Pain Questionnarie (MPQ) and Visual Analogue Scale (VAS) 2. Disability: Roland Morris Disability Index (RMDI) 3. Range of Motion: Inclinometry 4. Habitual activity levels 5. Lumbar multifidus Muscle CSA (LM) | |
| Berglund et al. (2017) | RCT | LMC n=33 Age 43.3 (10.3) BMI 25.0 (3.0) HLL n=32 Age 42.3 (9.8) BMI 25.4 (3.8) | Low Load Motor Control Exercises (LMC) | High-Load lifting (HLL) Exercise | Pain (VAS), Multifidus mucles thickness | |
| Pelvic Floor | Stuge et al. (2006) | RCT | Weight (kg) 69.5 (11.7) 67.3 (13.6) Height (cm) 169.6 (3.6) 164.5 (5.4) Body mass index 24.1 (3.3) 25.0 (5.4) Age of youngest child (months) 29.5 (2.9) 29.6 (3.6) | Volunteers with PGP (Pelvic Girdle Pain) + ASRL (Active Straigh Raise Leg) | Volunteers without PGP + ASRL | Abdominal muscles thicknes by ultrasound, pelvic floor muscles strength by pressure transducer, ability to perform ASLR test, Pain (VAS) |
| Bernardes et al. (2012) | RCT | Age (years) 51.9 (± 7.4) 56.7 (± 10.7) 58.7 (± 10.4) Body mass index (BMI, kg/m2) 29.9 (± 3.5) 28.8 (± 3.9) 29.7 (± 2.7) | Pelvic floor muscle training group (GI) | Hypopressive exercise group (GII) | Ano rectal muscle CSA, Length of urethra adn bladder neck by transperineal ultrasound. Pelvic organ prolapse (POP) classification | |
| McLean et al. (2013) | RCT | Control group 54.0 ±8.4 years, treatment group 49.5 ±8.2 years, body mass index (control group 28.6 ±11.3 kg/m2, treatment group 27.0 ±3.8 kg/m2) | 12 weekly sessions they learned contract their pelvic floor muscles (PFMs) and a home exercise program | No treament. | Baldder volume by trans-abdominal US, transperineal ultrasound for urethra morphology, Incontinence Impact Questionnaire (IIQ-7) and the Urogenital Distress Inventory (UDI-6) | |
| Johannessen et al. (2016) | RCT | Intervention group (n = 54) Control group (n = 55) Age (years), mean (SD) [range] 29.7 (4.3) [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38] 30.6 (3.8) [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40] Inclusion (days postpartum), mean (SD) 389 (122) 375 (141) Ethnicity: Norwegian 42 (77.8) 51 (92.4) European 8 (14.8) 3 (5.5) Asian 4 (7.4) 1 (1.8) | Individual physiotherapya of pelvic floor muscle exercises PFME (Pelvic Floor Muscle Exercise) | Written information of (PFME) | St. Mark’s score for Anal Incontinence, manometry measures of anal sphincter length and strength, endoanal ultrasound (EAUS) defect score and voluntary pelvic floor muscle contraction | |
| Legend: RCT: Randomized Clinical Trial, Q-RCT: Quasi Randomized Clinical Trial. EO (External Oblique), IO (Internal Oblique), TrA (Transversus Abdominus), US (Ultrasound), PBU (Pressure Biofeedback Unit), MCE (Motor Control Exercise). | ||||||
| Region | Author | Design | Title | Intervention | Control | Descriptive Statistics | Measured Outcomes |
|---|---|---|---|---|---|---|---|
| LUMBAR | Akbari et al. (2008) | RCT | The effect of motor control exercise versus general exercise on lumbar local stabilizing muscles thickness: Randomized controlled trial of patients with chronic low back pain | Motor Control Exercise (MCE) | General Exercise (GE) | MCE (n = 25) GE(n = 24). Age 39.6 ± 3.5b 40 ± 3.6. Height (cm) 171.2 ± 2.7 172.08 ± 2.2 0.2 Weight (kg) 73.7 ± 3.1 74.6 ± 2.4 0.26 BMI (kg/m2) 25.2 ± 1.7 25.21 ± 1.02 | 1. Muscle Thickness (TA and LM) 2. Activity limitation (AL) was assessed using Back Performance Scale (BPS). 3. Pain measurement Visual Analog Scale (VAS) |
| Berglund et al. (2017) | RCT | Effects of low-load motor control exercises and a high-load lifting exercise on lumbar multifidus thickness | Low Load Motor Control Exercises (LMC) | High-Load lifting (HLL) Exercise | LMC (n=33) Age: 43.3 (10.3) BMI: 25.0 (3.0) HLL (n=32) Age: 42.3 (9.8) BMI: 25.4 (3.8) | 1. VAS (Visual Analogue Scale) 2. Muscle Thickness | |
| ABDOMINAL | Ferreira et al. (2014) | RCT | Changes in recruitment of transversus abdominis correlate with disability in people with chronic low back pain | Motor Control Exercise (MCE) | General Exercise | MCE (n = 11) - GE (n = 10) - SMT (n = 13) Age, years (SD) 47.5 (17.3) 54.9 (11.3) 45.4 (17.7) Weight, kg (SD) 78.7 (13.0) 70.1 (12.0) 72.6 (10.2) Height, cm (SD) 171.0 (10.8) 160.7 (6.6) 165.0 (8.5) Female, n (%) 6 (55) 7 (70) 10 (77) | Global impression of recovery was measured on an 11-point scale. 1.Disability was measured using the 24-item version of the Roland Morris disability questionnaire. 2.Average pain intensity over the past week was measured on a numerical rating scale. 3.Function was measured with a modified patientspecific functional scale |
| Halliday et al. (2016) | RCT | A Randomized Controlled Trial comparing the Mckenzie method to motor control exercises in people with chronic low back pain and a directional preference. | Motor Control Exercise (MCE) | Mckenzie (MKZ) | Age (years) Mckenzie: 48.8 (12.1) MCE: 48.3 (14.2) Sex (males); n (%) McKenzie: Males 7 (20.0%) MCE: Males 7 (20.0%) | 1. Muscle Thickness (EO, IO, TrA) 2. Patient Specific Functional Scale 3. Pain (VAS) | |
| Shamsi et al. (2016) | Q-RCT | The effect of core stability and general exercise on abdominal muscle thickness in non-specific chronic low back pain using ultrasound imaging | Motor Control Exercise (MCE) | General Exercise | Core stability exercise group General exercise group N = 22 N = 21 Male: 7 Male: 6 Female: 15 Female: 15 Age (year) 39.2 11.7 Height (cm) 166.4 9.1 Weight (kg) 70.1 15.1 | Using ultrasound imaging, four transabdominal muscle thickness were measured before and after the intervention. Disability and pain were measured as secondary outcomes | |
| Nabavi et al. (2017) | RCT | The effect of 2 different exercise programs on pain intensity and muscle dimensions in patients with chronic low back pain: A randomized controlled trial | MCE (Motor Control Exercise) +electrotherapy (N=20) | General Exercise + Electrotherapy (N=21) | Stabilization Group Routine Group Mean Standard Age (y) 40.75 8.23 34.05 10.75 Height (m) 1.68 0.08 1.65 0/08 Weight (kg) 70.15 14.53 72.05 10.77 Body mass index (kg/m2) 24.86 ±4.39 26.39 ±3.21 | Pain intensity, using a visual analog scale, and muscle dimensions of both right and left transverse abdominis and lumbar multifidus muscles, using rehabilitativeultrasonography | |
| Legend: RCT: Randomized Clinical Trial, Q-RCT: Quasi Randomized Clinical Trial. | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Carnero, S.; Martin-Saborido, C.; Achalandabaso Ochoa-Ruiz de Mendoza, A.; Ferragut-Garcias, A.; Cuenca-Zaldivar, J.N.; Leal-Quiñones, A.; Calvo-Lobo, C.; Gallego-Izquierdo, T. The Role of Rehabilitative Ultrasound Imaging Technique in the Lumbopelvic Region as a Diagnosis and Treatment Tool in Physiotherapy: Systematic Review, Meta-Analysis and Meta-Regression. J. Clin. Med. 2021, 10, 5699. https://doi.org/10.3390/jcm10235699
Fernández-Carnero S, Martin-Saborido C, Achalandabaso Ochoa-Ruiz de Mendoza A, Ferragut-Garcias A, Cuenca-Zaldivar JN, Leal-Quiñones A, Calvo-Lobo C, Gallego-Izquierdo T. The Role of Rehabilitative Ultrasound Imaging Technique in the Lumbopelvic Region as a Diagnosis and Treatment Tool in Physiotherapy: Systematic Review, Meta-Analysis and Meta-Regression. Journal of Clinical Medicine. 2021; 10(23):5699. https://doi.org/10.3390/jcm10235699
Chicago/Turabian StyleFernández-Carnero, Samuel, Carlos Martin-Saborido, Alexander Achalandabaso Ochoa-Ruiz de Mendoza, Alejandro Ferragut-Garcias, Juan Nicolás Cuenca-Zaldivar, Alejandro Leal-Quiñones, Cesar Calvo-Lobo, and Tomas Gallego-Izquierdo. 2021. "The Role of Rehabilitative Ultrasound Imaging Technique in the Lumbopelvic Region as a Diagnosis and Treatment Tool in Physiotherapy: Systematic Review, Meta-Analysis and Meta-Regression" Journal of Clinical Medicine 10, no. 23: 5699. https://doi.org/10.3390/jcm10235699
APA StyleFernández-Carnero, S., Martin-Saborido, C., Achalandabaso Ochoa-Ruiz de Mendoza, A., Ferragut-Garcias, A., Cuenca-Zaldivar, J. N., Leal-Quiñones, A., Calvo-Lobo, C., & Gallego-Izquierdo, T. (2021). The Role of Rehabilitative Ultrasound Imaging Technique in the Lumbopelvic Region as a Diagnosis and Treatment Tool in Physiotherapy: Systematic Review, Meta-Analysis and Meta-Regression. Journal of Clinical Medicine, 10(23), 5699. https://doi.org/10.3390/jcm10235699








