The Effect of Ultrasonic Agitation on the Porosity Distribution in Apically Perforated Root Canals Filled with Different Bioceramic Materials and Techniques: A Micro-CT Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Selection and Preparation
2.2. Root Canal Obturation
- BR/SC group—the root canals were filled with BioRoot RCS sealer and single ProTaper NEXT size X5 gutta-percha point (Dentsply Sirona, Ballaigues, Switzerland). The apical 4 mm of the gutta-percha point was cut with a sterile scalpel to fit the gutta-percha with a tug-back effect at a length 2 mm shorter than the perforated apical foramen. The sealer was mixed according to the manufacturer’s instructions, inserted into the Skini syringe (Ultradent Products Inc., South Jordan, UT, USA) and subsequently delivered into the root canal via attached plastic Capillary Tip cannula (Ultradent Products Inc., South Jordan, UT, USA). The tip was inserted approximately 2 mm shorter than the perforation site, and the plunger of the syringe was gently pressed while withdrawing the plastic cannula until reaching the orifice level. After the injection of BioRoot RCS, the pre-fitted gutta-percha point was coated with a thin amount of the sealer and gently inserted into the root canal 2 mm short of the perforated apex.
- BR/SC-UA group—the root canals were filled with BioRoot RCS sealer and single ProTaper NEXT size X5 gutta-percha point using ultrasonic agitation. The selection and adaptation of the gutta-percha point and the injection of the sealer were accomplished identically to the BR/SC group. After delivering the sealer into the root canal, an Ultrawave ET25 ultrasonic tip (Ultradent Products Inc., South Jordan, UT, USA) attached to an Ultrawave XS ultrasonic device (Ultradent Products Inc., South Jordan, UT, USA) was directly inserted into the root canal and BioRoot RCS sealer 2 mm short of the WL. The ultrasonic tip was activated for 10 s at the medium power using Reflex technology (Ultradent Products Inc.), capable of automatic real-time frequency adjustment of 28–36 kHz. The pre-fitted gutta-percha point was subsequently coated with a small amount of the sealer and slowly inserted into the root canal 2 mm shorter than the apical foramen.
- MF group—the root canals were filled with MTA Flow cement. A total of 0.19 g of powder and 3 drops of liquid were mixed according to the manufacturer’s recommendations to get a thin consistency of the cement. The mixed material was inserted into the clear Skini syringe, and the flowability of the material was checked by extruding the small amount of the cement through the attached 29-G NaviTip needle. The filling material was delivered into the root canal by slowly pressing the plunger of the syringe and withdrawing the tip, which was inserted 2 mm short of the perforated apex.
- MF-UA group—the root canals were filled with MTA Flow cement using ultrasonic agitation. The filling material was prepared and injected into the root canal in the same manner as in the MF group. Afterwards, the Ultrawave ET25 ultrasonic tip was directly inserted into the root canal and MTA Flow cement 2 mm short of the perforation site and activated for 10 s at the 28–36 kHz frequency and the power described previously.
2.3. Micro-CT Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bueno, M.R.; Estrela, C.; De Figueiredo, J.A.; Azevedo, B.C. Map-reading strategy to diagnose root perforations near metallic intracanal posts by using cone beam computed tomography. J. Endod. 2011, 37, 85–90. [Google Scholar] [CrossRef]
- Sarao, S.K.; Berlin-Broner, Y.; Levin, L. Occurrence and risk factors of dental root perforations: A systematic review. Int. Dent. J. 2021, 71, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Siew, K.; Lee, A.H.; Cheung, G.S. Treatment outcome of repaired root perforation: A systematic review and meta-analysis. J. Endod. 2015, 41, 1795–1804. [Google Scholar] [CrossRef]
- Gorni, F.G.; Andreano, A.; Ambrogi, F.; Brambilla, E.; Gagliani, M. Patient and clinical characteristics associated with primary healing of iatrogenic perforations after root canal treatment: Results of a long-term Italian study. J. Endod. 2016, 42, 211–215. [Google Scholar] [CrossRef]
- Estrela, C.; Decurcio, D.A.; Rossi-Fedele, G.; Silva, J.A.; Guedes, O.A.; Borges, Á.H. Root perforations: A review of diagnosis, prognosis and materials. Braz. Oral. Res. 2018, 32, e73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsesis, I.; Fuss, Z. Diagnosis and treatment of accidental root perforations. Endod. Top. 2006, 13, 95–107. [Google Scholar] [CrossRef]
- Lopes, F.C.; Zangirolami, C.; Mazzi-Chaves, J.F.; Silva-Sousa, A.C.; Crozeta, B.M.; Silva-Sousa, Y.T.C.; Sousa-Neto, M.D. Effect of sonic and ultrasonic activation on physicochemical properties of root canal sealers. J. Appl. Oral. Sci. 2019, 27, e20180556. [Google Scholar] [CrossRef]
- Sisli, S.N.; Ozbas, H. Comparative micro-computed tomographic evaluation of the sealing quality of ProRoot MTA and MTA Angelus apical plugs placed with various techniques. J. Endod. 2017, 43, 147–151. [Google Scholar] [CrossRef]
- Mondelli, J.A.S.; Hoshino, R.A.; Weckwerth, P.H.; Cerri, P.S.; Leonardo, R.T.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M.; da Silva, G.F. Biocompatibility of mineral trioxide aggregate flow and biodentine. Int. Endod. J. 2019, 52, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, B.M.; Vivan, R.R.; Piazza, B.; Alcalde, M.P.; Bramante, C.M.; Duarte, M.A.H. Chemical-physical properties and apatite-forming ability of mineral trioxide aggregate flow. J. Endod. 2017, 43, 1692–1696. [Google Scholar] [CrossRef]
- Ultradent. Ultradent Products, Inc. Proudly Introduces MTA Flow™ Repair Cement. Available online: https://www.ultradent.com/company/newsroom/article/ultradent-products-inc-proudly-introduces-mta-flow-repair-cement (accessed on 25 October 2021).
- Liu, M.; He, L.; Wang, H.; Su, W.; Li, H. Comparison of in vitro biocompatibility and antibacterial activity of two calcium silicate-based materials. J. Mater. Sci. Mater. Med. 2021, 32, 52. [Google Scholar] [CrossRef] [PubMed]
- Drukteinis, S. Bioceramic Materials for Management of Endodontic Complications. In Bioceramic Materials in Clinical Endodontics, 1st ed.; Drukteinis, S., Camilleri, J., Eds.; Spinger: Cham, Germany, 2021; pp. 59–85. [Google Scholar]
- Siboni, F.; Taddei, P.; Zamparini, F.; Prati, C.; Gandolfi, M.G. Properties of BioRoot RCS, a tricalcium silicate endodontic sealer modified with povidone and polycarboxylate. Int. Endod. J. 2017, 50, 120–136. [Google Scholar] [CrossRef] [Green Version]
- Sfeir, G.; Zogheib, C.; Patel, S.; Giraud, T.; Nagendrababu, V.; Bukiet, F. Calcium silicate-based root canal sealers: A narrative review and clinical perspectives. Materials 2021, 14, 3965. [Google Scholar] [CrossRef] [PubMed]
- Bardini, G.; Casula, L.; Ambu, E.; Musu, D.; Mercadè, M.; Cotti, E. A 12-month follow-up of primary and secondary root canal treatment in teeth obturated with a hydraulic sealer. Clin. Oral Investig. 2021, 25, 2757–2764. [Google Scholar] [CrossRef]
- Zavattini, A.; Knight, A.; Foschi, F.; Mannocci, F. Outcome of root canal treatments using a new calcium silicate root canal sealer: A non-randomized clinical trial. J. Clin. Med. 2020, 9, 782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drukteinis, S.; Bilvinaite, G.; Tusas, P.; Shemesh, H.; Peciuliene, V. Microcomputed tomographic assessment of the single cone root canal fillings performed by undergraduate student, postgraduate student and specialist endodontist. J. Clin. Med. 2021, 10, 1080. [Google Scholar] [CrossRef]
- Drukteinis, S.; Peciuliene, V.; Shemesh, H.; Tusas, P.; Bendinskaite, R. Porosity distribution in apically perforated curved root canals filled with two different calcium silicate based materials and techniques: A micro-computed tomography study. Materials 2019, 12, 1729. [Google Scholar] [CrossRef] [Green Version]
- El-Ma’aita, A.M.; Qualtrough, A.J.; Watts, D.C. A micro-computed tomography evaluation of mineral trioxide aggregate root canal fillings. J. Endod. 2012, 38, 670–672. [Google Scholar] [CrossRef]
- da Silva Machado, A.P.; Câncio Couto de Souza, A.C.; Lima Gonçalves, T.; Franco Marques, A.A.; da Fonseca Roberti Garcia, L.; Antunes Bortoluzzi, E.; Acris de Carvalho, F.M. Does the ultrasonic activation of sealer hinder the root canal retreatment? Clin. Oral Investig. 2021, 25, 4401–4406. [Google Scholar] [CrossRef]
- Aguiar, B.A.; Frota, L.M.A.; Taguatinga, D.T.; Vivan, R.R.; Camilleri, J.; Duarte, M.A.H.; de Vasconcelos, B.C. Influence of ultrasonic agitation on bond strength, marginal adaptation, and tooth discoloration provided by three coronary barrier endodontic materials. Clin. Oral Investig. 2019, 23, 4113–4122. [Google Scholar] [CrossRef]
- Wiesse, P.E.B.; Silva-Sousa, Y.T.; Pereira, R.D.; Estrela, C.; Domingues, L.M.; Pécora, J.D.; Sousa-Neto, M.D. Effect of ultrasonic and sonic activation of root canal sealers on the push-out bond strength and interfacial adaptation to root canal dentine. Int. Endod. J. 2018, 51, 102–111. [Google Scholar] [CrossRef]
- Dinçer, A.N.; Güneşer, M.B.; Sisli, S.N. Micro-CT analysis of the marginal adaptation and porosity associated with ultrasonic activation of coronally placed tricalcium silicate-based cements. Aust. Endod. J. 2020, 46, 323–329. [Google Scholar] [CrossRef]
- Kim, J.A.; Hwang, Y.C.; Rosa, V.; Yu, M.K.; Lee, K.W.; Min, K.S. Root Canal Filling Quality of a Premixed Calcium Silicate Endodontic Sealer Applied Using Gutta-percha Cone-mediated Ultrasonic Activation. J. Endod. 2018, 44, 133–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Jang, Y.E.; Kim, B.S.; Pang, E.K.; Shim, K.; Jin, H.R.; Son, M.K.; Kim, Y. Effects of ultrasonic activation on root canal filling quality of single-cone obturation with calcium silicate-based sealer. Materials 2021, 14, 1292. [Google Scholar] [CrossRef]
- An, H.J.; Yoon, H.; Jung, H.I.; Shin, D.H.; Song, M. Comparison of obturation quality after MTA orthograde filling with various obturation techniques. J. Clin. Med. 2021, 10, 1719. [Google Scholar] [CrossRef] [PubMed]
- Milanovic, I.; Milovanovic, P.; Antonijevic, D.; Dzeletovic, B.; Djuric, M.; Miletic, V. Immediate and long-term porosity of calcium silicate-based sealers. J. Endod. 2020, 46, 515–523. [Google Scholar] [CrossRef]
- Schneider, S.W. A comparison of canal preparations in straight and curved root canals. Oral Surg. Oral Med. Oral Pathol. 1971, 32, 271–275. [Google Scholar] [CrossRef]
- Ansari, I.; Maria, R. Managing curved canals. Contemp Clin. Dent. 2012, 3, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Schafer, E.; Dammaschke, T. Development and sequelae of canal transportation. Endod. Top. 2006, 15, 75–90. [Google Scholar] [CrossRef]
- Abdelmotelb, M.A.; Gomaa, Y.F.; Khattab, N.M.A.; Elheeny, A.A.H. Premixed bioceramics versus mineral trioxide aggregate in furcal perforation repair of primary molars: In vitro and in vivo study. Clin. Oral Investig. 2021, 25, 4915–4925. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.M.V.; Marins, F.C.; Belladonna, F.G.; Souza, E.M.; De-Deus, G.; Lopes, R.T.; Silva, E.J.N.L. Untouched canal areas and debris accumulation after root canal preparation with rotary and adaptive systems. Aust. Endod. J. 2018, 44, 260–266. [Google Scholar] [CrossRef]
- Nagendrababu, V.; Jayaraman, J.; Suresh, A.; Kalyanasundaram, S.; Neelakantan, P. Effectiveness of ultrasonically activated irrigation on root canal disinfection: A systematic review of in vitro studies. Clin. Oral Investig. 2018, 22, 655–670. [Google Scholar] [CrossRef]
- Selem, L.C.; Li, G.H.; Niu, L.N.; Bergeron, B.E.; Bortoluzzi, E.A.; Chen, J.H.; Pashley, D.H.; Tay, F.R. Quality of obturation achieved by a non-gutta-percha-based root filling system in single-rooted canals. J. Endod. 2014, 40, 2003–2008. [Google Scholar] [CrossRef]
- Torres, F.F.E.; Guerreiro-Tanomaru, J.M.; Bosso-Martelo, R.; Espir, C.G.; Camilleri, J.; Tanomaru-Filho, M. Solubility, porosity, dimensional and volumetric change of endodontic sealers. Braz. Dent. J. 2019, 30, 368–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dioguardi, M.; Quarta, C.; Sovereto, D.; Troiano, G.; Zhurakivska, K.; Bizzoca, M.E.; Lo Muzio, L.; Lo Russo, L. Calcium silicate cements vs. epoxy resin based cements: Narrative review. Oral 2021, 1, 23–35. [Google Scholar] [CrossRef]
- Antonijevic, D.; Zelic, K.; Djuric, M. Novel calcium silicate based dental material with the addition of biologically active soy compound. In Proceedings of the 2015 IEEE 15th International Conference on Bioinformatics and Bioengineering (BIBE), Belgrade, Serbia, 2–4 November 2015. [Google Scholar] [CrossRef]
- Guerrero, F.; Berástegui, E. Porosity analysis of MTA and Biodentine cements for use in endodontics by using micro-computed tomography. J. Clin. Exp. Dent. 2018, 10, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Orhan, K.; Jacobs, R.; Celikten, B.; Huang, Y.; de Faria Vasconcelos, K.; Nicolielo, L.F.P.; Buyuksungur, A.; Van Dessel, J. Evaluation of threshold values for root canal filling voids in micro-CT and nano-CT images. Scanning 2018, 2018, 9437569. [Google Scholar] [CrossRef] [PubMed]
- Bueno, C.R.E.; Vasques, A.M.V.; Cury, M.T.S.; Sivieri-Araújo, G.; Jacinto, R.C.; Gomes-Filho, J.E.; Cintra, L.T.A.; Dezan-Junior, E. Biocompatibility and biomineralization assessment of mineral trioxide aggregate flow. Clin. Oral Investig. 2019, 23, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilleri, J.; Grech, L.; Galea, K.; Keir, D.; Fenech, M.; Formosa, L.; Damidot, D.; Mallia, B. Porosity and root dentine to material interface assessment of calcium silicate-based root-end filling materials. Clin. Oral Investig. 2014, 18, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Coomaraswamy, K.S.; Lumley, P.J.; Hofmann, M.P. Effect of bismuth oxide radioopacifier content on the material properties of an endodontic Portland cement-based (MTA-like) system. J. Endod. 2007, 33, 295–298. [Google Scholar] [CrossRef]
- Li, X.; Yoshihara, K.; De Munck, J.; Cokic, S.; Pongprueksa, P.; Putzeys, E.; Pedano, M.; Chen, Z.; Van Landuyt, K.; Van Meerbeek, B. Modified tricalcium silicate cement formulations with added zirconium oxide. Clin. Oral Investig. 2017, 21, 895–905. [Google Scholar] [CrossRef]
- Camilleri, J. BioRoot RCS. Endo Sealer or Biological Filler? 2019. Available online: https://www.septodont.com.ru/sites/ru/files/2019-07/Septodont_BioRoot_Endo%20sealer%20or%20biological%20filler_JC.pdf (accessed on 25 October 2021).
- Camilleri, J. Will bioceramics be the future root canal filling materials? Curr. Oral Health Rep. 2017, 4, 228–238. [Google Scholar] [CrossRef]
- Kalantar Motamedi, M.R.; Mortaheb, A.; Zare Jahromi, M.; Gilbert, B.E. Micro-CT evaluation of four root canal obturation techniques. Scanning 2021, 2021, 6632822. [Google Scholar] [CrossRef] [PubMed]
- Acris De Carvalho, F.M.; Silva-Sousa, Y.T.C.; Saraiva Miranda, C.E.; Miller Calderon, P.H.; Barbosa, A.F.S.; Domingues De Macedo, L.M.; Abi Rached-Junior, F.J. Influence of ultrasonic activation on the physicochemical properties of calcium silicate-based cements. Int. J. Dent. 2021, 2021, 6697988. [Google Scholar] [CrossRef]
- Yeung, P.; Liewehr, F.R.; Moon, P.C. A quantitative comparison of the fill density of MTA produced by two placement techniques. J. Endod. 2006, 32, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alfayate, R.; Algar-Pinilla, J.; Mercade, M.; Foschi, F. Sonic activation improves bioceramic sealer’s penetration into the tubular dentin of curved root canals: A confocal laser scanning microscopy investigation. Appl. Sci. 2021, 11, 3902. [Google Scholar] [CrossRef]
- Atmeh, A.R.; AlShwaimi, E. The effect of heating time and temperature on epoxy resin and calcium silicate-based endodontic sealers. J. Endod. 2017, 43, 2112–2118. [Google Scholar] [CrossRef]
- Benavides-García, M.; Hernández-Meza, E.; Reyes-Carmona, J. Ex vivo analysis of MTA FLOW® biomineralization and push-out strength: A pilot study. Int. J. Dent. Sci. 2021, 23, 76–90. [Google Scholar] [CrossRef]
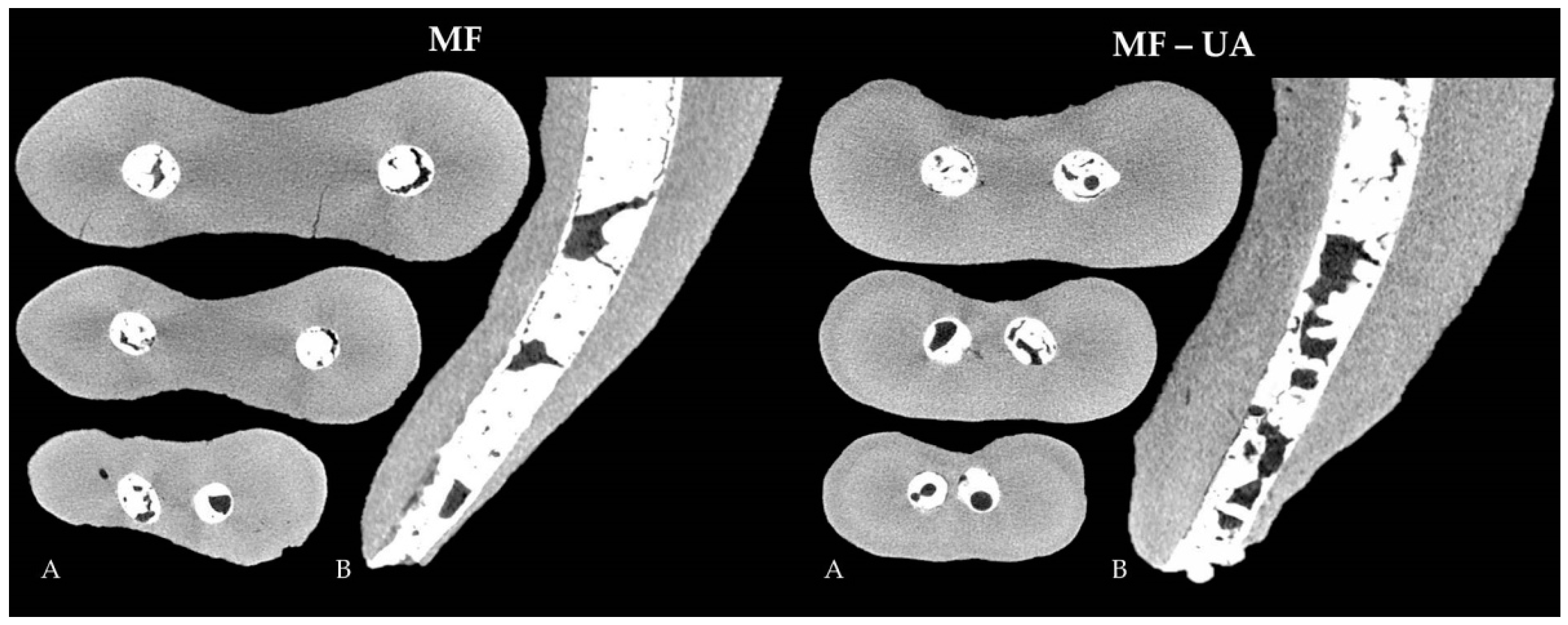
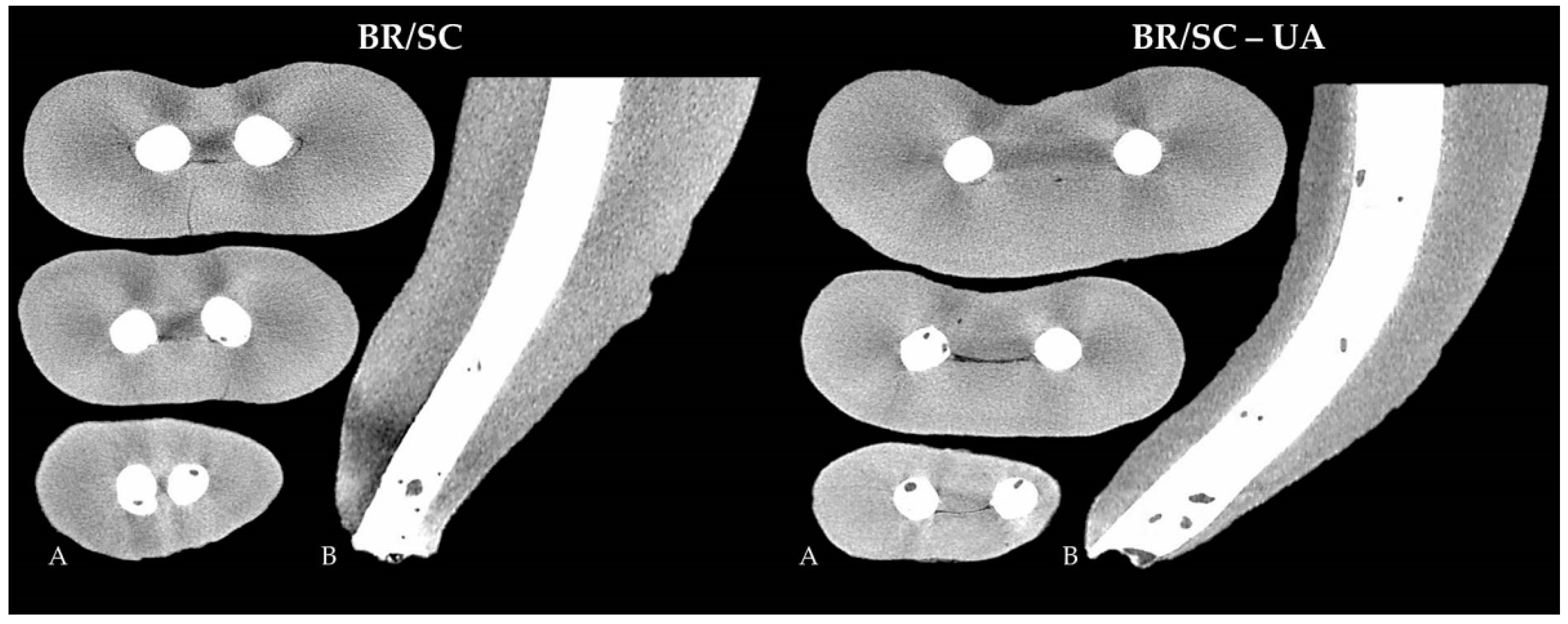
| Group | N | Open Pores | Closed Pores |
|---|---|---|---|
| BR/SC | 20 | 3.374 ± 2.751 A | 0.061 ± 0.080 A |
| BR/SC-UA | 20 | 3.390 ± 3.428 A | 0.066 ± 0.070 A |
| MF | 20 | 18.832 ± 3.334 B | 0.292 ± 0.226 B |
| MF-UA | 20 | 29.075 ± 9.440 C | 0.923 ± 0.684 C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drukteinis, S.; Bilvinaite, G.; Shemesh, H.; Tusas, P.; Peciuliene, V. The Effect of Ultrasonic Agitation on the Porosity Distribution in Apically Perforated Root Canals Filled with Different Bioceramic Materials and Techniques: A Micro-CT Assessment. J. Clin. Med. 2021, 10, 4977. https://doi.org/10.3390/jcm10214977
Drukteinis S, Bilvinaite G, Shemesh H, Tusas P, Peciuliene V. The Effect of Ultrasonic Agitation on the Porosity Distribution in Apically Perforated Root Canals Filled with Different Bioceramic Materials and Techniques: A Micro-CT Assessment. Journal of Clinical Medicine. 2021; 10(21):4977. https://doi.org/10.3390/jcm10214977
Chicago/Turabian StyleDrukteinis, Saulius, Goda Bilvinaite, Hagay Shemesh, Paulius Tusas, and Vytaute Peciuliene. 2021. "The Effect of Ultrasonic Agitation on the Porosity Distribution in Apically Perforated Root Canals Filled with Different Bioceramic Materials and Techniques: A Micro-CT Assessment" Journal of Clinical Medicine 10, no. 21: 4977. https://doi.org/10.3390/jcm10214977
APA StyleDrukteinis, S., Bilvinaite, G., Shemesh, H., Tusas, P., & Peciuliene, V. (2021). The Effect of Ultrasonic Agitation on the Porosity Distribution in Apically Perforated Root Canals Filled with Different Bioceramic Materials and Techniques: A Micro-CT Assessment. Journal of Clinical Medicine, 10(21), 4977. https://doi.org/10.3390/jcm10214977







