Does the Use of Peripheral Immune-Related Markers Indicate Whether to Administer Pazopanib, Trabectedin, or Eribulin to Advanced Soft Tissue Sarcoma Patients?
Abstract
:1. Introduction
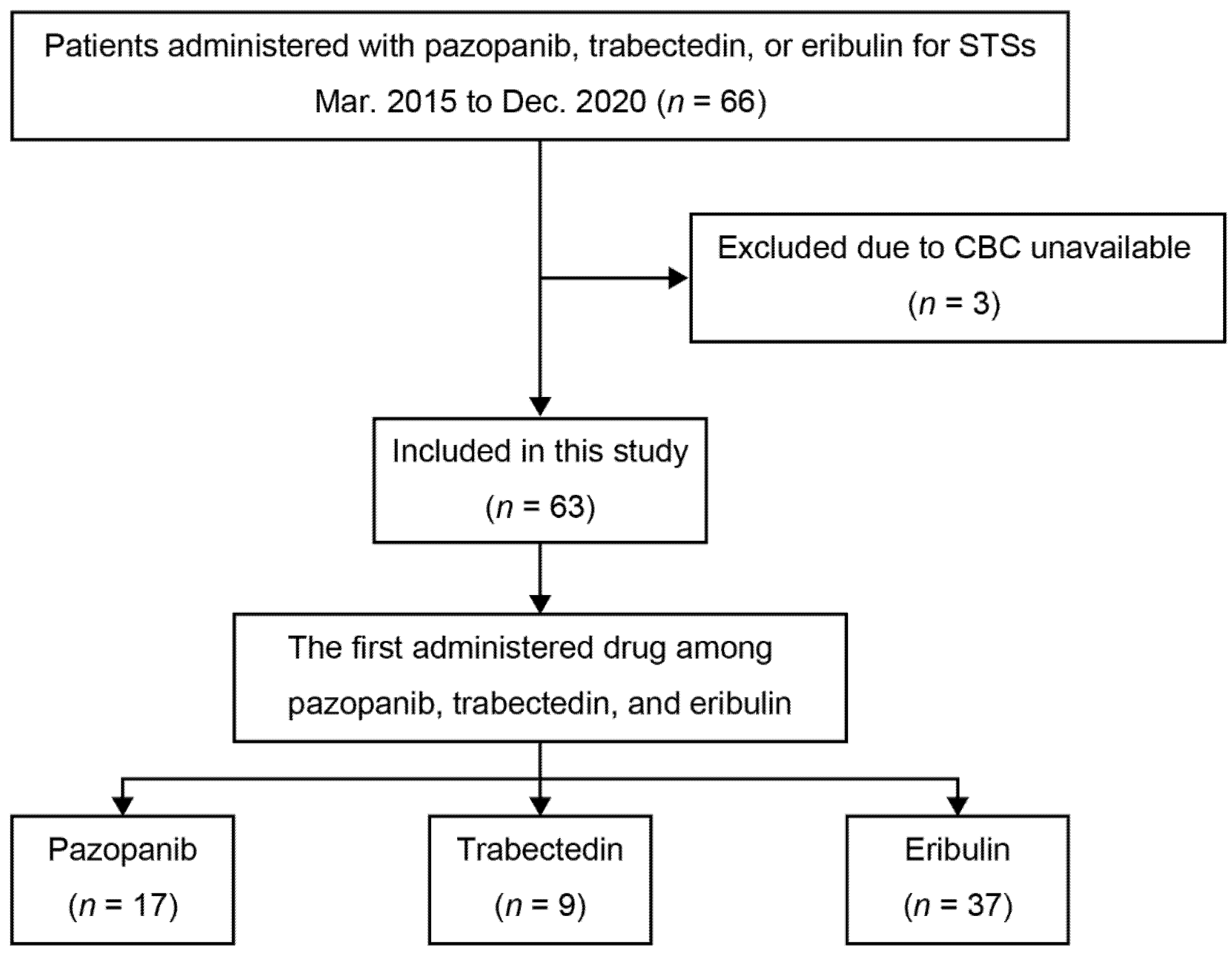
2. Materials and Methods
2.1. Patients
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. Patients and Group Demographics
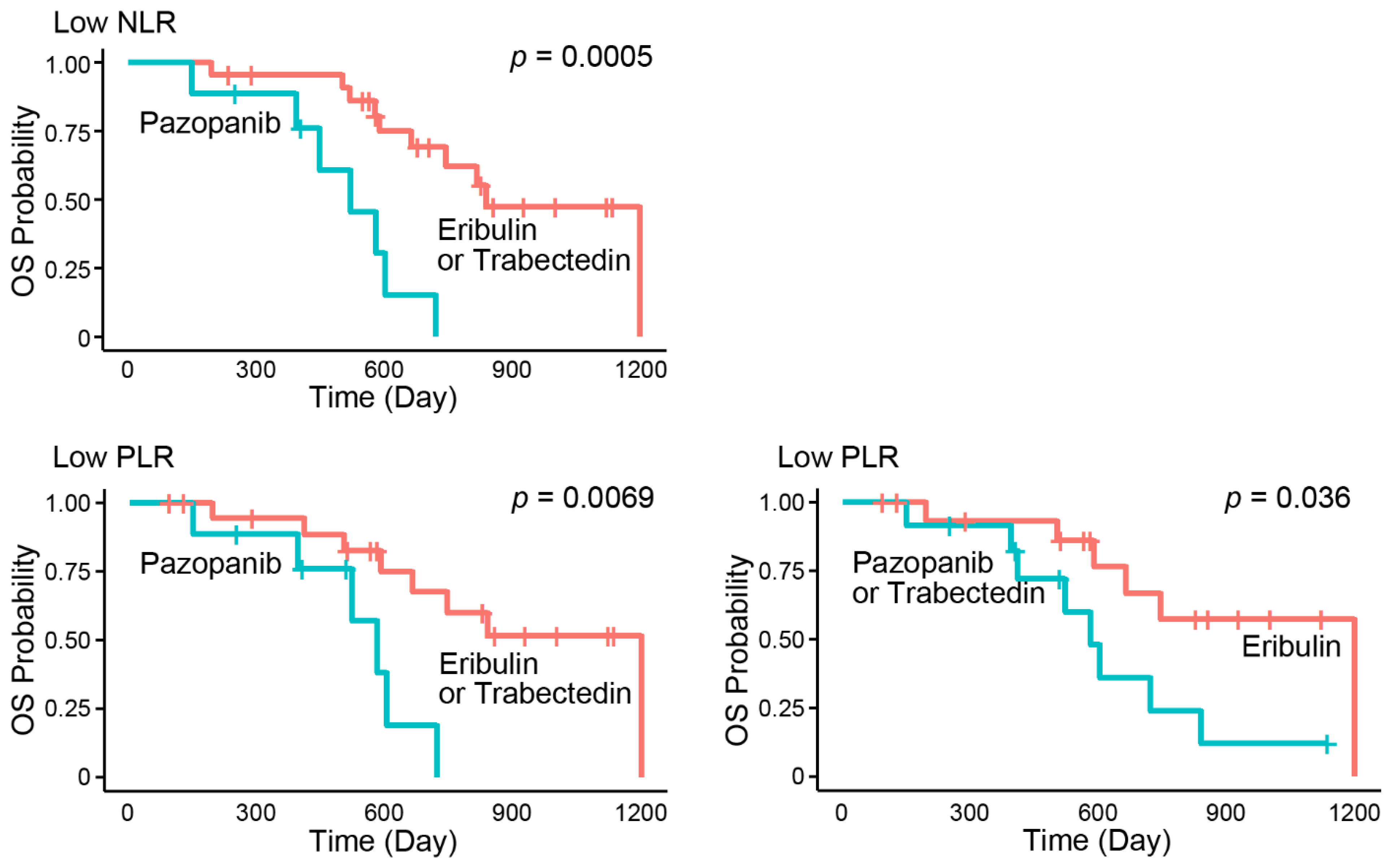
3.2. Subgroup Analysis of Peripheral Immune-Related Markers for Survival
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smrke, A.; Wang, Y.; Simmons, C. Update on systemic therapy for advanced soft-tissue sarcoma. Curr Oncol. 2020, 27 (Suppl. 1), 25–33. [Google Scholar] [CrossRef] [PubMed]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.-Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- Seddon, B.; Strauss, S.J.; Whelan, J.; Leahy, M.; Woll, P.J.; Cowie, F.; Rothermundt, C.; Wood, Z.; Benson, C.; Ali, N.; et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): A randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1397–1410. [Google Scholar] [CrossRef] [Green Version]
- van der Graaf, W.T.; Blay, J.-Y.; Chawla, S.P.; Kim, D.-W.; Bui-Nguyen, B.; Casali, P.G.; Schöffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Kawai, A.; Araki, N.; Sugiura, H.; Ueda, T.; Yonemoto, T.; Takahashi, M.; Morioka, H.; Hiraga, H.; Hiruma, T.; Kunisada, T.; et al. Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation-related sarcoma: A randomised, open-label, phase 2 study. Lancet Oncol. 2015, 16, 406–416. [Google Scholar] [CrossRef]
- Schöffski, P.; Chawla, S.; Maki, R.G.; Italiano, A.; Gelderblom, H.; Choy, E.; Grignani, G.; Camargo, V.; Bauer, S.; Rha, S.Y.; et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open-label, multicentre, phase 3 trial. Lancet 2016, 387, 1629–1637. [Google Scholar] [CrossRef]
- Walsh, S.R.; Cook, E.J.; Goulder, F.; Justin, T.A.; Keeling, N.J. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J. Surg Oncol. 2005, 91, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Khoja, L.; Atenafu, E.G.; Templeton, A.; Qye, Y.; Chappell, M.A.; Saibil, S.; Hogg, D.; Butler, M.O.; Joshua, A.M. The full blood count as a biomarker of outcome and toxicity in ipilimumab-treated cutaneous metastatic melanoma. Cancer Med. 2016, 5, 2792–2799. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, A.; Kurata, T.; Yoshioka, H.; Takeyasu, Y.; Niki, M.; Kibata, K.; Satsutani, N.; Ogata, M.; Miyara, T.; Nomura, S. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int. J. Clin. Oncol. 2018, 23, 634–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunakawa, Y.; Yang, D.; Cao, S.; Zhang, W.; Moran, M.; Astrow, S.H.; Hsiang, J.; Stephens, C.; Tsuji, A.; Takahashi, T.; et al. Immune-related Genes to Dominate Neutrophil-lymphocyte Ratio (NLR) Associated With Survival of Cetuximab Treatment in Metastatic Colorectal Cancer. Clin. Colorectal Cancer 2018, 17, e741–e749. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Zhang, S.; Liu, Y.; Ma, L.; Zhu, J.; Xin, Y.; Wang, Y.; Yang, C.; Cheng, Y. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J. Clin. Lab. Anal. 2019, 33, e22964. [Google Scholar] [CrossRef] [PubMed]
- Zahorec, R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001, 102, 5–14. [Google Scholar]
- Liang, Y.; Wang, W.; Li, J.; Guan, Y.; Que, Y.; Xiao, W.; Zhang, X.; Zhou, Z. Combined Use of the Neutrophil-Lymphocyte and Platelet-Lymphocyte Ratios as a Prognostic Predictor in Patients with Operable Soft Tissue Sarcoma. J. Cancer 2018, 9, 2132–2139. [Google Scholar] [CrossRef]
- Cheng, Y.; Mo, F.; Pu, L.; Li, Q.; Ma, X. Pretreatment Inflammatory Indexes as Prognostic Predictors of Survival in Patients Suffering From Synovial Sarcoma. Front. Oncol. 2019, 9, 955. [Google Scholar] [CrossRef]
- Teck Seo, S.; Singh, V.A.; Yasin, N.F. Preoperative lymphocyte count in relation to sarcoma prognosis. J. Orthop Surg 2019, 27, 2309499019854957. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Nakano, K.; Fukuda, N.; Wang, X.; Urasaki, T.; Ohmoto, A.; Yunokawa, M.; Ono, M.; Tomomatsu, J.; Hayakawa, K.; et al. Pre-treatment Neutrophil-to-Lymphocyte Ratio Predicts Efficacy of Eribulin for Soft-tissue Sarcoma. Anticancer Res. 2021, 41, 527–532. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Yoshimura, Y.; Saito, K.; Muramoto, K.; Sugawara, M.; Alexis, K.; Nomoto, K.; Nakamura, S.; Saeki, T.; Watanabe, J.; et al. High absolute lymphocyte counts are associated with longer overall survival in patients with metastatic breast cancer treated with eribulin-but not with treatment of physician’s choice-in the EMBRACE study. Breast Cancer 2020, 27, 706–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raungkaewmanee, S.; Tangjitgamol, S.; Manusirivithaya, S.; Srijaipracharoen, S.; Thavaramara, T. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J. Gynecol. Oncol. 2012, 23, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, T.; Ishizuka, M.; Shiraki, T.; Sakuraoka, Y.; Mori, S.; Abe, A.; Iso, Y.; Takagi, K.; Aoki, K.; Kubota, K. The clinical influence of the preoperative lymphocyte-to-monocyte ratio on the postoperative outcome of patients with early-stage gastrointestinal cancer. Ann. Gastroenterol Surg. 2020, 4, 580–590. [Google Scholar] [CrossRef]
- Ito, K.; Hamamichi, S.; Abe, T.; Akagi, T.; Shirota, H.; Kawano, S.; Asano, M.; Asano, O.; Yokoi, A.; Matsui, J.; et al. Antitumor effects of eribulin depend on modulation of the tumor microenvironment by vascular remodeling in mouse models. Cancer Sci. 2017, 108, 2273–2280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Romero, P.; Palucka, A.K.; Marincola, F.M. Tumour immunity: Effector response to tumour and role of the microenvironment. Lancet 2008, 371, 771–783. [Google Scholar] [CrossRef]
- Galmarini, C.M.; D’Incalci, M.; Allavena, P. Trabectedin and plitidepsin: Drugs from the sea that strike the tumor microenvironment. Mar. Drugs 2014, 12, 719–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ocana, A.; Nieto-Jimenez, C.; Pandiella, A.; Templeton, A.J. Neutrophils in cancer: Prognostic role and therapeutic strategies. Mol. Cancer 2017, 16, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoiber, D.; Assinger, A. Platelet-Leukocyte Interplay in Cancer Development and Progression. Cells 2020, 9, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchihashi, K.; Kusaba, H.; Yoshihiro, T.; Fujiwara, T.; Setsu, N.; Endo, M.; Matsumoto, Y.; Imajima, T.; Shinohara, Y.; Ito, M.; et al. Eribulin as a first-line treatment for soft tissue sarcoma patients with contraindications for doxorubicin. Sci. Rep. 2020, 10, 20896. [Google Scholar] [CrossRef] [PubMed]

| Eribulin (n = 37) | Pazopanib (n = 17) | Trabectedin (n = 9) | p–Value | ||
|---|---|---|---|---|---|
| Age | ≥65 | 19 (51.4%) | 9 (52.9%) | 4 (44.4%) | 1.00 |
| <65 | 18 (48.6%) | 8 (47.1%) | 5 (55.6%) | ||
| Gender | Male | 22 (59.5%) | 7 (41.2%) | 5 (55.6%) | 0.49 |
| Female | 15 (40.5%) | 10 (58.8%) | 4 (44.4%) | ||
| Histology | L-sarcoma | 24 (64.9%) | 3 (17.7%) | 6 (66.7%) | 0.0036 |
| Non–L–sarcoma | 13 (35.1%) | 14 (82.3%) | 3 (33.3%) | ||
| Primary lesion | Extremities | 12 (32.4%) | 4 (23.5%) | 4 (44.4%) | 0.55 |
| Others | 25 (67.6%) | 13 (76.5%) | 5 (55.6%) | ||
| Resection | Yes | 27 (73.0%) | 11 (64.7%) | 8 (88.9%) | 0.45 |
| No | 10 (27.0%) | 6 (35.3%) | 1 (11.1%) | ||
| ECOG PS | 0 | 5 (13.5%) | 3 (17.7%) | 1 (11.1%) | 0.88 |
| ≥1 | 32 (86.5%) | 14 (82.3%) | 8 (88.9%) | ||
| Previous doxorubicin | Yes | 23 (62.2%) | 12 (70.6%) | 7 (77.8%) | 0.70 |
| No | 14 (37.8%) | 5 (29.4%) | 2 (22.2%) | ||
| Previous ifosfamide | Yes | 14 (37.8%) | 8 (47.1%) | 5 (55.6%) | 0.59 |
| No | 23 (62.2%) | 9 (52.9%) | 4 (44.4%) | ||
| No. of previous chemotherapy | 0–1 | 29 (78.4%) | 13 (76.5%) | 6 (66.7%) | 0.77 |
| ≥2 | 8 (21.6%) | 4 (23.5%) | 3 (33.3%) | ||
| No. of following chemotherapy | 0–1 | 26 (70.3%) | 13 (76.5%) | 5 (55.6%) | 0.58 |
| ≥2 | 11 (29.7%) | 4 (23.5%) | 4 (44.4%) | ||
| Radiotherapy | Yes | 16 (43.2%) | 8 (47.1%) | 6 (66.7%) | 0.53 |
| No | 21 (56.8%) | 9 (52.9%) | 3 (33.3%) | ||
| Carbon-ion radiotherapy | Yes | 8 (21.6%) | 3 (17.7%) | 0 (0.0%) | 0.46 |
| No | 29 (78.4%) | 14 (82.3%) | 9 (100%) | ||
| ALC | Low | 27 (73.0%) | 10 (58.8%) | 8 (88.9%) | 0.27 |
| High | 10 (27.0%) | 7 (41.2%) | 1 (11.1%) | ||
| NLR | Low | 19 (51.3%) | 9 (52.8%) | 4 (44.4%) | 1.00 |
| High | 18 (48.7%) | 8 (47.2%) | 5 (55.6%) | ||
| PLR | Low | 17 (45.9%) | 9 (52.8%) | 3 (33.3%) | 0.68 |
| High | 20 (54.1%) | 8 (47.2%) | 6 (66.7%) | ||
| LMR | Low | 17 (45.9%) | 7 (41.2%) | 5 (55.6%) | 0.78 |
| High | 20 (54.1%) | 10 (58.8%) | 4 (44.4%) |
| Parameter | HR (95% CI) | p-Value | |
|---|---|---|---|
| Age | ≥65 (vs. <65) | 0.61 (0.32–1.19) | 0.15 |
| Gender | Male (vs. Female) | 1.06 (0.55–2.04) | 0.87 |
| Histology | L-sarcoma (vs. non–L–sarcoma) | 0.58 (0.30–1.14) | 0.11 |
| Primary lesion | Extremities (vs. others) | 0.90 (0.45–1.83) | 0.78 |
| Resection | Yes (vs. No) | 0.57 (0.28–1.15) | 0.12 |
| ECOG PS | 0 (vs. ≥1) | 0.48 (0.15–1.57) | 0.22 |
| Previous doxorubicin | Yes (vs. No) | 1.11 (0.55–2.25) | 0.77 |
| Previous ifosfamide | Yes (vs. No) | 1.11 (0.58–2.11) | 0.76 |
| No. of previous chemotherapy | 0–1 (vs. ≥2) | 0.70 (0.34–1.46) | 0.34 |
| Radiation | Yes (vs. No) | 1.01 (0.53–1.93) | 0.97 |
| Carbon-ion radiotherapy | Yes (vs. No) | 1.00 (0.41–2.41) | 1.00 |
| First drug | Eribulin (vs. others) | 0.60 (0.31–1.14) | 0.12 |
| Pazopanib (vs. others) | 1.98 (0.99–3.96) | 0.053 | |
| Trabectedin (vs. others) | 0.93 (0.36–2.40) | 0.88 | |
| ALC | Low (vs. High) | 1.18 (0.58–2.40) | 0.64 |
| NLR | Low (vs. High) | 0.50 (0.26–0.96) | 0.037 |
| PLR | Low (vs. High) | 0.52 (0.27–1.02) | 0.057 |
| LMR | Low (vs. High) | 2.24 (1.17–4.31) | 0.015 |
| Low NLR | Low PLR | ||||
|---|---|---|---|---|---|
| Parameter | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | ≥65 (vs. <65) | 0.94 (0.35–2.54) | 0.91 | 0.96 (0.31–2.93) | 0.94 |
| Gender | Male (vs. Female) | 0.49 (0.18–1.34) | 0.17 | 0.70 (0.22–2.17) | 0.53 |
| Histology | L-sarcoma (vs. non–L–sarcoma) | 0.48 (0.17–1.31) | 0.15 | 0.59 (0.19–1.81) | 0.36 |
| Primary lesion | Extremities (vs. others) | 0.67 (0.23–1.94) | 0.46 | 0.88 (0.29–2.70) | 0.83 |
| Resection | Yes (vs. No) | 0.63 (0.22–1.82) | 0.39 | 0.79 (0.22–2.86) | 0.71 |
| ECOG PS | 0 (vs. ≥1) | 1.34 (0.38–4.71) | 0.65 | 1.33 (0.36–4.86) | 0.67 |
| Previous doxorubicin | Yes (vs. No) | 1.68 (0.58–4.90) | 0.34 | 1.45 (0.47–4.48) | 0.52 |
| Previous ifosfamide | Yes (vs. No) | 1.54 (0.57–4.15) | 0.39 | 1.01 (0.31–3.30) | 0.98 |
| No. of previous chemotherapy | 0–1 (vs. ≥2) | 0.90 (0.20–4.09) | 0.89 | 0.90 (0.29–2.81) | 0.86 |
| Radiotherapy | Yes (vs. No) | 0.79 (0.29–2.18) | 0.65 | 0.73 (0.24–2.24) | 0.58 |
| Carbon-ion radiotherapy | Yes (vs. No) | 1.43 (0.46–4.47) | 0.54 | 1.10 (0.30–4.02) | 0.89 |
| First drug | Eribulin (vs. others) | 0.40 (0.15–1.08) | 0.072 | 0.32 (0.10–0.98) | 0.046 |
| Pazopanib (vs. others) | 5.93 (1.94–18.13) | 0.0018 | 4.71 (1.38–16.04) | 0.013 | |
| Trabectedin (vs. others) | 0.38 (0.15–2.87) | 0.35 | 0.96 (0.21–4.41) | 0.96 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimada, E.; Endo, M.; Matsumoto, Y.; Tsuchihashi, K.; Ito, M.; Kusaba, H.; Nabeshima, A.; Nawata, T.; Maekawa, A.; Matsunobu, T.; et al. Does the Use of Peripheral Immune-Related Markers Indicate Whether to Administer Pazopanib, Trabectedin, or Eribulin to Advanced Soft Tissue Sarcoma Patients? J. Clin. Med. 2021, 10, 4972. https://doi.org/10.3390/jcm10214972
Shimada E, Endo M, Matsumoto Y, Tsuchihashi K, Ito M, Kusaba H, Nabeshima A, Nawata T, Maekawa A, Matsunobu T, et al. Does the Use of Peripheral Immune-Related Markers Indicate Whether to Administer Pazopanib, Trabectedin, or Eribulin to Advanced Soft Tissue Sarcoma Patients? Journal of Clinical Medicine. 2021; 10(21):4972. https://doi.org/10.3390/jcm10214972
Chicago/Turabian StyleShimada, Eijiro, Makoto Endo, Yoshihiro Matsumoto, Kenji Tsuchihashi, Mamoru Ito, Hitoshi Kusaba, Akira Nabeshima, Tomoya Nawata, Akira Maekawa, Tomoya Matsunobu, and et al. 2021. "Does the Use of Peripheral Immune-Related Markers Indicate Whether to Administer Pazopanib, Trabectedin, or Eribulin to Advanced Soft Tissue Sarcoma Patients?" Journal of Clinical Medicine 10, no. 21: 4972. https://doi.org/10.3390/jcm10214972
APA StyleShimada, E., Endo, M., Matsumoto, Y., Tsuchihashi, K., Ito, M., Kusaba, H., Nabeshima, A., Nawata, T., Maekawa, A., Matsunobu, T., Setsu, N., Fujiwara, T., Iida, K., Nakagawa, M., Hirose, T., Kanahori, M., Oyama, R., Isobe, T., Ariyama, H., ... Nakashima, Y. (2021). Does the Use of Peripheral Immune-Related Markers Indicate Whether to Administer Pazopanib, Trabectedin, or Eribulin to Advanced Soft Tissue Sarcoma Patients? Journal of Clinical Medicine, 10(21), 4972. https://doi.org/10.3390/jcm10214972







