Hyo-Mental Angle and Distance: An Important Adjunct in Airway Assessment of Adult Mucopolysaccharidosis
Abstract
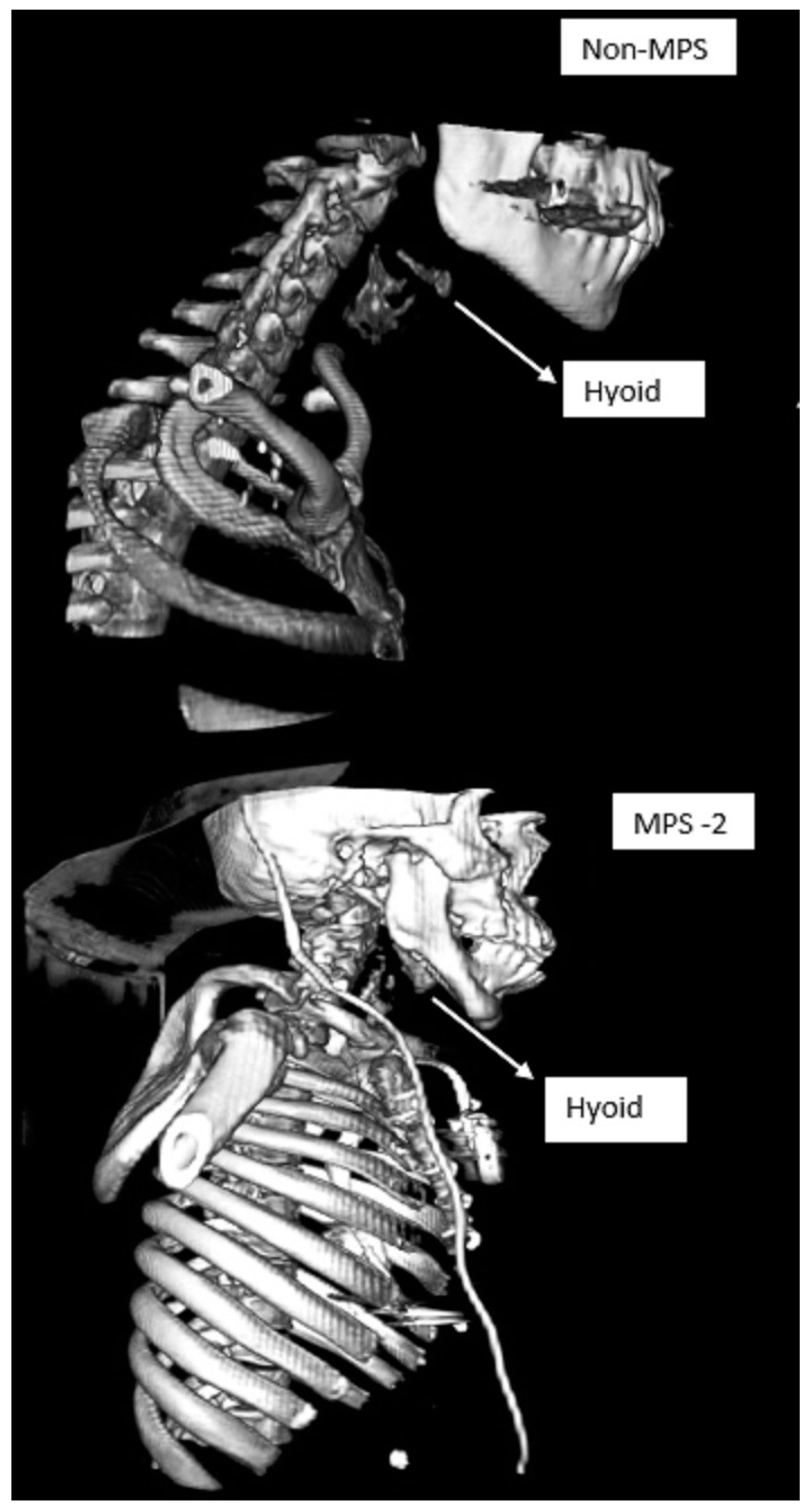
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Difficult Airway
4.2. Clinical Measures of Difficult Airway
4.3. Limitations of the Study
4.3.1. Head and Neck Position
4.3.2. Thyro-Hyoid Distance
4.3.3. Numbers
4.4. Wider Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehta, A.B.; Winchester, B. Lysosomal Storage Disorders: A Practical Guide; Wiley-Blackwell Chichester: Hoboken, NJ, USA, 2012. [Google Scholar]
- Mucopolysaccharidoses Fact Sheet. Available online: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Mucopolysaccharidoses-Fact-Sheet (accessed on 31 July 2020).
- Taylor, M.; Khan, S.; Stapleton, M.; Wang, J.; Chen, J.; Wynn, R.; Yabe, H.; Chinen, Y.; Boelens, J.J.; Mason, R.W. Hematopoietic stem cell transplantation for mucopolysaccharidoses: Past, present, and future. Biol. Blood Marrow Transplant. 2019, 25, e226–e246. [Google Scholar] [CrossRef] [PubMed]
- Concolino, D.; Deodato, F.; Parini, R. Enzyme replacement therapy: Efficacy and limitations. Ital. J. Pediatrics 2018, 44, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Gadepalli, C.; Stepien, K.M.; Sharma, R.; Jovanovic, A.; Tol, G.; Bentley, A. Airway Abnormalities in Adult Mucopolysaccharidosis and Development of Salford Mucopolysaccharidosis Airway Score. J. Clin. Med. 2021, 10, 3275. [Google Scholar] [CrossRef]
- Apfelbaum, J.; Hagberg, C.; Caplan, R.; Blitt, C.; Connis, R.; Nickinovich, D.; Benumof, J.; Berry, F. American Society of Anesthesiologists Task Force on Management of the Difficult Airway Practice guidelines for management of the difficult airway: An updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2013, 118, 251–270. [Google Scholar]
- Patil, V. Predicting the difficulty of intubation utilizing an intubation gauge. Anesth. Rev. 1983, 10, 32–33. [Google Scholar]
- Braunlin, E.A.; Harmatz, P.R.; Scarpa, M.; Furlanetto, B.; Kampmann, C.; Loehr, J.P.; Ponder, K.P.; Roberts, W.C.; Rosenfeld, H.M.; Giugliani, R. Cardiac disease in patients with mucopolysaccharidosis: Presentation, diagnosis and management. J. Inherit. Metab. Dis. 2011, 34, 1183–1197. [Google Scholar] [CrossRef] [Green Version]
- Neufeld, E.; Muenzer, J. The mucopolysaccharidoses. In The Metabolic and Molecular Bases of Inherited Diseases, 8th ed.; Scriver, C.R., Beaudet, A.L., Sly, W.S., Valle, D., Childs, R., Kinzler, K.W., Eds.; McGraw-Hill: New York, NY, USA, 2001; pp. 3421–3452. [Google Scholar]
- Valayannopoulos, V.; Nicely, H.; Harmatz, P.; Turbeville, S. Mucopolysaccharidosis vi. Orphanet J. Rare Dis. 2010, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Berger, K.I.; Fagondes, S.C.; Giugliani, R.; Hardy, K.A.; Lee, K.S.; McArdle, C.; Scarpa, M.; Tobin, M.J.; Ward, S.A.; Rapoport, D.M. Respiratory and sleep disorders in mucopolysaccharidosis. J. Inherit. Metab. Dis. 2013, 36, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Muhlebach, M.S.; Wooten, W.; Muenzer, J. Respiratory manifestations in mucopolysaccharidoses. Paediatr. Respir. Rev. 2011, 12, 133–138. [Google Scholar] [CrossRef]
- Crawley, S.; Dalton, A. Predicting the difficult airway. BJA Educ. 2015, 15, 253–257. [Google Scholar] [CrossRef]
- Frerk, C.; Mitchell, V.S.; McNarry, A.F.; Mendonca, C.; Bhagrath, R.; Patel, A.; O’Sullivan, E.P.; Woodall, N.M.; Ahmad, I. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br. J. Anaesth. 2015, 115, 827–848. [Google Scholar] [CrossRef] [Green Version]
- Shiga, T.; Wajima, Z.i.; Inoue, T.; Sakamoto, A. Predicting difficult intubation in apparently normal patients: A meta-analysis of bedside screening test performance. J. Am. Soc. Anesthesiol. 2005, 103, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.; Berger, K.I.; Borgo, A.; Braunlin, E.A.; Burton, B.K.; Ghotme, K.A.; Kircher, S.G.; Molter, D.; Orchard, P.J.; Palmer, J. Unique medical issues in adult patients with mucopolysaccharidoses. Eur. J. Intern. Med. 2016, 34, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Hendriksz, C.J.; Burton, B.; Fleming, T.R.; Harmatz, P.; Hughes, D.; Jones, S.A.; Lin, S.-P.; Mengel, E.; Scarpa, M.; Valayannopoulos, V. Efficacy and safety of enzyme replacement therapy with BMN 110 (elosulfase alfa) for Morquio A syndrome (mucopolysaccharidosis IVA): A phase 3 randomised placebo-controlled study. J. Inherit. Metab. Dis. 2014, 37, 979–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, L.A.; Wraith, J.E.; Beck, M.; Kolodny, E.H.; Pastores, G.M.; Muenzer, J.; Rapoport, D.M.; Berger, K.I.; Sidman, M.; Kakkis, E.D. Long-term efficacy and safety of laronidase in the treatment of mucopolysaccharidosis I. Pediatrics 2009, 123, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Harmatz, P.; Giugliani, R.; Schwartz, I.V.D.; Guffon, N.; Teles, E.L.; Miranda, M.C.S.; Wraith, J.E.; Beck, M.; Arash, L.; Scarpa, M. Long-term follow-up of endurance and safety outcomes during enzyme replacement therapy for mucopolysaccharidosis VI: Final results of three clinical studies of recombinant human N-acetylgalactosamine 4-sulfatase. Mol. Genet. Metab. 2008, 94, 469–475. [Google Scholar] [CrossRef]
- Aldenhoven, M.; Wynn, R.F.; Orchard, P.J.; O’Meara, A.; Veys, P.; Fischer, A.; Valayannopoulos, V.; Neven, B.; Rovelli, A.; Prasad, V.K. Long-term outcome of Hurler syndrome patients after hematopoietic cell transplantation: An international multicenter study. Blood J. Am. Soc. Hematol. 2015, 125, 2164–2172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, P.; Depuydt, A.; Thompson, J. Thyromental distance measurement–fingers don’t rule. Anaesthesia 2009, 64, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Al Ramadhani, S.; Mohamed, L.; Rocke, D.; Gouws, E.; Ramadhani, S. Sternomental distance as the sole predictor of difficult laryngoscopy in obstetric anaesthesia. Br. J. Anaesth. 1996, 77, 312–316. [Google Scholar] [CrossRef]
- Braunlin, E.; Steinberger, J.; DeFor, T.; Orchard, P.; Kelly, A.S. Metabolic syndrome and cardiovascular risk factors after hematopoietic cell transplantation in severe mucopolysaccharidosis type I (Hurler syndrome). Biol. Blood Marrow Transpl. 2018, 24, 1289–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.-Y.; Lee, C.-L.; Chiu, P.C.; Niu, D.-M.; Tsai, F.-J.; Hwu, W.-L.; Lin, S.J.; Lin, J.-L.; Chang, T.-M.; Chuang, C.-K. Relationships among Height, Weight, Body Mass Index, and Age in Taiwanese children with different types of mucopolysaccharidoses. Diagnostics 2019, 9, 148. [Google Scholar] [CrossRef] [Green Version]
- Wilson, M.; Spiegelhalter, D.; Robertson, J.; Lesser, P. Predicting difficult intubation. Br. J. Anaesth. 1988, 61, 211–216. [Google Scholar] [CrossRef]
- Mallampati, S.R.; Gatt, S.P.; Gugino, L.D.; Desai, S.P.; Waraksa, B.; Freiberger, D.; Liu, P.L. A clinical sign to predict difficult tracheal intubation; a prospective study. Can. Anaesth. Soc. J. 1985, 32, 429–434. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.-H.; Lee, M.-S.; Shih, Y.-L.; Chu, H.-C.; Huang, T.-Y.; Hsieh, T.-Y. Modified Mallampati classification as a clinical predictor of peroral esophagogastroduodenoscopy tolerance. BMC Gastroenterol. 2011, 11, 12. [Google Scholar] [CrossRef] [Green Version]
- Dalewski, B.; Kamińska, A.; Syrico, A.; Kałdunska, A.; Pałka, Ł.; Sobolewska, E. The Usefulness of Modified Mallampati Score and CT Upper Airway Volume Measurements in Diagnosing OSA among Patients with Breathing-Related Sleep Disorders. Appl. Sci. 2021, 11, 3764. [Google Scholar] [CrossRef]
- Cormack, R.; Lehane, J. Difficult tracheal intubation in obstetrics. Anaesthesia 1984, 39, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Yentis, S. The effects of single-handed and bimanual cricoid pressure on the view at laryngoscopy. Anaesthesia 1997, 52, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Cook, T. A new practical classification of laryngeal view. Anaesthesia 2000, 55, 274–279. [Google Scholar] [CrossRef]
- Jain, K.; Gupta, N.; Yadav, M.; Thulkar, S.; Bhatnagar, S. Radiological evaluation of airway—What an anaesthesiologist needs to know! Indian J. Anaesth. 2019, 63, 257–264. [Google Scholar] [PubMed]
- Hui, C.; Tsui, B. Sublingual ultrasound as an assessment method for predicting difficult intubation: A pilot study. Anaesthesia 2014, 69, 314–319. [Google Scholar] [CrossRef] [Green Version]
- Ezri, T.; Gewürtz, G.; Sessler, D.; Medalion, B.; Szmuk, P.; Hagberg, C.; Susmallian, S. Prediction of difficult laryngoscopy in obese patients by ultrasound quantification of anterior neck soft tissue. Anaesthesia 2003, 58, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Tol, G.; Stepien, K.; Yadthore, S.; Watson, S.; Samraj, P.; Gadepalli, C. Role of 3-dimensional (3D) reconstruction of radiology images and virtual endoscopy in the assessment of airways in adult mucopolysaccharidosis patients. Mol. Genet. Metab. 2020, 129, S147–S148. [Google Scholar] [CrossRef]
- Gadepalli, C.; Tol, G.; Yadthore, S.; Sharma, R.; Jovanovic, A.; Palmer, J.; Stepien, K.M. Nasendoscopy findings in adult patients with mucopolysaccharidosis: A tertiary UK centre experience. Mol. Genet. Metab. 2020, 129, S59–S60. [Google Scholar] [CrossRef]
- Han, Y.; Tian, Y.; Zhang, H.; Zhao, Y.; Xu, M.; Guo, X. Radiologic indicators for prediction of difficult laryngoscopy in patients with cervical spondylosis. Acta Anaesthesiol. Scand. 2018, 62, 474–482. [Google Scholar] [CrossRef] [PubMed]
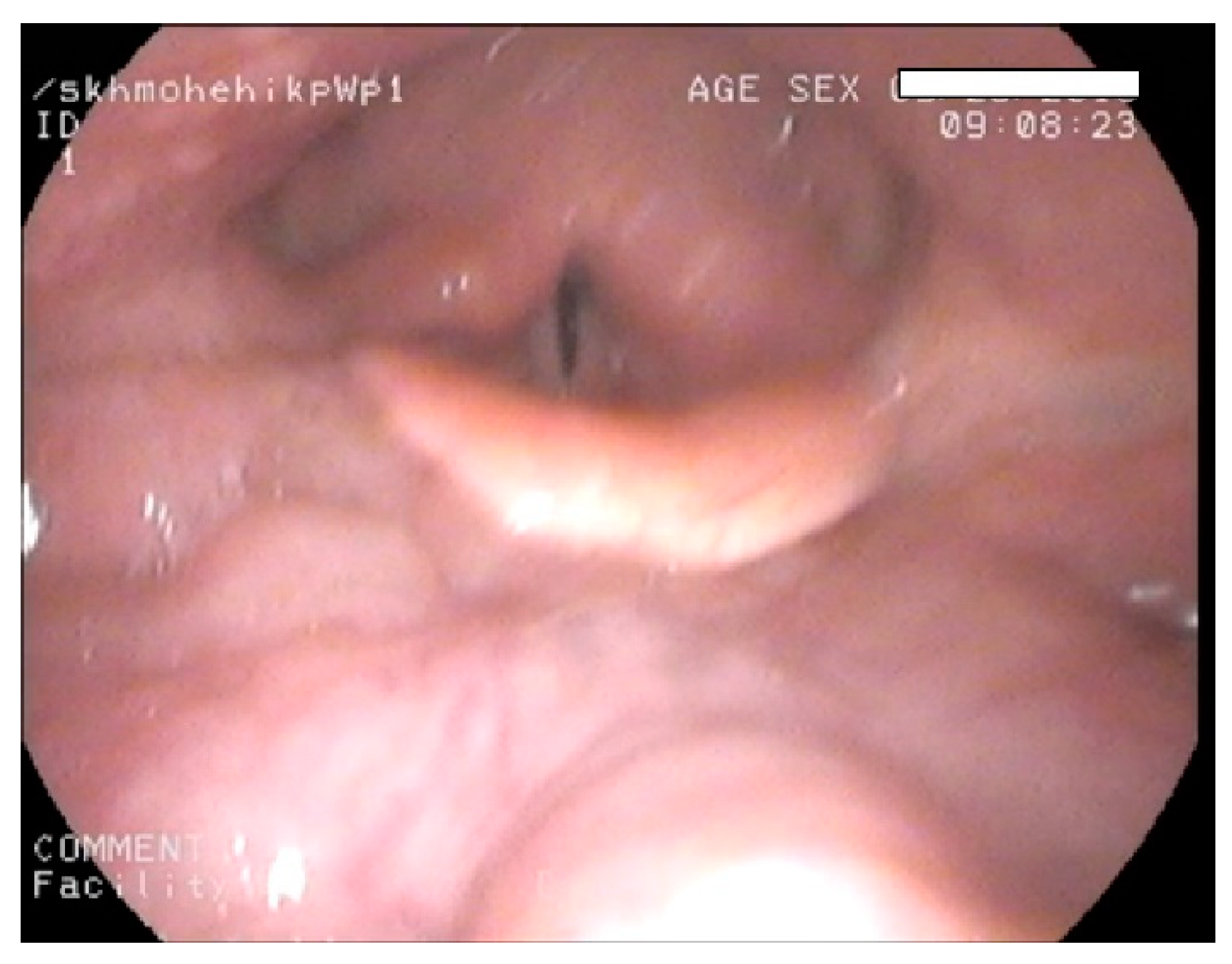

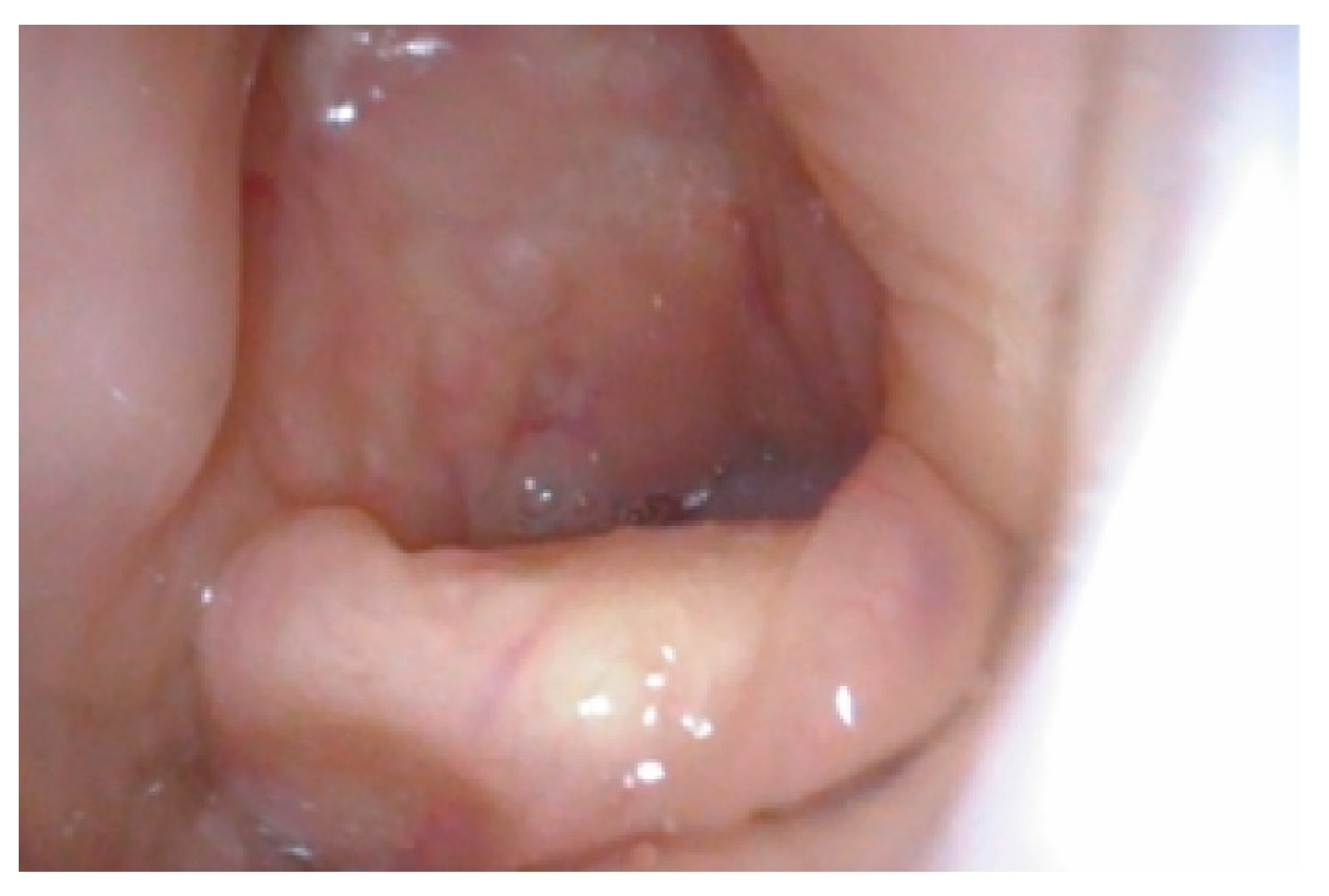
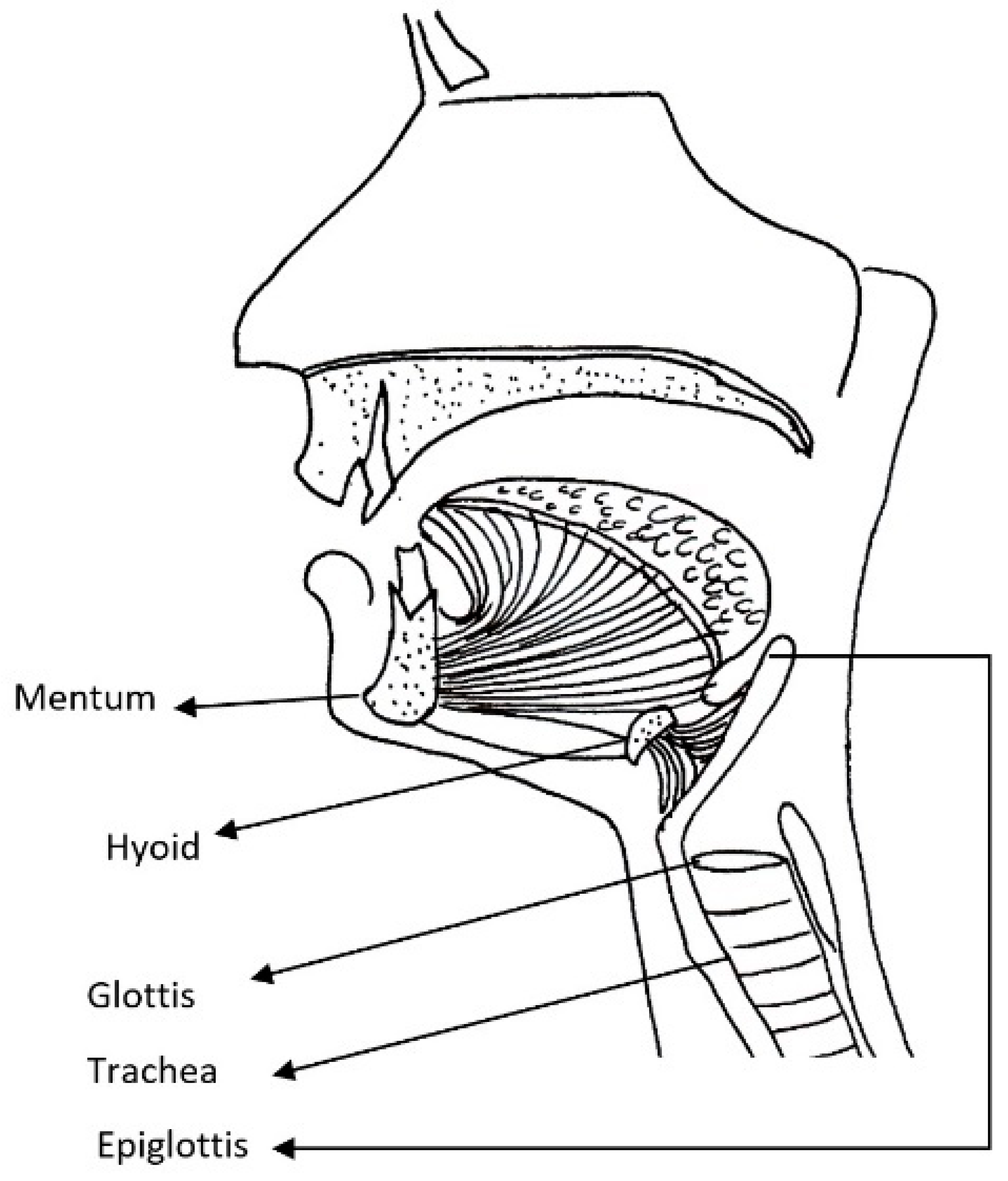
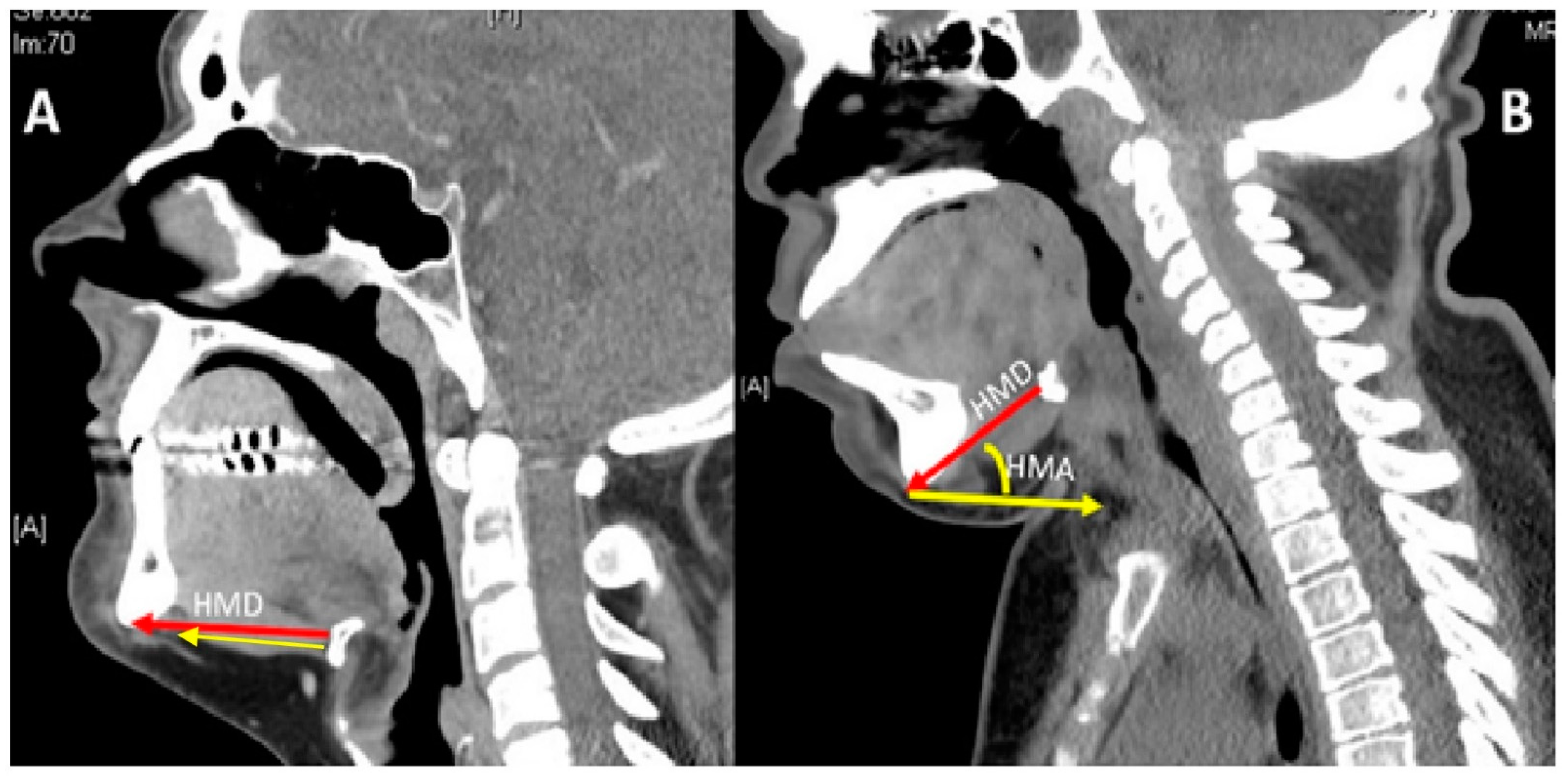
| MPS Type (Eponym) | Incidence per 105 Live Births; Inheritance Pattern | Typical Age at Diagnosis | Typical Life Expectancy If Untreated | Enzyme Deficiency | GAG |
|---|---|---|---|---|---|
| MPS I Hurler (H) MPS I Hurler-Scheie (H-S) MPS I Scheie (S) | 0.11–1.67; AR | H: <1 year H-S: 3–8 years S: 10–20 years | H: death in childhood H-S: death in teens or early adulthood S: normal to slightly reduced lifespan | α-L-iduronidase | DS, HS |
| MPS II (Hunter) | 0.1–1.07; XR | 1–2 years when rapidly progressing | rapidly progressing: death < 15 years slowly progressing: death in adulthood | iduronate-2-sulfatase | DS, HS |
| MPS III (Sanfilippo) A-B-C-D | 0.39–1.89; AR | 4–6 years | death in puberty or early adulthood | heparan sulfamidase (A) N-acetyl-α-D-glucosaminidase (B) acetyl-CoA-α-glucosaminidase N-acetyltransferase (C) N-acetylglucosamine-6-sulfatase (D) | HS |
| MPS IV (Morquio) A-B | 0.15–0.47; AR | 1–3 years | death in childhood- middle age | N-acetylgalactosamine-6-sulfatase (A) β-galactosidase (B) | CS, KS (A) KS (B) |
| MPS VI (Maroteaux-Lamy) | 0–0.38; AR | rapidly progressing: 1–9 years slowly progressing: >5 years | rapidly progressing: death in 2nd–3rd decade slowly progressing: death in 4–5th decade | N-acetylgalactosamine-4-sulfatase | DS |
| MPS VII (Sly) | 0–0.29; AR | neonatal to adulthood | death in infancy-4th decade ** | β-D-glucuronidase | CS, DS, HS |
| MPS IX (Natowicz) * | unknown | adolescence | unknown | hyaluronidase | CS |
| MPS | Non-MPS | |
|---|---|---|
| Number of patients | 50 | 50 |
| Males | 32 | 25 |
| Females | 18 | 25 |
| Age range in years | 19–66 | 22–84 |
| Mean age in years | 31.7 | 59.9 |
| Mean Body Mass index | 26.8 | 26.5 |
| Pathology | Number | Males | Females |
|---|---|---|---|
| MPS group | |||
| MPSI | 16 | 8 | 8 |
| MPSII | 13 | 13 | 0 |
| MPSIII | 1 | 1 | 0 |
| MPSIV | 14 | 6 | 8 |
| MPSVI | 6 | 4 | 2 |
| Total | 50 | 32 | 18 |
| Non-MPS group | |||
| Subglottic stenosis | 10 | 1 | 9 |
| Tracheal stenosis | 8 | 5 | 3 |
| Vasculitis | 7 | 4 | 3 |
| Malignancy not involving supraglottis, oropharynx | 16 | 12 | 4 |
| Bilateral vocal fold immobility | 5 | 0 | 5 |
| Vocal cord leukoplakia | 4 | 3 | 1 |
| Total | 50 | 25 | 25 |
| MPS | ||||||
| Age in Years | HMD in Millimeters | HMA in Degrees | Height in Centimeters | Weight in Kilograms | Body Mass Index | |
| N = 50 | ||||||
| Mean | 31.74 | 46.5 | 27.6 | 135.8 | 50.47 | 26.8 |
| Median | 29.50 | 48.5 | 25.0 | 136.8 | 48 | 25.7 |
| Range | 19–66 | 25.7–66.0 | −10.0 to 50 | 91.0–182 | 17.4–125.2 | 16.5–43.6 |
| Non-MPS | ||||||
| Age in Years | HMD in Millimeters | HMA in Degrees | Height in Centimeters | Weight in Kilograms | Body Mass Index | |
| N = 50 | ||||||
| Mean | 59.9 | 41.9 | 2.420 | 166.1 | 72.6 | 26.5 |
| Median | 63 | 40.9 | 0.0 | 166.0 | 71.9 | 26.9 |
| Range | 22–99 | 27.0–60.3 | −35.0 to 28 | 150.0–188 | 39.3–130 | 14.2–47.3 |
| Correlations MPS | ||||||
| HMD | HMA | HT | WT | BMI | ||
| HMD | rho | 1 | −0.2 | 0.28 * | 0.3 * | 0.2 |
| p-value | 0.15 | 0.05 * | 0.009 * | 0.13 | ||
| HMA | rho | −0.2 | 1 | −0.45 * | −0.41 * | −0.1 |
| p-value | 0.146 | 0.0001 * | 0.003 * | 0.73 | ||
| Correlations Non-MPS | ||||||
| HMD | HMA | HT | WT | BMI | ||
| HMD | rho | 1 | 0.15 | 0.1 | 0.35 | 0.27 |
| p-value | 0.3 | 0.49 | 0.014 | 0.06 | ||
| HMA | rho | 0.15 | 1 | −0.03 | −0.03 | 0.02 |
| p-value | 0.296 | 0.85 | 0.862 | 0.91 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadepalli, C.; Stepien, K.M.; Tol, G. Hyo-Mental Angle and Distance: An Important Adjunct in Airway Assessment of Adult Mucopolysaccharidosis. J. Clin. Med. 2021, 10, 4924. https://doi.org/10.3390/jcm10214924
Gadepalli C, Stepien KM, Tol G. Hyo-Mental Angle and Distance: An Important Adjunct in Airway Assessment of Adult Mucopolysaccharidosis. Journal of Clinical Medicine. 2021; 10(21):4924. https://doi.org/10.3390/jcm10214924
Chicago/Turabian StyleGadepalli, Chaitanya, Karolina M. Stepien, and Govind Tol. 2021. "Hyo-Mental Angle and Distance: An Important Adjunct in Airway Assessment of Adult Mucopolysaccharidosis" Journal of Clinical Medicine 10, no. 21: 4924. https://doi.org/10.3390/jcm10214924
APA StyleGadepalli, C., Stepien, K. M., & Tol, G. (2021). Hyo-Mental Angle and Distance: An Important Adjunct in Airway Assessment of Adult Mucopolysaccharidosis. Journal of Clinical Medicine, 10(21), 4924. https://doi.org/10.3390/jcm10214924







