Abstract
Background and Aims: Niemann–Pick disease (NPD) types A (NPA) and B (NPB) are caused by deficiency of the acid sphingomyelinase enzyme, which is encoded by the SMPD1 gene, resulting in progressive pathogenic accumulation of lipids in tissues. Trehalose has been suggested as an autophagy inducer with therapeutic neuroprotective effects. We performed a single-arm, open-label pilot study to assess the potential efficacy of trehalose treatment in patients with NPA and NPB patients. Methods: Five patients with NPD type A and B were enrolled in an open-label, single-arm clinical trial. Trehalose was administrated intravenously (IV) (15 g/week) for three months. The efficacy of trehalose in the management of clinical symptoms was evaluated in patients by assessing the quality of life, serum biomarkers, and high-resolution computed tomography (HRCT) of the lungs at the baseline and end of the interventional trial (day 0 and week 12). Results: The mean of TNO-AZL Preschool children Quality of Life (TAPQOL) scores increased in all patients after intervention at W12 compared to the baseline W0, although the difference was not statistically significant. The serum levels of lyso-SM-509 and lyso-SM were decreased in three and four patients out of five, respectively, compared with baseline. Elevated ALT and AST levels were decreased in all patients after 12 weeks of treatment; however, changes were not statistically significant. Pro-oxidant antioxidant balance (PAB) was also decreased and glutathione peroxidase (GPX) activity was increased in serum of patients at the end of the study. Imaging studies of spleen and lung HRCT showed improvement of symptoms in two patients. Conclusions: Positive trends in health-related quality of life (HRQoL), serum biomarkers, and organomegaly were observed after 3 months of treatment with trehalose in patients with NPA and NPB. Although not statistically significant, due to the small number of patients enrolled, these results are encouraging and should be further explored.
1. Introduction
Niemann–Pick disease (NPD) is a lysosomal storage disorder (LSDs) caused by the deficiency of acid sphingomyelinase activity (ASM) NP type A and B or cholesterol transporter function (NP type C) leading to lipid accumulation in different tissues and organs [1]. The estimated prevalence of NPA and NPB is 0.4–0.6 in 100,000 individuals [2]. Hepatosplenomegaly, pulmonary insufficiency, and profound central nervous system (CNS) involvement can lead to death in untreated patients within the first few years of life in NPA [3]. In contrast, NPB is the non-neuropathic form of the disorder with milder symptoms and clinical manifestations starting at later ages, with most patients reaching adulthood [4]. Low levels or total deficiency of ASM is the main cause of sphingomyelin accumulation and lipid abnormalities as well as downstream cell signaling pathways that affect ceramide generation as an important secondary pathway [1]. A common histopathological occurrence in NP patients is lipid-laden macrophages, also called foam cells, in the liver, spleen, lung airways, bone marrow, and cerebral cortex that lead to progressive destruction of target tissues [5]. Early diagnosis and treatment are required to attenuate outcome and to improve the quality of life in NP patients; however, bone marrow transplantation (BMT), enzyme replacement therapy (ERT), and other therapeutic approaches are still in stages of research and have not been adequately effective [6,7,8].
Trehalose is a natural non reducing (1–1 α-linkage) disaccharide in various organisms, from bacteria to animals, that exerts cell-protective effects under tensions, such as temperature, drought, and oxidative stress [9]. Trehalose has been recognized as a safe additive by the Joint WHO/FOA Expert Committee on Food Additive (JECFA) and U.S. Food and Drug Administration (FDA) in 2000, and was approved for use in food in Europe in 2001 [10]. Apart from basic and experimental evidence [11,12,13,14,15,16], several clinical trials were performed to evaluate the safety and efficacy of trehalose in healthy subjects or patients with different diseases, both orally and intravenously [17,18]. At doses up to 50 g, trehalose is safe for humans, and no adverse effect has been reported in most subjects; however, gastrointestinal side effects may occur in trehalose-deficient individuals [19].
In addition, trehalose has also been reported to prevent neuronal damage and attenuate neurodegenerative disorders caused by LSDs [19,20]. Antiaggregant, anti-inflammatory, and antioxidant properties, along with autophagy inducer, might be proposed as potential mechanisms of neuroprotective activities of trehalose in both cell cultures and in-vivo animal models [21,22]. Several lines of evidence suggest the chaperone-like activity of trehalose to prevent protein misfolding or aggregation and to contribute to clearance of accumulated proteins through promoting autophagy in neurodegenerative diseases (NDs) [21]. As such, trehalose is emerging as a novel therapeutic alternative to repressing oxidative stress and inflammation by decreasing the production of reactive oxygen species (ROS) and proinflammatory cytokines, such as interleukin 1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α), respectively [23]. Deposition of sphingomyelin and other lipids [24], neuroinflammation [25], and oxidative stress [26] have been considered as leading causes of NP. Therefore, trehalose might be effective at attenuating the negative outcomes in NP patients by reducing lipid accumulation, inflammation, and oxidative damage [9,27]. Trehalose can be used by either oral or intravenous (IV) administration; however, its absorption is decreased to 0.5% in the oral route due to enzymatic metabolization with Trehalase exhibiting in the intestinal brush border, and (IV) trehalose administration is more efficient for clinical trials [10,28]. Nevertheless, oral administration of trehalose in both preclinical and clinical studies of oculopharyngeal muscular dystrophy (OPMD) and Machado–Joseph disease (MJD) can stabilize neurological impairment and improve the severity of clinical disease scores [17,18].
This study reports clinical research aimed to investigate the efficacy of intravenous trehalose infusion (15 g/week) for a period of 12 weeks in five NPA and NPB patients.
2. Patients and Methods
2.1. Study Design and Participants
A single-arm, open-label pilot study was performed to assess trehalose therapeutic potential in NPA and NPB patients. All patients received IV trehalose infusions once a week (15 gr) for 90 min during three months of treatment. Follow-up visits were also conducted weekly during the study period. This clinical research was approved by the Ethics Committee of the Mashhad University of Medical Sciences, registered in the Iranian Registry of Clinical Trials (Code: IRCT20130829014521N16). Five patients aged 2–12 years old who had been diagnosed with NPA and NPB (confirmation by genotype and clinical examination) were considered for enrollment in the present study. The parents or legal guardians of the children signed the informed consent forms before any procedures were performed.
2.2. Test Substances
For our research, the pharmaceutical grade of trehalose has been used as a form of aqueous 15% solution in 100 mL sterile sealed vials manufactured by Dr. Rajabi Pharmaceutical Company, Khorasan Razavi, Iran.
2.3. Endpoints and Assessments
The main objective was to determine the therapeutic efficacy of trehalose in patients with NPA and NPB. Primary endpoints included quality of life assessment and reduction in serum biomarker levels (lysosphingomyelin-lysoSM, and lysosphingomyelin-509 (lysoSM-509)). The secondary endpoints of the study were to assess the condition of the liver, spleen, and lung, and measurement of the aminotransferases enzymes (AST and ALT levels), as well as oxidative stress status at the baseline and end of the interventional trial (day 0 and week 12).
2.3.1. Primary Endpoints
Quality of life assessment: TAPQOL (TNO-AZL Preschool children Quality of Life) index was used during this research to evaluate the physical, social, emotional, and cognitive function of patients. TAPQOL is a multidimensional questionnaire-parent form with 43 items comprising 12 scales, which was developed to measure health-related quality of life (HRQoL) in preschool children (aged 2–48 months) [29].
Sample preparation and lyso-SM and lyso-SM-509 quantification: Blood samples were obtained from all patients before and after treatment. Samples were collected in tubes containing serum gel separator and were centrifuged at 750× g for 20 min to obtain serum. Serum samples were aliquoted and were stored at −80 °C until required for measurements. Changes in serum lyso-SM and lyso-SM-509 levels were measured by ultra-performance liquid chromatography tandem mass spectrometry (UPLC–MS/MS) in a Xevo TQ-S micro (Waters Technology, Milford, MA, USA) at baseline (day 0) and the end of the study (week 12). The method used for the quantification of lyso-SM and lyso-SM-509 was adapted from Polo et al., 2019 [30].
2.3.2. Secondary Endpoints
Liver, spleen, and lung scans: Serum levels of alanine aminotransferase (ALT) and aspartate transaminase (AST) were measured by the kinetic method using a colorimetric assay kit to assess liver function at baseline and the end of the trehalose treatment period. Moreover, the spleen and liver size were measured using ultrasonography, and volumetric analyses were performed at the baseline and end of the study. Chest high-resolution computed tomography (HRCT) was also performed to compare the lung condition of patients between the W0 and W12.
Oxidative stress status: To evaluate whether trehalose could improve the antioxidant status, investigation of (anti)oxidant parameters pre- and post-treatment were performed by commercial kits (Kiazist; Iran). In this study, PAB (pro-oxidant antioxidant balance) was measured to evaluate the total oxidants and antioxidants in a single measurement simultaneously according to the previously described method [31], which is based on the oxidation of the chromogen 3,3′,5,5′-tetramethylbenzidine (TMB) to a color cation by pro-oxidants in an enzymatic reaction and reduction of the TMB cation to a colorless compound in a chemical reaction. The antioxidant enzyme activity of the glutathione peroxidase (GPx) was also assayed based on the reduction of hydrogen peroxide to water accompanied by the oxidation of glutathione.
2.3.3. Statistical Analysis
Statistical analysis was performed with GraphPad Prism version 8 software and Microsoft Excel (2019) The results were analyzed using paired t-test to evaluate the significance of differences before and after the treatment period. Results with p < 0.05 were considered statistically significant.
3. Results
3.1. Clinical Characteristics of Patients
Five male patients with a mean age of 4.4 years (range = 2–12 years of age) were enrolled who were diagnosed clinically and genetically with Niemann–Pick (NP) type A and B, genotype analysis results were homozygous. All children were born from consanguineous families. No subjects discontinued from the study, and all patients received all 12 of their scheduled doses.
3.2. Quality of Life Assessment
TAPQOL Test: To determine whether trehalose treatment could improve the health status in patients, we compared the TAPQOL score, which was used to assess the patients’ health-related quality of life between the W12 and W0. The TAPQOL index score can vary from 0–100, and higher scores indicate better quality of life. The TAPQOL score was elevated in all patients, and the mean score for quality of life was increased after intervention at W12 compared to the baseline (difference between means ± standard error mean (SEM): 6/000 ± 2/449), although the difference was not statistically significant. The results suggested an improvement in health-related quality of life after 12 weeks of trehalose treatment (Figure 1) in patients 2–5.
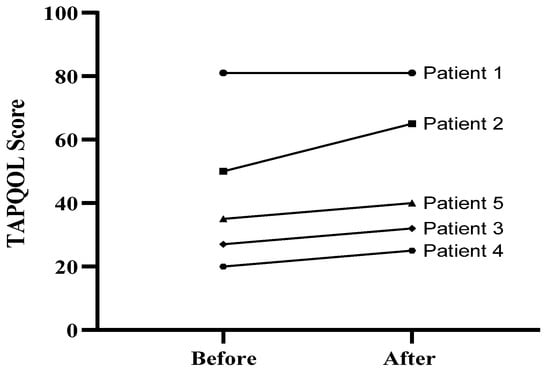
Figure 1.
TAPQOL score of five patients at baseline (week 0) and at the end of treatment (week 12).
3.3. Serum Lysosphingomyelin Levels (lyso-SM and lyso-SM509)
The levels of serum lysosphingomyelin are shown at baseline and week 12 (Figure 2). The average of lyso-SM-509 at the baseline was 30.511 (nmoL/L), while the average post-treatment is 25.051 (nmoL/L); the average of lyso-SM at the baseline was 72 (nmoL/L) while the average post-treatment was 12 (nmoL/L). Overall, out of five patients there was a reduction in the levels of lyso-SM-509 in three patients, and a reduction in levels of lyso-SM in four patients (Figure 2). However, the changes were not statistically significant.
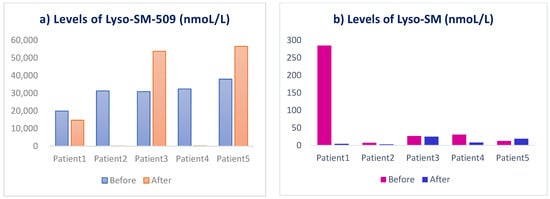
Figure 2.
Levels of serum lysosphingomyelin (a) lyso-SM509 and (b) lyso-SM in patients before and after 3 months treatment with trehalose.
3.4. Serum ALT and AST Levels
The average of ALT at the baseline was 94.40 (IU/L), while the average post-treatment was 14.40 (IU/L); the average of AST at the baseline was 94.20 (IU/L), while the average post-treatment was 21.80 (IU/L). Overall, there was a reduction in the levels of ALT and AST post-treatment (Figure 3). Although changes were not statistically significant, improved results (reduction in ALT and AST levels) showed improvement in liver function after trehalose treatment.
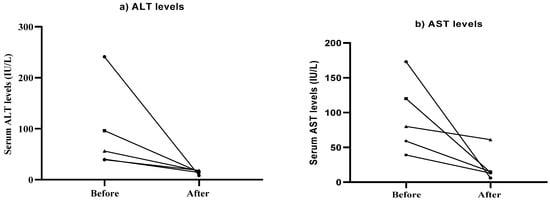
Figure 3.
Alterations in serum ALT (a) and AST (b) levels in patients following 12 weeks of treatment with trehalose.
3.5. Oxidative Stress Index (OSI)
The mean of pro-oxidant-antioxidant balance (PAB) before treatment was 10.734 (HK unit), while post-treatment was 11.018 (HK unit) (Figure 4b). The level of GPX activity before treatment was 7.93 (mU/mL), while post-treatment was 9.09 (mU/mL) (Figure 4a). Although, differences were not statistically significant neither in PAB values nor in GPX activity.
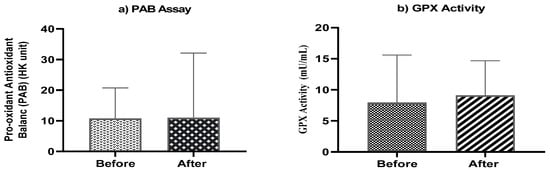
Figure 4.
(a) Mean serum pro-oxidant antioxidant balance (PAB) and (b) mean serum activities of GPX (mU/mL) at baseline and end of the study.
3.6. Sonographic Liver and Spleen Dimensions
Table 1 includes the alteration of spleen and liver size in patients pre-and post-treatment with trehalose. Although spleen size was found to have decreased in two patients (patients 2 and 4) compared with baseline, a progressive increase in the mean splenic length and the average liver volume was observed at the end of the study. It is worth mentioning that the liver diameter was reported by measuring the liver span below the costal margin in the midclavicular line by using the ultrasound scan because it could assist clinicians to confirm these changes in practice.

Table 1.
Spleen and liver diameter changes pre-and post-treatment with trehalose.
3.7. Lung HRCT
Follow-up HRCT chest was carried out in all patients. Improvement of symptoms in HRCT chest findings were observed in two patients out of five after 3 months treatment with trehalose (Figure 5).
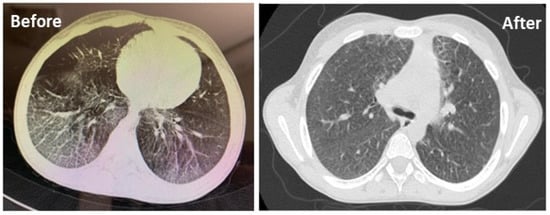
Figure 5.
HRCT scan of the chest showing ground–glass changes in both lung fields before and after 3 months of treatment with trehalose in a patient.
4. Discussion
ASMD (acid sphingomyelinase deficiency), also known as Niemann–Pick disease, is a rare autosomal recessive LSD that includes two subtypes (A and B) associated with lipid metabolism abnormalities and intracellular deposition of glycosphingolipids [32]. Abnormal lipid accumulation due to a deficiency of specific lysosomal enzymes has been shown to impact morphologic alterations in different tissues, leading to multi-organ failure and early death in children with NPA and NPB [3]. Currently, no effective treatment is available for NPA/NPB patients [33]. A considerable body of evidence suggests the role of impaired autophagy in the pathophysiology and progression of lipid storage disorders [34,35,36]. Therefore, the possible use of the autophagy-inducing compounds in decreasing lipid accumulation has been proposed to attenuate severe LSDs manifestations [37]. In recent years, trehalose has been described as a natural non-reducing disaccharide that promotes the autophagy process in both in vitro and in vivo models by activating transcription factor EB (TFEB) and enhancing target genes such as GLA, LAMP2A, MCOLN1, CTSB. Furthermore, it can also induce the autophagy process via the mTOR-independent pathway in cells of the nervous system [38,39,40,41]. This study aimed to evaluate the potential efficacy of IV trehalose (at a dose of 15 mg/week) in NPA and NPB patients. The dose of 15 mg/week was selected based on recent evidence and a previous similar clinical study showing the safety and efficacy of 15 mg IV trehalose in patients with Machado–Joseph disease (MJD) [18]. We hypothesized that trehalose could slow disease progression and improve neuropathologic features by decreasing sphingolipid deposition post three months of treatment. Disruption of sphingolipid homeostasis leads to several pathological consequences, and the accumulation of these metabolites can trigger a high level of apoptosis by activating proapoptotic genes and proteins [42,43]. Elevated levels of lysosphingolipids (lyso-SM and lyso-SM-509) have been identified as specific and reliable biomarkers for the diagnosis of NP and early assessment of drug effects during the treatment process in all types of NP (A/B and C), which might be detected via different methods in plasma, serum, or dried blood spots of patients [30,44]. The results of this study might suggest that treatment with trehalose could potentially lead to a decrease in the levels of both lyso-SM and lyso-SM-509; however, additional studies are required to further elucidate the efficacy of trehalose treatment and to confirm if the mechanism is associated with the autophagy-inducing effect of this small molecule that contributes to the clearance of accumulated lysosomal lipid substrates.
In addition, as important signaling mediators involved in the control of cell survival, sphingolipids also have an essential role in regulating proinflammatory cytokines and inflammation processes. Sphingosine-1-phosphate can induce interleukin 8 (IL-8) expression and activated protein 1 (AP-1) inflammatory transcriptional action via activating ERK and p38 MAPK pathways, which are involved in many inflammatory responses, particularly in lung inflammation and progressive respiratory failure [45,46]. Pulmonary involvement is considered one of the main causes of morbidity and mortality in NPA and NPB patients [47]. It has been shown that trehalose can attenuate inflammation in different animal models by reducing the production of inflammatory cytokines, such as TNF-α, MCP-1 (monocyte chemotactic protein-1) and PAI-1 (plasminogen activator inhibitor–1) [23]. Our lung function tests and high-resolution computed tomography (HRCT) findings showed improved lung function in two patients during three months of trehalose treatment that might be due to anti-inflammatory effects of trehalose and modulation of pro-inflammatory cytokines. Furthermore, recent studies have uncovered the link between sphingolipid deposition and cellular stress responses, such as ER and oxidative stress [48,49]. The accumulation of complex sphingolipid inositol phosphorylceramide (IPC) can increase ROS generation in mitochondria, which in turn decreases mitochondrial mass by activating Ras and affecting Snf1/AMPK pathways [49]. It has also been suggested that trehalose might exhibit a protective effect against oxidative stress by either upregulation of antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPX) [50,51], or scavenging ROS [52]. In line with this, we investigated pro-oxidant antioxidant balance and GPX activity after treatment to evaluate if trehalose has antioxidant effects. A decrease in levels of PAB and increased GPX activity could be due to the antioxidant activity of trehalose in the serum of NPA and NPB patients.
Besides anti-inflammatory and antioxidant properties, neuroprotective effects of trehalose to ameliorate neurological pathologies have been established in several experimental models of neurodegenerative diseases (NDs) [19]. Significant improvement has been observed on multiple behavioral tasks along with a marked increase in synaptophysin, doublecortin, and progranulin in the hippocampus and cortex of mice treated with oral administration of 2% trehalose for one month [53,54,55]. Moreover, a clinical study showed the effect of trehalose in patients with MJD with the optimal dose of 15 mg/week to improve disease severity and clinical symptoms [18]. Neurological involvement in NP varies in frequency and severity of disease, loss of mental abilities, and cognitive impairment more prominent in NPA, while type B patients tend to have milder symptoms with later-onset [56]. Our data confirmed previous similar reports in the literature and demonstrated significant improvement in health-related quality of life assessment through increased TAPQOL scores in four out of five patients after three months of treatment.
Hepatosplenomegaly accompanied by liver failure is another typical sign in NP patients [56]. Two clinical studies indicated liver dysfunction and elevated transaminase levels (ALT and AST) in 51% to 75% of NP patients [57,58]. Our results showed improvements in liver transaminase levels, and a reduction in the levels of both ALT and AST were observed in all patients treated with trehalose. Furthermore, a slight decrease in the spleen dimensions were found in two patients.
Our study has several limitations, including it being an open-label pilot research with limited sample size. Larger controlled, blind studies are required to demonstrate whether trehalose is effective in NPA and NPB patients. The length of treatment in our research is also not long enough to evaluate trehalose’s effects on behavioral problems. Finally, future dose-ranging studies are needed to indicate the optimal therapeutic dose of trehalose.
In conclusion, the treatment of NPA and NPB in patients with 15 mg/week of trehalose may be effective to reduce serum levels of sphingomyelins and possibly improving disease symptoms caused by lipid accumulation, although large-scale randomized trials with longer follow-up are needed to confirm whether trehalose has clinical efficacy in patients with LSDs.
Author Contributions
Conceptualization, M.M., M.K. and A.S.; data curation, F.K. and M.V.; investigation, R.V.; methodology, S.V. and S.J.S.; project administration, S.A.A.; validation, S.A.E.; writing–original draft, S.R., P.E., T.J. and O.R.; writing–review and editing, Ž.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Mashhad University of Medical Sciences (grant no. 981217) and the Iran National Science Foundation (grant no. 99014887).
Institutional Review Board Statement
This study was approved by the Ethics Committee of the Mashhad University of Medical Sciences (Ethics ID: IR.MUMS.REC.1398.257). Trial registration: IRCT20130829014521N16.
Informed Consent Statement
The parents or legal guardians of the children signed the informed consent forms before any procedures were performed.
Data Availability Statement
Data are available from the corresponding author upon a reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schuchman, E.H.; Desnick, R.J. Types A and B niemann-pick disease. Mol. Genet. Metab. 2017, 120, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Cerón-Rodríguez, M.; Vázquez-Martínez, E.R.; García-Delgado, C.; Ortega-Vázquez, A.; Valencia-Mayoral, P.; Ramírez-Devars, L.; Arias-Villegas, C.; Monroy-Muñoz, I.E.; López, M.; Cervantes, A.; et al. Niemann-Pick disease A or B in four pediatric patients and SMPD1 mutation carrier frequency in the Mexican population. Ann. Hepatol. 2019, 18, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Schuchman, E.H.; Wasserstein, M.P. Types A and B niemann-pick disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 237–247. [Google Scholar] [CrossRef]
- Ordieres-Ortega, L.; Galeano-Valle, F.; Mallén-Pérez, M.; Muñoz-Delgado, C.; Apaza-Chavez, J.E.; Menárguez-Palanca, F.J.; Alvarez-Sala Walther, L.A.; Demelo-Rodríguez, P. Niemann-Pick disease type-B: A unique case report with compound heterozygosity and complicated lipid management. BMC Med. Genet. 2020, 21, 94. [Google Scholar] [CrossRef] [PubMed]
- Pick, L., II. Niemann-Pick’s disease and other forms of so-called xanthomatosis. Am. J. Med. Sci. 1933, 185, 615–616. [Google Scholar] [CrossRef]
- McGovern, M.M.; Wasserstein, M.P.; Kirmse, B.; Duvall, W.L.; Schiano, T.; Thurberg, B.L.; Richards, S.; Cox, G.F. Novel first-dose adverse drug reactions during a phase I trial of olipudase alfa (recombinant human acid sphingomyelinase) in adults with Niemann-Pick disease type B (acid sphingomyelinase deficiency). Genet. Med. Off. J. Am. Coll. Med. Genet. 2016, 18, 34–40. [Google Scholar] [CrossRef]
- Coelho, G.R.; Praciano, A.M.; Rodrigues, J.P.; Viana, C.F.; Brandão, K.P.; Valenca, J.T., Jr.; Garcia, J.H. Liver transplantation in patients with niemann-pick disease--single-center experience. Transplant. Proc. 2015, 47, 2929–2931. [Google Scholar] [CrossRef]
- Victor, S.; Coulter, J.; Besley, G.; Ellis, I.; Desnick, R.; Schuchman, E.; Vellodi, A. Niemann–Pick disease: Sixteen-year follow-up of allogeneic bone marrow transplantation in a type B variant. J. Inherit. Metab. Dis. 2003, 26, 775–785. [Google Scholar] [CrossRef]
- Tang, Q.; Zheng, G.; Feng, Z.; Chen, Y.; Lou, Y.; Wang, C.; Zhang, X.; Zhang, Y.; Xu, H.; Shang, P.; et al. Trehalose ameliorates oxidative stress-mediated mitochondrial dysfunction and ER stress via selective autophagy stimulation and autophagic flux restoration in osteoarthritis development. Cell Death Dis. 2017, 8, e3081. [Google Scholar] [CrossRef]
- Richards, A.B.; Krakowka, S.; Dexter, L.B.; Schmid, H.; Wolterbeek, A.P.; Waalkens-Berendsen, D.H.; Shigoyuki, A.; Kurimoto, M. Trehalose: A review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem. Toxicol. 2002, 40, 871–898. [Google Scholar] [CrossRef]
- DeBosch, B.J.; Heitmeier, M.R.; Mayer, A.L.; Higgins, C.B.; Crowley, J.R.; Kraft, T.E.; Chi, M.; Newberry, E.P.; Chen, Z.; Finck, B.N.; et al. Trehalose inhibits solute carrier 2A (SLC2A) proteins to induce autophagy and prevent hepatic steatosis. Sci. Signal. 2016, 9, ra21. [Google Scholar] [CrossRef]
- Nazari-Robati, M.; Akbari, M.; Khaksari, M.; Mirzaee, M. Trehalose attenuates spinal cord injury through the regulation of oxidative stress, inflammation and GFAP expression in rats. J. Spinal Cord Med. 2019, 42, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour-Moghaddam, K.; Caraglia, M.; Sahebkar, A. Autophagy induction by trehalose: Molecular mechanisms and therapeutic impacts. J. Cell. Physiol. 2018, 233, 6524–6543. [Google Scholar] [CrossRef] [PubMed]
- Khalifeh, M.; Barreto, G.E.; Sahebkar, A. Trehalose as a promising therapeutic candidate for the treatment of Parkinson’s disease. Br. J. Pharmacol. 2019, 176, 1173–1189. [Google Scholar] [CrossRef] [PubMed]
- Khalifeh, M.; Read, M.I.; Barreto, G.E.; Sahebkar, A. Trehalose against Alzheimer’s disease: Insights into a potential therapy. BioEssays 2020, 42, 1900195. [Google Scholar] [CrossRef]
- Sahebkar, A.; Hatamipour, M.; Tabatabaei, S.A. Trehalose administration attenuates atherosclerosis in rabbits fed a high-fat diet. J. Cell. Biochem. 2019, 120, 9455–9459. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E.; Sarkar, S.; Rubinsztein, D.C. Trehalose reduces aggregate formation and delays pathology in a transgenic mouse model of oculopharyngeal muscular dystrophy. Hum. Mol. Genet. 2005, 15, 23–31. [Google Scholar] [CrossRef]
- Zaltzman, R.; Elyoseph, Z.; Lev, N.; Gordon, C.R. Trehalose in machado-joseph disease: Safety, tolerability, and efficacy. Cerebellum 2020, 19, 672–679. [Google Scholar] [CrossRef]
- Khalifeh, M.; Barreto, G.E.; Sahebkar, A. Therapeutic potential of trehalose in neurodegenerative diseases: The knowns and unknowns. Neural Regen. Res. 2021, 16, 2026–2027. [Google Scholar] [CrossRef]
- Lotfi, P.; Tse, D.Y.; Di Ronza, A.; Seymour, M.L.; Martano, G.; Cooper, J.D.; Pereira, F.A.; Passafaro, M.; Wu, S.M.; Sardiello, M. Trehalose reduces retinal degeneration, neuroinflammation and storage burden caused by a lysosomal hydrolase deficiency. Autophagy 2018, 14, 1419–1434. [Google Scholar] [CrossRef]
- Emanuele, E. Can trehalose prevent neurodegeneration? Insights from experimental studies. Curr. Drug Targets 2014, 15, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Assoni, G.; Frapporti, G.; Colombo, E.; Gornati, D.; Perez-Carrion, M.D.; Polito, L.; Seneci, P.; Piccoli, G.; Arosio, D. Trehalose-based neuroprotective autophagy inducers. Bioorg. Med. Chem. Lett. 2021, 40, 127929. [Google Scholar] [CrossRef]
- Taya, K.; Hirose, K.; Hamada, S. Trehalose inhibits inflammatory cytokine production by protecting IkappaB-alpha reduction in mouse peritoneal macrophages. Arch. Oral Biol. 2009, 54, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Gabandé-Rodríguez, E.; Boya, P.; Labrador, V.; Dotti, C.G.; Ledesma, M.D. High sphingomyelin levels induce lysosomal damage and autophagy dysfunction in Niemann Pick disease type A. Cell Death Differ. 2014, 21, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Cologna, S.M.; Cluzeau, C.V.; Yanjanin, N.M.; Blank, P.S.; Dail, M.K.; Siebel, S.; Toth, C.L.; Wassif, C.A.; Lieberman, A.P.; Porter, F.D. Human and mouse neuroinflammation markers in Niemann-Pick disease, type C1. J. Inherit. Metab. Dis. 2014, 37, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cañamás, A.; Benvegnù, S.; Rueda, C.B.; Rábano, A.; Satrústegui, J.; Ledesma, M.D. Sphingomyelin-induced inhibition of the plasma membrane calcium ATPase causes neurodegeneration in type A Niemann-Pick disease. Mol. Psychiatry 2017, 22, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Yaribeygi, A.; Sathyapalan, T.; Sahebkar, A. Molecular mechanisms of trehalose in modulating glucose homeostasis in diabetes. Diabetes Metab. Syndr. 2019, 13, 2214–2218. [Google Scholar] [CrossRef]
- Ohtake, S.; Wang, Y.J. Trehalose: Current use and future applications. J. Pharm. Sci. 2011, 100, 2020–2053. [Google Scholar] [CrossRef]
- Bunge, E.; Essink-Bot, M.-L.; Kobussen, M.; van Suijlekom-Smit, L.; Moll, H.; Raat, H. Reliability and validity of health status measurement by the TAPQOL. Arch. Dis. Child. 2005, 90, 351–358. [Google Scholar] [CrossRef]
- Polo, G.; Burlina, A.P.; Ranieri, E.; Colucci, F.; Rubert, L.; Pascarella, A.; Duro, G.; Tummolo, A.; Padoan, A.; Plebani, M.; et al. Plasma and dried blood spot lysosphingolipids for the diagnosis of different sphingolipidoses: A comparative study. Clin. Chem. Lab. Med. 2019, 57, 1863–1874. [Google Scholar] [CrossRef]
- Ghayour-Mobarhan, M.; Alamdari, D.H.; Moohebati, M.; Sahebkar, A.; Nematy, M.; Safarian, M.; Azimi-Nezhad, M.; Parizadeh, S.M.; Tavallaie, S.; Koliakos, G.; et al. Determination of prooxidant—Antioxidant balance after acute coronary syndrome using a rapid assay: A pilot study. Angiology 2009, 60, 657–662. [Google Scholar] [CrossRef]
- Von Ranke, F.M.; Pereira Freitas, H.M.; Mançano, A.D.; Rodrigues, R.S.; Hochhegger, B.; Escuissato, D.; Araujo Neto, C.A.; da Silva, T.K.; Marchiori, E. Pulmonary involvement in Niemann-Pick disease: A state-of-the-art review. Lung 2016, 194, 511–518. [Google Scholar] [CrossRef]
- Santos-Lozano, A.; Villamandos García, D.; Sanchis-Gomar, F.; Fiuza-Luces, C.; Pareja-Galeano, H.; Garatachea, N.; Nogales Gadea, G.; Lucia, A. Niemann-Pick disease treatment: A systematic review of clinical trials. Ann. Transl. Med. 2015, 3, 360. [Google Scholar] [CrossRef]
- Myerowitz, R.; Puertollano, R.; Raben, N. Impaired autophagy: The collateral damage of lysosomal storage disorders. EBioMedicine 2021, 63, 103166. [Google Scholar] [CrossRef]
- Pacheco, C.D.; Lieberman, A.P. The pathogenesis of Niemann–Pick type C disease: A role for autophagy? Expert Rev. Mol. Med. 2008, 10, e26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Canonico, B.; Cesarini, E.; Salucci, S.; Luchetti, F.; Falcieri, E.; Di Sario, G.; Palma, F.; Papa, S. Defective autophagy, mitochondrial clearance and lipophagy in Niemann-Pick type B lymphocytes. PLoS ONE 2016, 11, e0165780. [Google Scholar] [CrossRef] [PubMed]
- Rusmini, P.; Cortese, K.; Crippa, V.; Cristofani, R.; Cicardi, M.E.; Ferrari, V.; Vezzoli, G.; Tedesco, B.; Meroni, M.; Messi, E.; et al. Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy 2019, 15, 631–651. [Google Scholar] [CrossRef]
- Chen, X.; Li, M.; Li, L.; Xu, S.; Huang, D.; Ju, M.; Huang, J.; Chen, K.; Gu, H. Trehalose, sucrose and raffinose are novel activators of autophagy in human keratinocytes through an mTOR-independent pathway. Sci. Rep. 2016, 6, 28423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Higgins, C.B.; Mayer, A.L.; Mysorekar, I.U.; Razani, B.; Graham, M.J.; Hruz, P.W.; DeBosch, B.J. TFEB-dependent induction of thermogenesis by the hepatocyte SLC2A inhibitor trehalose. Autophagy 2018, 14, 1959–1975. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ren, J. mTOR-Independent autophagy inducer trehalose rescues against insulin resistance-induced myocardial contractile anomalies: Role of p38 MAPK and Foxo1. Pharmacol. Res. 2016, 111, 357–373. [Google Scholar] [CrossRef]
- Evans, T.D.; Jeong, S.-J.; Zhang, X.; Sergin, I.; Razani, B. TFEB and trehalose drive the macrophage autophagy-lysosome system to protect against atherosclerosis. Autophagy 2018, 14, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Phan, V.H.; Herr, D.R.; Panton, D.; Fyrst, H.; Saba, J.D.; Harris, G.L. Disruption of sphingolipid metabolism elicits apoptosis-associated reproductive defects in Drosophila. Dev. Biol. 2007, 309, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Mignard, V.; Dubois, N.; Lanoé, D.; Joalland, M.P.; Oliver, L.; Pecqueur, C.; Heymann, D.; Paris, F.; Vallette, F.M.; Lalier, L. Sphingolipid distribution at mitochondria-associated membranes (MAMs) upon induction of apoptosis. J. Lipid Res. 2020, 61, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, M.; Jinnoh, I.; Matsumoto, Y.; Narita, A.; Mashima, R.; Takahashi, H.; Iwahori, A.; Saigusa, D.; Fujii, K.; Abe, A.; et al. Structural determination of lysosphingomyelin-509 and discovery of novel class lipids from patients with Niemann–Pick disease type C. Int. J. Mol. Sci. 2019, 20, 5018. [Google Scholar] [CrossRef]
- Ghidoni, R.; Caretti, A.; Signorelli, P. Role of sphingolipids in the pathobiology of lung inflammation. Mediat. Inflamm. 2015, 2015, 487508. [Google Scholar] [CrossRef]
- Chandru, H.; Boggaram, V. The role of sphingosine 1-phosphate in the TNF-alpha induction of IL-8 gene expression in lung epithelial cells. Gene 2007, 391, 150–160. [Google Scholar] [CrossRef]
- Uyan, Z.S.; Karadağ, B.; Ersu, R.; Kiyan, G.; Kotiloğlu, E.; Sirvanci, S.; Ercan, F.; Dağli, T.; Karakoç, F.; Dağli, E. Early pulmonary involvement in Niemann-Pick type B disease: Lung lavage is not useful. Pediatric Pulmonol. 2005, 40, 169–172. [Google Scholar] [CrossRef]
- Park, W.J.; Park, J.W. The role of sphingolipids in endoplasmic reticulum stress. FEBS Lett. 2020, 594, 3632–3651. [Google Scholar] [CrossRef] [PubMed]
- Knupp, J.; Martinez-Montañés, F.; Van Den Bergh, F.; Cottier, S.; Schneiter, R.; Beard, D.; Chang, A. Sphingolipid accumulation causes mitochondrial dysregulation and cell death. Cell Death Differ. 2017, 24, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Mizunoe, Y.; Kobayashi, M.; Sudo, Y.; Watanabe, S.; Yasukawa, H.; Natori, D.; Hoshino, A.; Negishi, A.; Okita, N.; Komatsu, M.; et al. Trehalose protects against oxidative stress by regulating the Keap1-Nrf2 and autophagy pathways. Redox Biol. 2018, 15, 115–124. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, Q.; Xiao, Y.; Liu, X.; Li, Y.; Zhang, J.; Pan, J.; Zhang, Z. Trehalose targets Nrf2 signal to alleviate d-galactose induced aging and improve behavioral ability. Biochem. Biophys. Res. Commun. 2020, 521, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.F.; Kuo, Y.T.; Chen, T.Y.; Chien, C.T. Quercetin-rich guava (Psidium guajava) juice in combination with trehalose reduces autophagy, apoptosis and pyroptosis formation in the kidney and pancreas of type II diabetic rats. Molecules 2016, 21, 334. [Google Scholar] [CrossRef] [PubMed]
- Portbury, S.D.; Hare, D.J.; Sgambelloni, C.; Perronnes, K.; Portbury, A.J.; Finkelstein, D.I.; Adlard, P.A. Trehalose improves cognition in the transgenic Tg2576 mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 60, 549–560. [Google Scholar] [CrossRef]
- Portbury, S.D.; Hare, D.J.; Finkelstein, D.I.; Adlard, P.A. Trehalose improves traumatic brain injury-induced cognitive impairment. PLoS ONE 2017, 12, e0183683. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.; Marconi, M.; Musillo, C.; Chiarotti, F.; Bellisario, V.; Matarrese, P.; Gambardella, L.; Vona, R.; Lombardi, M.; Foglieni, C.; et al. Trehalose administration in C57BL/6N old mice affects healthspan improving motor learning and brain anti-oxidant defences in a sex-dependent fashion: A pilot study. Exp. Gerontol. 2020, 129, 110755. [Google Scholar] [CrossRef]
- McGovern, M.M.; Avetisyan, R.; Sanson, B.-J.; Lidove, O. Disease manifestations and burden of illness in patients with acid sphingomyelinase deficiency (ASMD). Orphanet J. Rare Dis. 2017, 12, 41. [Google Scholar] [CrossRef]
- McGovern, M.M.; Wasserstein, M.P.; Giugliani, R.; Bembi, B.; Vanier, M.T.; Mengel, E.; Brodie, S.E.; Mendelson, D.; Skloot, G.; Desnick, R.J.; et al. A prospective, cross-sectional survey study of the natural history of Niemann-Pick disease type B. Pediatrics 2008, 122, e341–e349. [Google Scholar] [CrossRef] [PubMed]
- Wasserstein, M.P.; Desnick, R.J.; Schuchman, E.H.; Hossain, S.; Wallenstein, S.; Lamm, C.; McGovern, M.M. The natural history of type B Niemann-Pick disease: Results from a 10-year longitudinal study. Pediatrics 2004, 114, e672–e677. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).